Abstract
Background:
Smoking is the main preventable cause of death in the United States and worldwide and is associated with serious cardiovascular health consequences, including thrombotic diseases. Recently, electronic cigarettes (e-cigarettes) and, in particular JUUL, have attained wide popularity among smokers, nonsmokers, pregnant females, and even the youth, which is alarming. Interestingly, there is/are no information/studies regarding the effect of JUUL on cardiovascular diseases, specifically in the context of modulation of platelet activation. Thus, it is important to discern the cardiovascular disease health risks associated with JUUL.
Methods and Results:
We used a passive e-vape vapor inhalation system where C57BL/6J mice (10-12 weeks old) were exposed to JUUL e-cigarette vape. Menthol flavored JUUL pods containing 5% nicotine by weight were used as the e-liquid. Mice were exposed to a total of 70 puffs daily for 2 weeks; 3-second puff duration, and 25-second puff interval. The effects of JUUL relative to clean air were analyzed, on mouse platelet function in vitro (eg, aggregation) and in vivo (eg, FeCl3-induced carotid artery injury thrombosis model). Our results indicate that short-term exposure to JUUL e-cigarette causes hyperactivation of platelets and shortens the thrombus occlusion as well as hemostasis/bleeding times, relative to clean air (medians of 14 vs. 200 seconds, P < .01 and 35 vs. 295 seconds, P < .001, respectively).
Conclusion:
Our findings document—for the first time—that short-term exposure to the JUUL e-cigarette increases the risk of thrombotic events, in part by modulating platelet function, such as aggregation and secretion, in mice.
Keywords: platelets, thrombosis, JUUL, e-cigarettes, vaping, cardiovascular disease
Introduction
It is well known that tobacco smoking is associated with cardiovascular disease (CVD), with the latter being the primary cause of death worldwide.1-3 In fact, tobacco smoking is the single most preventable risk factor for the development of CVD.2,4 While traditional cigarette smoking has been declining, new tobacco products have been, and continue to be introduced into the market.5,6 To this end, electronic cigarettes (e-cigarettes) have attained wide popularity in recent years, due to the perception of being a “safer” alternative to tobacco smoking.7 Thus, while usage of cigarettes is on the decline, the marketing of e-cigarettes, which deliver a similar addictive component and other toxicants, seems to have offset this decline. As the e-cigarette market keeps growing at a fast pace, hundreds of devices/models continue to emerge. One particular e-cigarette device that has gained significant popularity over the past 2 years is known as “JUUL,”8-10 reaching more than 50% of the retail market share of e-cigarettes.11 It has a novel slim USB-like design that makes it easy to use and carry, and it also comes with prefilled e-liquid pods that are available in various flavors.12,13 The unique design and appealing nature of the device and flavors along with the advertisement/marketing campaign have been attracting smokers, nonsmokers, and even the youth.14,15 Of particular concern is the fact that the youth has the highest increase in e-cigarettes usage,16,17 especially JUUL.13,18,19
JUUL has certain/unique features and does not resemble other traditional e-cigarette devices. For example, compared with other products in the market, the e-liquid composition of the JUUL pods contains nicotine salt and higher concentrations of nicotine (3% and 5% by volume).12,20 It has been claimed that these devices are “totally safe” and “much safer than cigarettes.”21 However, despite these claims, converging lines of evidence indicate that the concentrations of chemicals in the JUUL pods are cytotoxic and may have detrimental effects on human health.20
Based on these considerations, it is paramount to establish the safety of exposure to JUUL e-cigarette, particularly in the context of CVD. To this end, limited studies have evaluated the impact of traditional e-cigarettes on the genesis of thrombosis-based CVD. Nonetheless, we have previously shown—in mice—that short-term e-cigarette exposure modulates physiological hemostasis and elevates the risk of thrombosis via altering platelet function.22 In addition, a recent crossover study that was conducted on tobacco smokers and nonsmokers found that acute use of e-cigarettes enhanced platelet aggregation.23 However, to the best of our knowledge, there is no information regarding the impact of JUUL exposure on the cardiovascular system, particularly in the context of platelet function. Therefore, this study aims to characterize the impact of JUUL exposure on thrombogenesis and platelet function by using a mouse whole-body exposure system and a protocol that mimics real-life exposure scenarios of daily JUUL user(s). Our findings demonstrate that 2-week exposure to the JUUL e-cigarette does indeed enhance the risk of thrombosis and potentiate hemostasis and that this is due to its capacity to enhance platelet activity. These findings indicate that the JUUL e-cigarette is not safe as people curently believe.
Materials and Methods
Materials and Reagents
JUUL device and JUUL Pods (5% nicotine and menthol flavoring) were purchased from the JUUL official website. Absolute 0 e-liquid (18-mg Nicotine, 30% PG, and 70% VG with a menthol flavor) was obtained from The Vapor Chef. Adenosine diphosphate (ADP) and thrombin were purchased from Sigma Aldrich. The anti-P-selectin and Annexin V antibodies were fluorescein isothiocyanate (FITC)–conjugated and purchased from Cell Signaling Technology, Inc. The JON/A antibody was purchased from Emfret analytics. The Mouse/Rat Cotinine enzyme-linked immunosorbent assay (ELISA) kit was from Calbiotech. The stir bars and other experimental disposable material was obtained from Chrono-Log Corporation. The rest of the reagents were of analytical grade.
Animals
C57BL/6J (10- to 12-week-old male) mice were obtained from Jackson Laboratory. The mice were housed under standard conditions (24 °C; 12/12 light/dark cycles; ad libitum access to water and food), with 1 to 5 mice in each group. The Institutional Animal Care and Use Committee of The University of Texas at El Paso approved all of our animal experimental protocols.
