Background
On March 9, 2020, Canada confirmed its first death related to COVID-19. Soon after, the World Health Organization (WHO) declared the global outbreak of COVID-19 a pandemic.1 This pandemic has put escalating pressure on Canada’s health care system, increasingly straining the supply of qualified health care providers.2 Pharmacists are currently among those health care professionals on the front line, providing essential health care services such as dispensing, counselling and various clinical services.
Most individuals infected with the COVID-19 virus have been reported to experience mild to moderate respiratory illness and recover without requiring special treatment. Common symptoms among reported cases in Canada included cough (77%), headache (57%) and general weakness (56%).3 Older individuals and those with underlying medical conditions such as cardiovascular disease, diabetes, chronic respiratory disease and cancer are more likely to develop serious illness, resulting in the majority of hospitalizations, ICU admissions and deaths.4
Drug shortages have been an issue for many years before COVID-19. A drug shortage is defined as a situation “in which the manufacturer that sets out the drug identification number assigned for a drug is unable to meet the demand for the drug.”5 Drug shortages are not a novel concern and have been an ongoing serious and growing problem within the Canadian health care system and globally. Drug shortages can occur for many reasons, including manufacturing and quality assurance issues, delays and discontinuations. These shortages have been shown to increase the risk of medication errors, adverse events and patient nonadherence, and they cause considerable stress for patients and pharmacists alike.6,7
Health Canada lists and updates all notifications on the Drug Shortages Canada (DSC) website, where their prioritization is categorized in tiers. Each tier mandates different actions from various stakeholders, including drug manufacturers, government authorities, supply chain and health care professionals. Tier 1 refers to anticipated drug shortages, where future supply by the drug manufacturer or importer may not meet current demands, thereby resulting in an early communication for all stakeholders. Tier 2 involves actual drug shortages, where a drug shortage is definitely expected to occur. Tier 3 are actual drug shortages of increased severity due to the drug in question being produced by only one manufacturer and for which there are limited or no alternative therapies.8
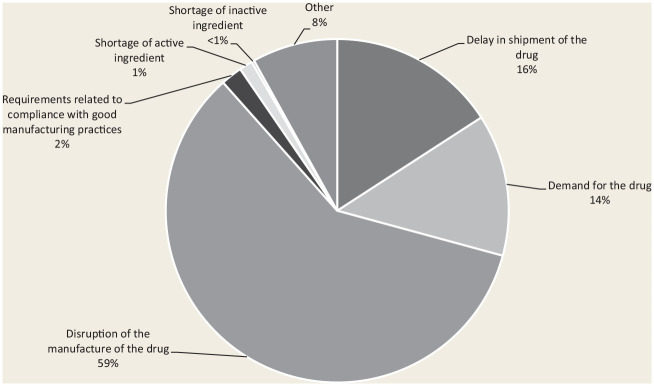
Between January 1, 2020, and March 31, 2020, the majority (59%) of drug shortages reported were a result of manufacturing disruption (Figure 1). The majority of manufacturers in Canada use single-source foreign suppliers, primarily in China and India, for raw materials.6,7 Further, many manufacturers source these materials from the same suppliers. Therefore, when raw material supply or manufacturing processes are halted in a major international manufacturing facility, the local manufacturer is subsequently confronted with a lack of supply. On January 23, 2020, nearly a month after the first case reports of COVID-19 were relayed to the WHO, China placed Hubei province, home to 42 pharmaceutical contract manufacturing facilities, on lockdown. According to the WHO, about 74% of COVID-19 infections diagnosed in China were in Hubei province, as of February 8, 2020 (Wuhan is the capital of Hubei). The resulting epidemic led the Chinese government to impose a curfew on Hubei and to isolate the entire province from the rest of China.
Figure 1.