JUUL E-Cigarette and Traditional E-Cigarette Exposure Protocol
We exposed the C57BL/6 mice (10-12 weeks old males) to 70 puffs/day of JUUL for a period of 2 weeks. The duration of each puff was 3 seconds (with a puff volume of 50 mL) with puff intervals of 25 seconds. These experimental variables were designed in order to mimic real-life exposure scenarios.22 With regard to the control mice—which were exposed to clean air—they were males and matched in terms of age to the JUUL e-cigarette exposed mice. The JUUL e-cigarette exposed and control mice were deprived of food and water during exposure. The apparatus used to generate the aerosol (e-vape) is a bench-top e-Vape™ vapor inhalation system for rodents (custom-made by the La Jolla Alcohol Research, Inc). The system has 4 chambers each connected to its own “mode box.” The “mode box” is custom made with a USB outlet to be compatible with the JUUL device and is connected to a controller that allows us to automatically predetermine the following experimental conditions: the puff time/duration, puff interval (time between puffs), and number of puffs. In terms of the JUUL pods, they contain 0.7 mL of e-liquid, and the nicotine concentration was 5% (by weight) with menthol flavoring.8,24 Of note, the menthol flavor was chosen for consistency with our previous study on traditional e-cigarettes,22 as well as the fact that it is one of the 3 flavors still available in the market and has been used by adults. The traditional e-cigarette exposure protocol was similar to the JUUL protocol in terms of the number of puffs per day, the puff duration, and exposure duration. As for the device, we used the third-generation device as described previously,22 namely the SMOK TFV4 Mini Tanks, whereas we used the Vapor Chief e-liquid (18 mg/mL nicotine content) with a menthol flavor.
Cotinine Assay
The urinary cotinine levels, a known nicotine metabolite, were measured in both JUUL and clean air-exposed mice, by using an ELISA kit (the Cotinine Direct ELISA kit) as per the manufacturer’s (Calbiotech) instructions and as described previously.25
In Vivo Thrombosis Model
This experiment was performed as previously described.22,25,26 JUUL e-cigarette and clean air-exposed mice were anesthetized by IP injections of avertin. Next, we exposed/cleaned the left carotid artery, before measuring its baseline blood flow using a 0.5-mm micro-flow probe (Transonic Systems Inc). After the blood flow stabilized, 7.5% ferric chloride (FeCl3) was applied onto a 1-mm diameter filter paper disc. The filter paper was placed immediately onto the left carotid artery for a total of 3 minutes. The carotid artery blood flow was monitored until its stoppage ([stable occlusion] ie, zero blood flow for a total of 2 minutes), with the monitoring lasting no longer than 30 minutes. The findings were documented, with the time to the carotid artery blockade (occlusion) calculated as the difference in time between stable occlusion and removal of the FeCl3 filter paper. As indicated, a 30-minutes occlusion time served as the cutoff time for statistical analysis purposes.
Tail Bleeding Time
Physiological hemostasis was assessed by using the standard tail bleeding/transection method.22,25,27 Briefly, mice were anesthetized before 5 mm of their tail was transected from the tip, using a scalpel. Immediately afterward, the tail was immersed in 37 °C saline, and the time it took for the bleeding to stop was measured, with 10-minute bleeding time serving as the cutoff for statistical analysis purposes.
Peripheral Blood Cell/Platelet Counts
The hematology profile, including platelets and white blood cells of the JUUL/clean air-exposed mice was performed on whole blood using a HEMAVET 950FS Multi-Species Hematology System (Erba Diagnostics).
Murine Platelet-Rich Plasma Preparation
The clean air-, the JUUL e-cigarette-, or the traditional e-cigarette-exposed mice (C57BL/6J; 10-12 weeks old) were anesthetized before their blood was harvested from the heart. A sodium citrate solution (0.38%; from Fisher Scientific) was used to inhibit blood coagulation. Next, the blood was centrifuged for 15 minutes, 237 g at room temperature (RT), before the platelet-rich plasma (PRP) was collected. The platelets in PRP were counted by using the HEMAVET and their count adjusted (7 × 107 platelets/mL) before initiating each of the specific experiments.
Washed Platelet Preparation
Washed platelets were prepared as described previously.25,28 Blood was drawn from mice as described earlier, and it was mixed with phosphate-buffered saline (pH 7.4), incubated with PGI2 (10 ng/mL; 5 minutes), to ensure there is no platelet activation. Platelets underwent centrifugation (10 minutes, RT; at 237g). Platelet-rich plasma was then collected before pelleting the platelets at 483g for 10 minutes at RT. Next, the platelet pellet was resuspended in HEPES/Tyrode buffer that was mixed with 1 mmol/L EGTA, 0.37 U/mL apyrase, as well as PGI2 (10 ng/mL). Platelets were washed and resuspended in HEPES/Tyrodes (pH 7.4) without EGTA, apyrase, or PGI2. Finally, the platelets were counted using the HEMAVET and the counts were adjusted.
In Vitro Platelet Aggregation
The PRP from the exposed mice (clean air, JUUL e-cigarettes, or traditional e-cigarettes) was activated using either 1 μM ADP or 0.1 U/mL thrombin. The aggregation of platelets was assessed using an aggregometer (turbidimetric method; model 700; Chrono-Log Corporation). Each experiment was repeated at least 3 times, with blood pooled from 5 to 8 mice each time.
Dense Granule/Adenosine Triphosphate Secretion
The PRP (250 μL) with count adjusted to 7 × 107/mL was placed into siliconized cuvettes and stirred at 37 °C for 5 minutes. Next, we added 12.5 µL of the Chrono-Log luciferase substrate/luciferase cocktail, which was followed by the addition of 1 μM ADP or 0.1 U/mL thrombin.
Flow Cytometric Analysis
The flow cytometry experiments were conducted using washed platelets as described previously.25 Thus, platelets were activated using 1 μM ADP or 0.1 U/mL thrombin for 3 minutes, after which the reactions were stopped using 2% formaldehyde (30 minutes; RT) to fix the platelets. Next, platelets were incubated with one of the following antibodies (in the dark at RT) for 30 minutes: JON/A/phycoerythrin-conjugated αIIbβ3, FITC-conjugated anti-P-selectin, or FITC-conjugated Annexin V. Platelets were diluted using HEPES/Tyrode buffer (pH 7.4; 2.5-fold), before being transferred to FACS tubes. Finally, the fluorescent intensities were measured by using a BD Accuri C6 flow cytometer. The data were analyzed using the CFlow Plus software (BD Biosciences).
Statistical Analysis
Experiments were conducted at least 3 times, as applicable. The data were analyzed using the GraphPad PRISM statistical software, version 7.0 and presented as mean ± SD. The difference in the mean of the bleeding and occlusion times was evaluated using the Mann–Whitney U test. Of note, the t test and/or 1-way analysis of variance (with Tukey’s multiple regression comparison as post hoc) were also used as applicable for comparative analysis purposes and comparable results were observed. The significance was accepted at P < .05, unless otherwise is stated.
Results
JUUL E-Cigarette Exposure Results in the Systematic Delivery of Nicotine
To verify that the whole-body exposure model does systematically deliver nicotine to the mice, cotinine, the major metabolite of nicotine, was measured, thus serving as a marker of its delivery.29 Therefore, ELISA was used to measure the urinary levels of cotinine, which was analyzed “immediately” after the conclusion of the 2-week exposure, and our data showed significantly elevated levels of cotinine in the urine of JUUL e-cigarette exposed mice, whereas it was undetectable in the control clean air mice (Figure 1).
Figure 1.
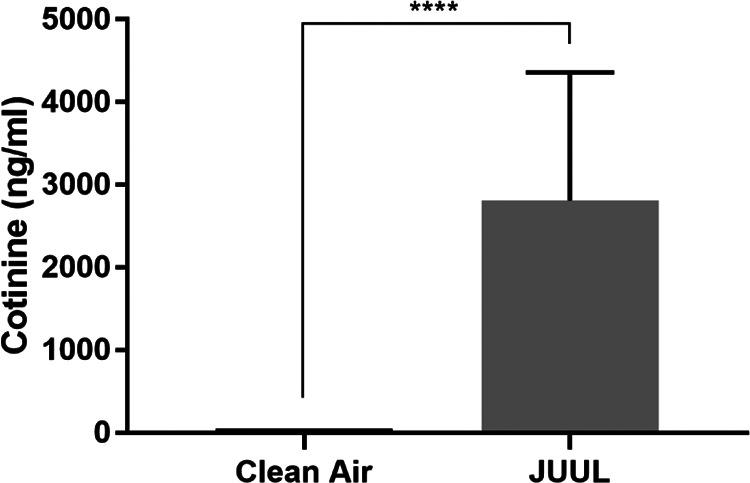
JUUL exposure results in delivering nicotine (cotinine) systemically. Urinary cotinine levels from JUUL and clean air-exposed mice (clean air, n = 5; and JUUL, n = 5; ****P < .0001; data are presented as mean ± SD).
JUUL E-Cigarette-Exposed Mice Exhibit Enhanced Physiological Hemostasis and Thrombus Formation
In terms of characterizing the negative cardiovascular effects of JUUL e-cigarette, we initially investigated its capacity to modulate physiological hemostasis by using the tail bleeding time assay. Our findings show that JUUL-exposed mice have significantly shortened tail bleeding time compared to mice exposed to clean air (Figure 2A; median of 35 vs. 295 seconds, respectively; P < .001). Next, the thrombotic effect of JUUL e-cigarette exposure was examined by performing the carotid artery injury-induced thrombosis model. It was observed that JUUL-exposed mice have remarkably shortened occlusion time compared to their controls (Figure 2B; median of 14 vs. 200 seconds, respectively; P < .01). Notably, there was no difference in the platelet and/or other blood cell counts between the JUUL and clean air-exposed mice (Table 1).
Figure 2.
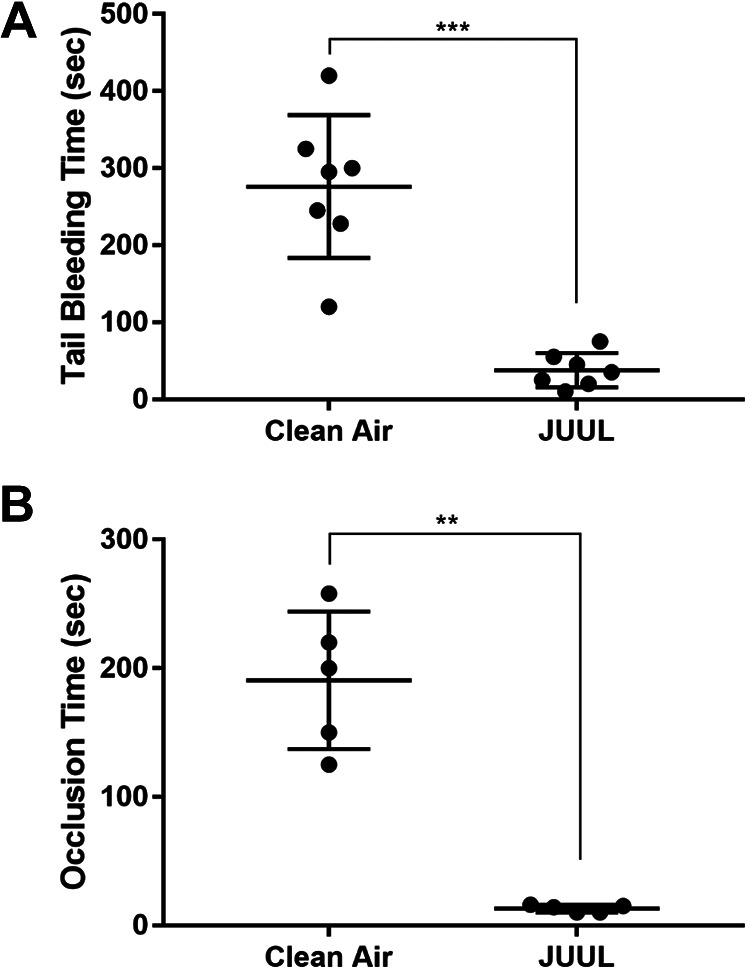
JUUL exposure shortens the bleeding time in the tail bleeding time assay, and the time to occlusion in the ferric chloride in vivo thrombosis model. A, The tail bleeding time assessment was performed on the clean air and JUUL e-cigarette exposed mice. Each point represents the tail bleeding time of a single animal (clean air, n = 7; and JUUL, n = 7; ***P < .001). B, The ferric chloride-induced thrombosis model was performed on the JUUL e-cigarette and clean air-exposed mice. Each point represents the occlusion time of a single animal (clean air, n = 5; and JUUL e-cigarette, n = 5; **P < .01).