Reasons for actual drug shortages reported on Drug Shortages Canada between January 1 and March 31, 2020
On March 3, India, which imports the majority of active pharmaceutical ingredients (APIs) from China, announced that it would pause the exportation of 26 drugs and APIs due to a shortage of raw ingredients from China.9,10 Since the pharmaceutical industry in Canada depends on China and India for these ingredients, it is likely that our market has been and will continue to be affected by this decrease in exports.11 Between March 1 and 31, 2020, DSC reported a total of 215 actual drug shortages, an increase since January and February, when there were 123 and 85, respectively.5 Through the month of March, a total of 180 medications were in shortage, whereas between April 1 and May 13, the total drug shortage was already 418. A drug listed on the DSC website may still be available in the supply chain, and a listing on DSC does not mean an immediate shortage at the pharmacy level.5
With the ongoing COVID-19 crisis, demand for drugs spanning a variety of therapeutic areas, including respiratory and infectious diseases, has escalated. Between January 1 and March 31, 2020, 14% of reported drug shortages, such as salbutamol metered-dose inhalers and antibiotics such as azithromycin, were a result of an increase in demand. Unanticipated increases in demand ultimately result in insufficient quantities to meet current and future needs.12 The reasons for the increase in demand are vast but are generally due to hoarding behaviour and the influence of media encouraging the use of pharmaceutical agents as preventive options for SARS-COV-2. Hydroxychloroquine, an antimalarial used to prevent and treat malaria, in addition to treating rheumatoid arthritis and lupus erythematosus, was recently publicized as a possible treatment for COVID-19, with only very weak evidence for its efficacy. This widespread attention may have influenced demand, resulting in an actual drug shortage of its generic brand on March 19, 2020. The response to this was to allocate inventory. From a clinical perspective, this drug shortage has affected patients for whom the drug is indicated for malaria and rheumatoid arthritis.13,14 Furthermore, patients diagnosed with lupus erythematosus could be facing a double whammy—both a shortage of hydroxychloroquine and a greater risk of becoming infected with COVID-19, given the immunologic nature of lupus. Other drugs in limited supply include paralyzing agents such as vecuronium, pancuronium and atracurium; inhalers such as salbutamol metered-dose inhalers; anesthetic agents such as ketamine and propofol; and analgesia injectables such as morphine, hydromorphone and fentanyl.15 The supply of over-the-counter drugs such as acetaminophen and medical supplies such as thermometers has also been heavily affected.
The COVID-19 pandemic can create domino shortages—where drug A becomes unavailable and drug B is used as a replacement, causing a shortage of drug B. A prime example is when Sandoz Canada recalled ranitidine in mid-September 2019 and the most common therapeutic alternative was famotidine.16 By September 26, 2019, famotidine was in short supply, with a proposed return date of late March 2020.5 From mid-March to April 2, 2020, several inhalation products containing salbutamol, beginning with the generic products, were listed as shortages. The brand product also faced this increase in demand, resulting in the decision to place the inventory on allocation.5
As of late March 2020, both salbutamol metered-dose inhalers and hydroxychloroquine have been listed as Tier 3 drug shortages, having the greatest potential impact on our health care system nationwide. In response to the COVID-19 pandemic, Health Canada has deemed these drugs eligible to be included as part of the interim order under the Food and Drugs Act allowing Canada to import drugs (and other medical supplies) under exceptional circumstances.16
Pharmacists are crucially important in identifying, reporting and managing potential cases of COVID-19 as well as providing safeguards for medication distribution amid escalating drug shortages. As the first point of contact with our health care system for many Canadians, pharmacists must remain current on COVID-19 research updates, including available treatment options. Now, more than ever, patients will rely on their most accessible health care provider for advice on navigating a media environment with overwhelming information and pseudoscience. Pharmacists must use their ethical and professional judgment to make allocation decisions supported by evidence-based recommendations. The importance of a particular drug to a given patient’s outcome is a critical factor to consider when making this decision. Pharmacists are the experts at finding alternatives when the ideal drug is not available and are the best resources for other health care professionals in these situations.
Strategies and recommendations to support the role of pharmacists to help combat the pandemic.
Managing drug shortages to ensure continuity of care
Pharmacists
Pharmacists should monitor drug supply chain closely to be informed of the reason for drug shortage so they can act swiftly and accordingly. Drugshortagescanada.ca is a reputable resource with updated information.