Table 1.
Peripheral Blood Cell Counts in JUUL- and Clean Air-Exposed Mice.a
| Cell type | Clean air | JUUL | P value |
|---|---|---|---|
| Platelets | 519 ± 115.2 | 535 ± 133 | .86 |
| MPV | 4.63 ± 0.21 | 4.68 ± 0.17 | .72 |
| Red blood cells | 5.81 ± 1.38 | 6.47 ± 1.73 | .58 |
| Lymphocytes | 2.79 ± 0.89 | 1.78 ± 0.58 | .11 |
| Monocytes | 0.18 ± 0.03 | 0.15 ± 0.08 | .50 |
| Granulocytes | 4.43 ± 2.15 | 3.4 ± 0.83 | .41 |
| HCT | 27.75 ± 6.92 | 31.4 ± 9.16 | .55 |
Abbreviation: MPV, mean platelet volume.
a Blood cells were counted as described in Methods. The counts are expressed as thousands per microliter, with the exception of the red blood cells that are expressed as millions per microliter. The data are presented as mean ± SD.
JUUL E-Cigarette Exposure Enhances ADP- and Thrombin-Induced Platelet Aggregation
We next sought to investigate whether the JUUL e-cigarette exposure would also produce platelet effects. Consequently, the effects of the JUUL e-cigarette exposure on ADP- and thrombin-induced platelet aggregation were assessed. Our data revealed that exposure to JUUL e-cigarettes results in higher ADP (1 µM)-induced platelet aggregation, in comparison to clean air control platelets (Figure 3A; inset shows data quantification). Similarly, 0.1 U/mL thrombin-induced platelet aggregation was also elevated/enhanced in the JUUL e-cigarette exposed platelets (Figure 3B; inset shows data quantification). Interestingly, furthermore, when compared with traditional e-cigarettes, it was found that JUUL enhances 1µM ADP-triggered aggregation to a much larger extent than traditional e-cigarettes (Figure 3C; inset shows data quantification).
Figure 3.
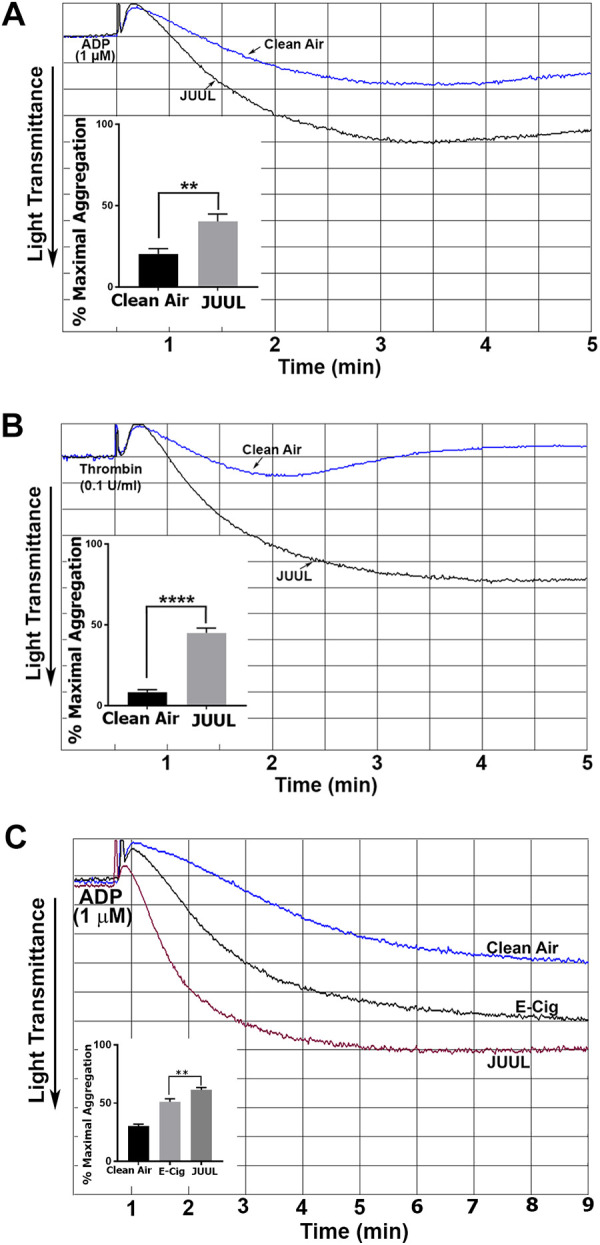
Platelet aggregation is potentiated in JUUL exposed mice. Platelets from JUUL e-cigarette and clean air-exposed mice were stimulated with 1 μM ADP (A) or 0.1 U/mL thrombin (B). Platelets from JUUL, a traditional e-cigarette (indicated as “E-Cig”) and clean air-exposed mice, were stimulated with 1 μM ADP (C). Aggregation was measured using an aggregometry system. Inset shows quantification of data; **P < .01 and ****P < .0001. Experiments were repeated 3 times, using blood that was pooled from 5 to 8 mice each time.
JUUL E-Cigarette Exposure Enhances ADP- and Thrombin-Induced Platelet Secretion
It was also determined if JUUL e-cigarette exposure exerts any impact on dense and α-granule secretion. In line with our aggregation data, ATP secretion from platelets obtained from the JUUL e-cigarette-exposed mice in response to ADP (1 µM; Figure 4A; inset shows data quantification) and thrombin (0.1 U/mL; Figure 4B; inset shows data quantification) agonist stimulation were found to be enhanced, when compared to platelets from the clean air-exposed mice. Moreover, JUUL e-cigarette exposed platelets had significantly higher ADP- and thrombin-triggered P-selectin expression (Figure 5A).
Figure 4.
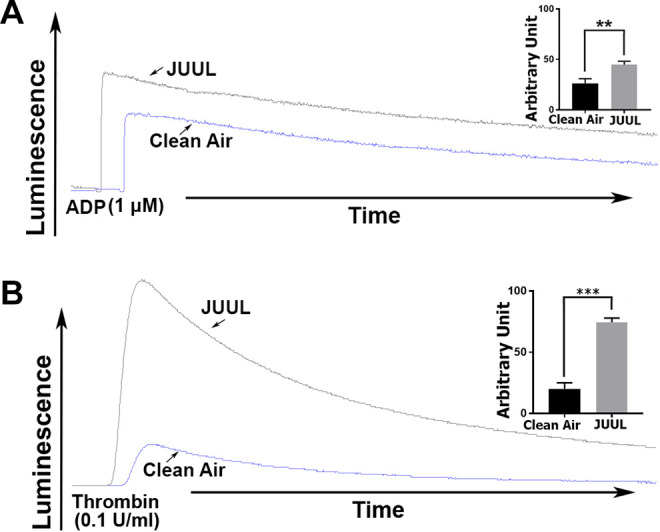
Platelet dense granule secretion is enhanced in JUUL exposed mice. Platelets from clean air- or JUUL e-cigarette-exposed mice were incubated with 12.5 µL of luciferase luciferin, before activation with 1 μM ADP (A) or 0.1 U/mL thrombin (B). Dense granule/ATP release was assessed by a lumi-aggregometer and detected as luminescence. Inset shows quantification of data; **P < .01 and ***P < .0001.These experiments were repeated 3 times, using blood that was pooled from 5 to 8 mice each time.
Figure 5.
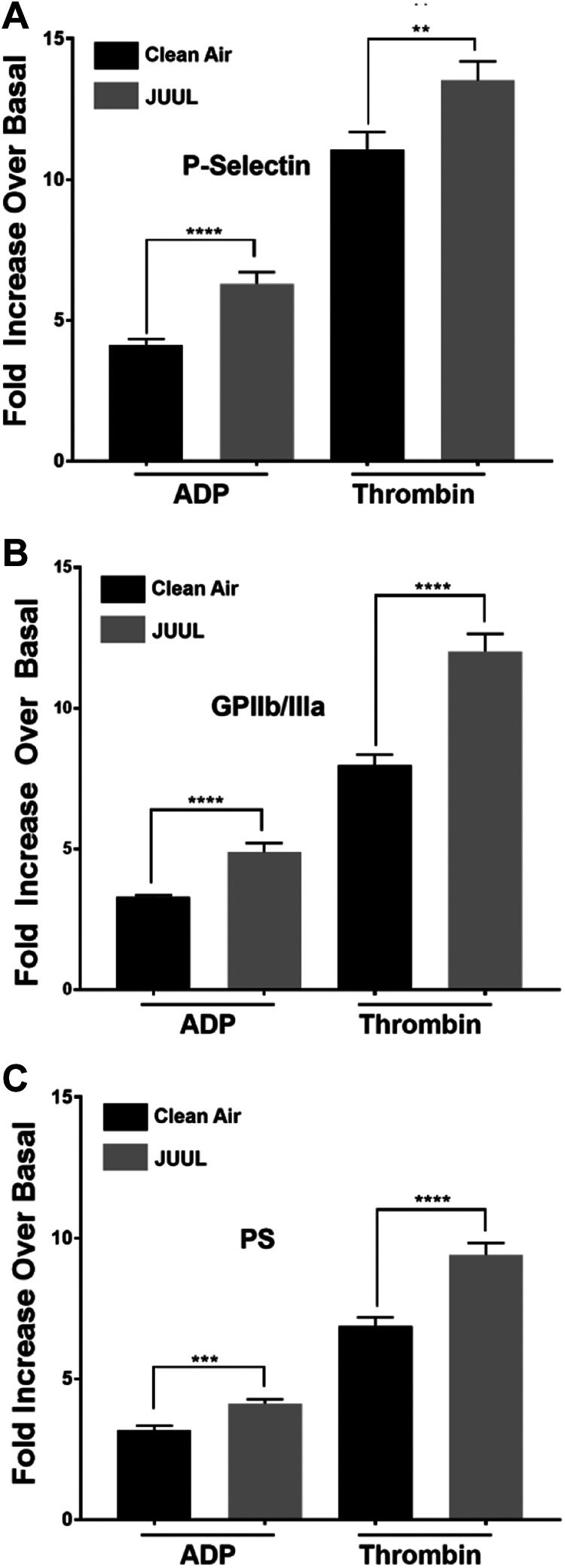
Platelets α granule secretion, integrin GPIIb/IIIa activation, and phosphatidylserine (PS) exposure are increased in JUUL-exposed mice. Washed platelets from clean air-exposed and JUUL e-cigarette-exposed mice were washed and activated with 1 μM ADP or 0.1 U/mL thrombin. Next, these platelets were incubated with FITC-conjugated CD62P antibody, before the fluorescence intensity was measured by flow cytometry (A). The average of the mean fluorescence intensities is shown (****P < .0001). Platelets were incubated with phycoerythrin-conjugated JON/A antibody, before the fluorescence intensity was measured by flow cytometry (B). The average of the mean fluorescence intensities is shown (****P < .0001). Platelets were incubated with fluorescein isothiocyanate–conjugated Annexin V antibody, before the fluorescence intensity was measured by flow cytometry (C). The average of the mean fluorescence intensities is shown (****P < .0001). These experiments were repeated 3 times, using blood that was pooled from 5 to 8 mice each time. Data are presented as mean ± SD.