Pharmacists should investigate and collaborate with prescribers regarding any prescriptions for off-label or office use of drugs such as hydroxychloroquine and should monitor updates on medications currently under investigation for combating COVID-19.
Community pharmacists should, in their professional and ethical judgment, prioritize prescriptions for the continuation of existing medication therapy, inpatient settings and other indications where there is no alternative therapy.
Pharmacists should advise patients against hoarding medications or stockpiling by adhering to a 30-day medication supply.17
Pharmacists should work with physicians and other health professionals during this difficult time to continue to provide excellent care to our immunocompromised patients who are more vulnerable to COVID-19 and to ensure they receive their prescribed medications.
Lifesaving drugs for symptom management such as salbutamol are on critical allocation. Pharmacists need to use their professional judgment in dispensing only 1 inhaler for each patient and to look into alternative therapy, if necessary. Pharmacists should ask all patients about their inhaler use prior to dispensing to ensure medications are taken as prescribed to avoid overuse. Overuse of a rescue inhaler may require the need to reevaluate therapy.
Pharmacists should continue to practise antimicrobial stewardship. Some antibiotics being used to treat COVID-19-associated pneumonia are currently on critical allocation, such as ceftriaxone, azithromycin and piperacillin-tazobactam. Pharmacists must use their expertise to suggest alternative therapies to available products with similar microbial coverage or suggest watchful waiting practices where clinically appropriate.
COVID-19 trial medications: No pharmacist should dispense hydroxychloroquine or chloroquine except when written as prescribed for an indication approved by Health Canada. The Infectious Diseases Society of America, in its recently released COVID-19 guidelines, recommended that because of uncertainty regarding the risks and benefits of hydroxychloroquine, its use should limited to clinical trials.18 Pharmacists must keep up to date on new COVID-19 treatment options.
No prescription should be dispensed for chloroquine or hydroxychloroquine unless all of the following apply (may vary between individual provinces depending on their respective governing body legislations): (a) the prescription bears a written diagnosis from the prescriber consistent with the evidence for its use; (b) the prescription is limited to no more than 14 days’ supply unless the patient was previously established on the medication; and (c) no refill may be permitted unless the current fill is finished. Prescriptions for chloroquine, hydroxychloroquine, mefloquine and azithromycin should be restricted in the outpatient setting and should require a diagnosis “consistent with the evidence for its use.”
Recent shortages have affected drugs such as proton pump inhibitors, which creates an opportunity to reassess whether therapy is still indicated or necessary.5 Pharmacists should leverage available resources (e.g., deprescribing.org and the Canadian Deprescribing Network) and collaborate with prescribers to consider deprescribing when possible. This will benefit the patient and alleviate the demand impact on current drug shortage. Virtual means of interprofessional communication (such as contacting prescribers remotely if they are not in office) should be considered in cases where in-person collaboration is made difficult during the pandemic.
Regulatory bodies and government entities
Health Canada should promote sharing of drug supply between provinces and sites across the country, as should pharmacy advocacy groups. We are all in this together.
Provincial government entities should collaborate with one another to ensure that reliable information is readily accessible to all practising members. The federal government should communicate lessons from countries around the world so we may learn from international experiences.
Pharmacy regulatory bodies, in partnership with governments, should authorize all frontline pharmacists, in the event of a drug shortage, to dispense a therapeutic substitute with documentation and communication to the prescriber. Pharmacists have demonstrated that they are capable of assessing individual situations and determining the most appropriate alternative therapy specific to a patient’s condition and current medication regimen.19 The expanded scope needs to be enacted in all provinces and territories. Recently, Health Canada issued a temporary exception, pursuant to the Controlled Drug and Substances Act, allowing pharmacists to renew and adapt a prescription for a controlled substance, including narcotics, in addition to accepting a verbal order and transferring a prescription for a controlled drug. This is only a small portion of what pharmacists can contribute to mitigate health care resource depletion.15
Professional regulatory and advocacy bodies across Canada should continue to work together as a collective voice to monitor any inappropriate prescribing and stockpiling of investigational coronavirus drugs (such as hydroxychloroquine, azithromycin and lopinavir/ritonavir), which are heavily mentioned in the media, to prevent shortages of these drugs, which are potentially harmful if used inappropriately. On March 23, 2020, the Registered Nurses’ Association of Ontario (RNAO), Ontario Medical Association (OMA) and Ontario Pharmacist Association (OPA), representing more than 110,000 health care providers, issued a joint statement addressing the use of a hydroxychloroquine/azithromycin combination for COVID-19 prophylaxis and treatment. In this statement, the group collectively advised members to be diligent in our efforts to not let blind hope drive decisions and reminded providers of their roles as antibiotic stewards and safeguards of limited supplies of medication.19
Health care leaders should further advocate for their frontline pharmacists to make strides towards increased clinical roles, and those roles could be enhanced by providing the time and approvals needed to support the recommendations made by pharmacists.