JUUL E-Cigarette Exposure Enhances Agonist-Induced Integrin GPIIb/IIIa Activation and Phosphatidylserine Expression
The next set of studies investigated if there is a commensurate increase in integrin GPIIb/IIa (αIIbβ3) activation in the JUUL e-cigarette exposed platelets, given their increased aggregation phenotype. It was found that JUUL e-cigarette-exposed platelets had an enhanced agonist-mediated activation of GPIIb/IIa, when compared to clean air-exposed platelets (Figure 5B). Moreover, agonist-mediated phosphatidylserine (PS) expression, which is a critical element for efficient activation of the coagulation cascade and an essential aspect of clot formation, was also found to be enhanced in the JUUL platelets (Figure 5C).
Discussion
The JUUL e-cigarette has been gaining popularity at an alarming rate since its introduction into the US market in 2015 and by 2018 has overtaken more than half of the e-cigarette market.9,14,12 In this connection, it was marketed by JUUL Labs, the manufacturer, as an e-cigarette device with a higher safety profile than traditional cigarettes,7 which we know is not supported by evidence. Its appealing USB-like design and pre-filled pods led to increased use, especially and of more concern, among the youth.24,30 It is also important to note that the usage of these devices is fundamentally different than traditional cigarettes. Thus, in traditional cigarettes, the cigarette burns away after a while and there is nothing “left” for the user to consume unless they light another cigarette, which provides a check/decision point for the user. On the other hand, JUUL/e-cigarettes do not have this checkpoint, until the pod/e-liquid is consumed. This means that with JUUL/e-cigarette, the user may consume the amount of nicotine in 1 pack of traditional cigarettes in a much shorter time (puffing behavior).
In terms of the cardiovascular safety of e-cigarettes, while we recently showed that exposure to traditional e-cigarettes enhances physiological hemostasis and increases the risk of thrombosis via modulating platelet function, in adult mice,22 whether JUUL exerts similar negative health effects remains to be investigated. This is important given its wide popularity and unique features. Thus, this issue was investigated by using a mouse whole-body in vivo exposure model and a protocol that mimics real-life exposure scenarios of a daily JUUL user.18 It has been recently reported that daily JUUL users consume 10 pods per 1 month, which equals 1 pod every 3 days. Since each pod can generate 200 puffs, it was decided to expose mice to 70 puffs per day to resemble human settings.18 Given that the JUUL e-cigarette has only recently been introduced into the market, human studies related to its effect on the general health, and the cardiovascular system in specific are nonexistent. Thus, mouse models can serve as “readily available” and practical tools to provide rather rapid answers to some of the safety questions, which may involve years of use for human studies. Furthermore, human studies are complicated by the variability of e-liquids and flavors, patterns of use, user’s experience, and the concomitant use of other tobacco products; all of which would make it difficult to draw conclusions. Notably, given these challenges in human studies, the current mouse model gives flexibility in mimicking many aspects of human use, and the literature does support the fact that mouse studies “map” very well to humans in the context of tobacco exposure.22
Initially, our model was validated by measuring the levels of cotinine, a key biomarker of nicotine exposure,31 in urine samples of JUUL e-cigarette and clean air-exposed mice. The cotinine levels increased significantly in JUUL e-cigarette-exposed mice, whereas it was undetectable in those exposed to clean air. Indeed, these high cotinine levels are not surprising given the fact that each JUUL pod (200 puffs) contains a high concentration of nicotine (5% by weight), which is equivalent to 1 pack of cigarettes.24,32 Furthermore, the use of nicotine salt results in high amounts of nicotine being delivered to users as the salt masks the bitter taste of nicotine.33 Consistent with our finding, a recent study showed that youth are exposed to higher concentrations of nicotine when they use JUUL, compared to combustible cigarettes.34 Taken together, JUUL e-cigarette delivers a high amount of nicotine to users, which raises concerns regarding the potential negative impact of nicotine, especially among youth users,32 which warrants investigation. Since JUUL exposure can increase the plasma levels of nicotine by many folds, this would be of particular concern in individuals with preexisting CVD. Additionally, one should also consider the concomitant use of other substances (eg, alcohol, stimulants, etc) and/or medications (eg, α-blockers, hormonal contraceptives, etc) that could potentiate nicotine’s negative effects.35 Of note, while e-cigarettes have (potential) toxicants such as nicotine, propylene glycol/glycerol, and flavors,36 as mentioned before, the JUUL pods contain nicotine salts, whose toxicity is virtually unknown. Notably, the effect of nicotine on thrombus formation is controversial, with some studies showing it to be increased,37 decreased,38 or even unaffected.39 However, our recent waterpipe/hookah exposure studies showed that nicotine does have the capacity to enhance biochemical markers of platelet activation.25 It is also important to note that nicotine has a short half-life in vivo, and hence it is rapidly metabolized, particularly into cotinine, and the latter was shown to enhance platelet activation.25 Also, while the toxicity of e-cigarettes continues to be under investigation, there is evidence suggesting that they may have become a gateway to the known and harmful tobacco cigarettes.40,41
As for the effects of JUUL on the genesis of thrombosis-dependent CVD, our mouse model data demonstrated for the first time that exposure to JUUL (2 weeks) does increase the risk of thrombosis, as reflected by the prolonged occlusion time, when compared to the clean air controls. In addition, JUUL exposure altered physiological hemostasis as evidenced by the shortened tail bleeding time. A similar prothrombotic phenotype was also observed in short-term exposure to traditional e-cigarettes.22 In support of these findings, a recent meta-analysis concluded that e-cigarettes should not be marketed as “cardiovascularly” safe devices as more evidence needs to be collected.42 It is noteworthy that endothelial cell dysfunction and oxidative stress, which also play important roles in the pathogenesis of cardiovascular/thrombotic disease, were also found to be associated with e-cigarettes.43,44 Collectively, our findings clearly indicate that JUUL e-cigarettes pose a negative cardiovascular (thrombotic) health impact, contrary to the claimed safety by JUUL Labs.7
In light of the observed prothrombotic phenotype and since platelets are key players in thrombus formation, we sought to investigate the impact/effects of JUUL e-cigarette on platelet function. Our data revealed that JUUL exposure enhances platelet function, including aggregation, secretion, integrin GPIIb/IIIa activation, and PS exposure, compared to the control. Furthermore, our studies also revealed that JUUL’s capacity to enhance platelet aggregation was significantly higher than that of traditional e-cigarettes. While the underlying cause(s) of these effects is/are unknown, they could derive from the different devices and/or concentrations of nicotine. These data indicate that JUUL e-cigarette-exposed platelets are hyperactive, which could explain, at least partially, the observed prothrombotic phenotype. Supportingly, our previously published data with traditional e-cigarette exposure showed similar trends of increased platelet function, in mice.22 These data are also consistent with a recent human study in which acute e-cigarette use was found to increase platelet activation.23 In addition, a separate in vitro study found that e-cigarette vapor extracts enhanced aggregation and adhesion responses45 of platelets from healthy humans. Of note, while our data clearly revealed a detrimental effect for JUUL, it does not address the role of nicotine, solvent and/or flavor, if any, which will be the scope of a future investigation.
In conclusion, our study documents for the first time that whole-body JUUL e-cigarette exposure alters physiological hemostasis and increases the risk of thrombosis, in mice, in part by modulating platelet function. Importantly, these findings provide clear/initial evidence that the JUUL e-cigarette is not safe as currently claimed and should be the focus of further investigations. In addition, our study is expected to promote public awareness of the negative health impact of these devices, especially among the youth, including those who had never smoked before but tried JUUL with the perception that these devices are safe. Given the current epidemic of e-cigarette use, specifically JUUL, our data are expected to inform and guide the FDA in developing scientifically/evidence-driven regulations to limit the use of JUUL devices.
Footnotes
Author Contributions: Ramirez performed experiments, analyzed data, and wrote manuscript. Karim performed experiments and analyzed data. Alarabi performed experiments and analyzed data. Ben Taleb wrote manuscript and assisted in data interpretation. Rivera contributed to conceptualization of studies and edited manuscript. Khasawneh and Rivera contributed to conceptualization of studies, analyzed data, and edited manuscript. Alshbool and Rivera contributed to conceptualization of studies, analyzed data, and wrote manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Environmental Health Sciences and the National Heart, Lung, and Blood Institute, of the National Institutes of Health under Awards Number R21ES029345 and R01HL145053. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Our studies were also funded using startup funds from The University of Texas at El Paso’s School of Pharmacy (to Alshbool).
ORCID iD: Zubair A. Karim  https://orcid.org/0000-0001-9376-551X
https://orcid.org/0000-0001-9376-551X
Fatima Z. Alshbool  https://orcid.org/0000-0002-2765-3250
https://orcid.org/0000-0002-2765-3250
References
- 1. Centers for Disease Control and Prevention. CDC death report 2014. 2014.