Pharmacy supply chain and retailers
Independent pharmacies should coordinate and work together to support patients in this crisis and not compete at this difficult time. This may include sharing medication supply and COVID-19 resources.
Drug suppliers should continue to be transparent and act in a just manner in distributing medications on shortage to community pharmacies and hospitals.
Prescribing for ambulatory conditions
Regulatory bodies may need to further expand pharmacists’ abilities in all provinces and territories to prescribe for ambulatory conditions. This will alleviate emergency room visits that could further expose a vulnerable patient to an overcapacity hospital environment. Pharmacists are also capable of ordering and interpreting laboratory results and discussing them with patients. Avoiding emergency room visits may decrease the demand on drugs that are already in shortage.
Reimbursement
Provincial drug benefit plans need to reimburse pharmacists for dispensing fees and remove the cap on numbers of claimable fees during this pandemic. This step will help to restrict stockpiling for those patients who are ordering more than a 1-month supply each time. Professional services such as medication reviews, typically conducted in-home or at the pharmacy, should be allowed to be delivered by telephone. This will allow pharmacists to continue to provide medication management services. Professional fees also need to be reimbursed for services such as providing therapeutic substitution, prescribing for ambulatory conditions and deprescribing. Moreover, pharmacies need to be supported by provinces to deliver medication to home-dwelling elderly patients who are vulnerable and to those in underserved areas.
Workflow
Health care leaders should authorize pharmacists and registered pharmacy technicians to work remotely to assist in medication entry and verification. Encouragement of telehealth services has an important role in supporting the understaffed pharmacies throughout Canada. Pharmacy regulatory bodies may need to approve out-of-province pharmacists and registered pharmacy technicians to allow provinces to assist each other if a shortage of staffing is encountered.
Conclusion
The COVID-19 pandemic has significantly affected drug shortages in Canada, and the crisis has caused more essential drugs to become unavailable to patients. Therefore, it is vital that all key players in the health care industry unite and work together to alleviate this urgent issue. Government entities, regulatory bodies, drug manufacturers and distributors all can use strategies to alleviate drug shortages. Pharmacists and other frontline health care professionals also have an obligation to support the fight against the COVID-19 pandemic. When there is a disruption in the supply of medications, pharmacists manage their inventory carefully to ensure that all their patients can receive a quantity of the medication to meet their immediate needs. Unnecessary stockpiling of medication can create unintended shortages and puts other patients’ health at risk. Pharmacists have an important role to play in limiting the supply of medications to 30 days and using their professional judgment if a larger supply is needed. Pharmacists also must continue using their expertise and skills to act in the best interest of patients. Health care leaders, organizations and regulatory bodies across Canada need to recognize, legislate and reimburse pharmacists for cognitive services performed when challenged by drug shortages. Pharmacists are among the most accessible health care professionals, and support from stakeholders should be available to facilitate pharmacists’ day-to-day workflow. Patients need our skills now more than ever before. We are all fighting against COVID-19 together to prevent inaccessibility to lifesaving drugs and, ultimately, to improve outcomes for all patients.
Footnotes
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Author Contributions:All authors approved the final version of the article.