- 2. Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of electronic cigarettes on the cardiovascular system. J Am Heart Assoc. 2017;6(9):e006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Cardiovascular diseases (CVDs). May 17, 2017. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)#targetText=Key%20facts,31%25%20of%20all%20global%20deaths
- 4. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control Prevention. Smoking & Tobacco Use. February 6, 2019. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm#targetText=Smoking%20is%20the%20leading%20cause%20of%20preventable%20death.&targetText=Cigarette%20smoking%20is%20responsible%20for,resulting%20from%20secondhand%20smoke%20exposure
- 6. Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco product use among middle and high school students—United States, 2011–2017. Morb Mortal Wkly Rep. 2018;67(22):629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U.S. Food & Drug Administration. WARNING LETTER—JUUL Labs, Inc. September 09, 2019 https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/juul-labs-inc-590950-09092019
- 8. Allem JP, Dharmapuri L, Unger JB, Cruz TB. Characterizing JUUL-related posts on Twitter. Drug Alcohol Depend. 2018;190:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brett EI, Stevens EM, Wagener TL, et al. A content analysis of JUUL discussions on social media: using Reddit to understand patterns and perceptions of JUUL use. Drug Alcohol Depend. 2019;194:358–362. [DOI] [PubMed] [Google Scholar]
- 10. Kavuluru R, Han S, Hahn EJ. On the popularity of the USB flash drive-shaped electronic cigarette Juul. Tob Control. 2019;28(1):110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28(2):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control. 2019;28(1):115–116. [DOI] [PubMed] [Google Scholar]
- 13. Fadus MC, Smith TT, Squeglia LM. The rise of e-cigarettes, pod mod devices, and JUUL among youth: factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019;201:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keamy-Minor E, McQuoid J, Ling PM. Young adult perceptions of JUUL and other pod electronic cigarette devices in California: a qualitative study. BMJ Open. 2019;9(4):e026306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Food & Drug Administration. Youth Tobacco Use: Results from the National Youth Tobacco Survey. May 29, 2019. https://www.fda.gov/tobacco-products/youth-and-tobacco/youth-tobacco-use-results-national-youth-tobacco-survey
- 16. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health; 2016. [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Youth and Tobacco Use. 2015
- 18. Leavens ELS, Stevens EM, Brett EI, et al. JUUL electronic cigarette use patterns, other tobacco product use, and reasons for use among ever users: results from a convenience sample. Addict Behav. 2019;95:178–183. [DOI] [PubMed] [Google Scholar]
- 19. Kim AE, Chew R, Wenger M, et al. Estimated ages of JUUL Twitter followers. JAMA Pediatr. 2019;173(7):690–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P. High-nicotine electronic cigarette products: toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol. 2019;32(6):1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. U.S. Food and Drug Administration. FDA warns JUUL Labs for marketing unauthorized modified risk tobacco products, including in outreach to youth. 2019.
- 22. Qasim H, Karim ZA, Silva-Espinoza JC, et al. Short-term E-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. J Am Heart Assoc. 2018;7(15):e009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nocella C, Biondi-Zoccai G, Sciarretta S, et al. Impact of tobacco versus electronic cigarette smoking on platelet function. Am J Cardiol. 2018;122(9):1477–1481. [DOI] [PubMed] [Google Scholar]
- 24. McKelvey K, Baiocchi M, Halpern-Felsher B. Adolescents’ and young adults’ use and perceptions of pod-based electronic cigarettes. JAMA Netw Open. 2018;1(6):e183535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alarabi AB, Karim ZA, Ramirez JEM, et al. Short-term exposure to waterpipe/hookah smoke triggers a hyperactive platelet activation state and increases the risk of thrombogenesis. Arterioscler Thromb Vasc Biol. 2020;40(2):335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hensch NR, Karim ZA, Pineda J, Mercado N, Alshbool FZ, Khasawneh FT. P2Y12 antibody inhibits platelet activity and protects against thrombogenesis. Biochem Biophys Res Commun. 2017;493(2):1069–1074. [DOI] [PubMed] [Google Scholar]
- 27. Qasim H, Karim ZA, Hernandez KR, Lozano D, Khasawneh FT, Alshbool FZ. Arhgef1 plays a vital role in platelet function and thrombogenesis. J Am Heart Assoc. 2019;8(9):e011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alshbool FZ, Karim ZA, Espinosa EVP, Lin OA, Khasawneh FT. Investigation of a thromboxane A2 receptor-based vaccine for managing thrombogenesis. J Am Heart Assoc. 2018;7(13):e009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leone A. Biochemical markers of cardiovascular damage from tobacco smoke. Curr Pharm Des. 2005;11(17):2199–2208. [DOI] [PubMed] [Google Scholar]
- 30. Krishnan-Sarin S, Jackson A, Morean M, et al. E-cigarette devices used by high-school youth. Drug Alcohol Depend. 2019;194:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raja M, Garg A, Yadav P, Jha K, Handa S. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04–EZ06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goniewicz ML, Boykan R, Messina CR, Eliscu A, Tolentino J. High exposure to nicotine among adolescents who use JUUL and other vape pod systems (‘pods’). Tob Control. 2019;28(6):676–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talih S, Salman R, El-Hage R, et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control. 2019;28(6):678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goniewicz ML, Boykan R, Messina CR, Eliscu A, Tolentino J. High exposure to nicotine among adolescents who use JUUL and other vape pod systems (‘pods’). Tob Control. 2019;28(6):676–677. tobaccocontrol-2018-054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917–1921. [DOI] [PubMed] [Google Scholar]
- 36. Sassano MF, Davis ES, Keating JE, et al. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16(3):e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hioki H, Aoki N, Kawano K, et al. Acute effects of cigarette smoking on platelet-dependent thrombin generation. Eur Heart J. 2001;22(1):56–61. [DOI] [PubMed] [Google Scholar]
- 38. Girdhar G, Xu S, Bluestein D, Jesty J. Reduced-nicotine cigarettes increase platelet activation in smokers in vivo: a dilemma in harm reduction. Nicotine Tob Res. 2008;10(12):1737–1744. [DOI] [PubMed] [Google Scholar]
- 39. Ljungberg LU, Persson K, Eriksson AC, Green H, Whiss PA. Effects of nicotine, its metabolites and tobacco extracts on human platelet function in vitro. Toxicol In Vitro. 2013;27(2):932–938. [DOI] [PubMed] [Google Scholar]
- 40. Lee P, Fry J. Investigating gateway effects using the PATH study. F1000Res. 2019;8:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watkins SL, Glantz SA, Chaffee BW. Association of noncigarette tobacco product use with future cigarette smoking among youth in the Population Assessment of Tobacco and Health (PATH) Study, 2013-2015. JAMA Pediatr. 2018;172(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skotsimara G, Antonopoulos AS, Oikonomou E, et al. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(11):1219–1228. [DOI] [PubMed] [Google Scholar]
- 43. Schweitzer KS, Chen SX, Law S, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L175–L187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fetterman JL, Weisbrod RM, Feng B, et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38(7):1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27(7):694–702. [DOI] [PubMed] [Google Scholar]