Funding:The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD:Ali Elbeddini  https://orcid.org/0000-0002-3339-6203
https://orcid.org/0000-0002-3339-6203
References
- 1. World Health Organization. Coronavirus. Available: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed March 31, 2020). [Google Scholar]
- 2. Arabi Y, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. March 3, 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Canadian Press. How to protect yourself and others from infection as COVID-19 cases increase. Available: https://www.cochranetoday.ca/lifestyle-news/how-to-protect-yourself-and-others-from-infection-as-covid-19-cases-increase-2207246 (accessed March 31, 2020).
- 4. Centers for Disease Control and Prevention. People at high risk for flu complications. Available: https://www.cdc.gov/flu/highrisk/index.htm (accessed April 15, 2020).
- 5. Health Canada. Drug shortages Canada. 2016. Available: https://www.drugshortagescanada.ca/ (accessed April 15, 2020).
- 6. Canadian Medical Association. CMA position statement on prescription drug shortages in Canada. Available: https://www.cma.ca/sites/default/files/2018-11/PD13-06-e.pdf (accessed April 15, 2020).
- 7. GlobalData Healthcare. Coronavirus epicentre Hubei has 42 pharma contract manufacturing facilities. February 14, 2020. Available: https://www.pharmaceutical-technology.com/comment/coronavirus-epicentre-hubei/ (accessed April 15, 2020).
- 8. The Multi-Stakeholder Steering Committee on Drug Shortages in Canada. Protocol for the notification and communication of drug shortages. Revised 2017. Available: https://www.drugshortagescanada.ca/files/MSSC_Protocol_2017.pdf (accessed April 15, 2020).
- 9. Clarke K. Coronavirus: delays, shortages predicted by Richmond business leaders. Available: https://www.richmond-news.com/news/coronavirus-delays-shortages-predicted-by-richmond-business-leaders-1.24095328 (accessed April 6, 2020).
- 10. Government of India. Notification No. 50 /2015-2020. Available: https://dgft.gov.in/sites/default/files/Noti%2050_0.pdf (accessed April 6, 2020).
- 11. Canadian Generic Pharmaceutical Association. Ensuring a consistent supply of safe, effective and high quality generic medicines for Canadians. October 17, 2017. Available: https://canadiangenerics.ca/wp-content/uploads/2016/11/10.17.16DeloitteGlobalSupplyChainReport_ENG_FINAL.pdf (accessed March 6, 2020).
- 12. Canadian Association for Pharmacy Distribution Management. Ensuring a safe supply of essential medications and healthcare supplies to Canadians during COVID-19. April 9, 2020. Available: https://www.capdm.ca/CAP/media/Images/CAPDM-Open-Letter-from-CEO-to-COVID-19-Stakeholders-April-9-20-Final.pdf (accessed April 15, 2020).
- 13. Centers for Disease Control and Prevention. Information for clinicians on therapeutic options for patients with COVID-19. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html (accessed April 6, 2020).
- 14. Sawa T. Doctors face sanctions for prescribing unproven COVID-19 drugs to friends and family, regulators warn. Available: https://www.cbc.ca/news/health/sanctions-canadian-doctors-experimental-drugs-1.5511244 (accessed April 6, 2020).
- 15. Health Canada. Exceptional importation and sale of drugs in relation to COVID-19: Tier 3 drug shortages. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/covid19-interim-order-drugs-medical-devices-special-foods/information-provisions-related-drugs-biocides/tier-3-shortages.html (April 15, 2020).
- 16. Health Canada. Sandoz Canada Ranitidine product recall (2019-09-17). Available: https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/71031r-eng.php (accessed March 6, 2020).
- 17. Canadian Pharmacists Association. A review of pharmacy services in Canada and the health and economic evidence. February 2016. Available: https://www.pharmacists.ca/cpha-ca/assets/File/cpha-on-the-issues/Pharmacy%20Services%20Report%201.pdf (accessed April 15, 2020).
- 18. Infectious Diseases Society of America. Guidelines on the treatment and management of patients with COVID-19. April 11, 2020. Available: https://www.idsociety.org/covid19guidelines (accessed April 15, 2020). [DOI] [PMC free article] [PubMed]
- 19. College of Pharmacists of British Columbia. Novel coronavirus (COVID-19). Updated May 7, 2020. Available: https://www.bcpharmacists.org/covid19 (accessed April 15, 2020).