Abstract
Methamphetamine (METH) addicts lose control over drug consumption despite suffering multiple adverse medicolegal consequences. To mimic the negative events associated with drug addiction in humans, we recently introduced a rat model of self-administration (SA) with response-contingent punishment on METH intake. These procedures allowed us to distinguish between two addiction-like phenotypes in rats, those that sustained METH taking despite negative consequences (shock-resistant, SR) and rats that significantly reduced their METH intake (shock-sensitive, SS). Here, we further developed our adverse consequence model and examined incubation of METH craving by measuring cue-induced drug seeking in SR and SS rats. Male Sprague–Dawley rats were trained to self-administer METH (0.1 mg/kg/injection) or saline intravenously (i.v.) during twenty-two 9-h sessions that consisted of 3 separate 3-hr sessions separated by 30 minutes. Subsequently, rats were subjected to incremental footshocks during thirteen additional 9-hr METH SA sessions performed in a fashion identical to the training phase. Cue-induced drug craving was then assessed at 2 and 21 days after the footshock phase. All rats escalated their intake of METH, with both phenotypes showing similar drug taking patterns during SA training. In addition, rats that continued their METH intake despite negative consequences showed even greater cue-induced drug craving following withdrawal than the rats that reduced METH intake following negative consequences. Taken together, our adverse consequence-based model highlights the possibility of identifying rats by addiction-like phenotypes and subsequent vulnerability to relapse-like behaviors. The use of similar SA models should help in the development of better therapeutic approaches to treat different stages of METH addiction.
Keywords: abstinence, drug addiction, foot-shock, self-administration
1. Introduction
Methamphetamine (METH) addiction is a serious neuropsychiatric disorder characterized by deficits in executive function, impairments in motor control, and in memory formation [1, 2]. Additionally, clinical research has shown that METH addicts can relapse to drug taking despite long periods of abstinence [3, 4]. These clinical observations have led to sustained research activities aimed at developing therapeutic approaches to reduce relapse rates [5, 6] that are related to both individual and environmental factors [7, 8]. Using self-administration (SA) procedures, several groups of investigators have measured cue-induced drug-seeking behaviors termed ‘incubation of drug craving’ [9, 10] as a rodent model to represent the propensity of human drug seeking after periods of sustained abstinence. In this model, animals show increases in the number of presses on drug-associated levers after various time intervals of withdrawal from drug SA [10–12]. Rats that undergo METH SA also show incubation of craving when presented with drug-associated cues and contexts [11–14]. Nevertheless, experimental approaches used to study cue-induced drug craving have not, for the most part, included contingent negative consequences known to be associated with drug addiction [10, 15]. These negative outcomes are among factors that can lead to abstinence in some patients [16]. Moreover, continuation of compulsive drug use despite adverse consequences is an important criterion often used to reach a diagnosis of drug addiction in humans [17–19].
To mimic the negative consequences associated with addiction in humans, we recently used contingent punishments with mild footshocks during METH SA. These procedures allowed us to distinguish between two distinct phenotypes in rats, namely animals that continue to take METH despite negative outcomes (shock-resistant, SR) and rats that significantly reduce their METH intake (shock-sensitive, SS) [20, 21]. In the present study, we used the same model in order to identify potential differences in relapse-like behaviors in the two phenotypes. We thought it likely that SR rats which show compulsive METH taking behaviors in the presence of mild footshocks might also show higher cue-induced drug seeking than SS rats which developed abstinence. To test this possibility, we measured cue-induced drug-seeking behavior at two different intervals of withdrawal from METH SA. Herein, we report that compulsive drug-taking is positively correlated with greater incubation of METH craving. In agreement with our previous work, these results further implicate the notion of developing more clinically relevant models to study the human condition of drug addiction.
2. Materials and methods
2.1. Animals and Intravenous Surgery
Male Sprague-Dawley rats (Charles River, Raleigh, NC), weighing 350–400 g, were used in these experiments. Rats were group-housed (two per cage) for 1 week before surgery and maintained in the animal facility under a reversed 12:12h light/ dark cycle with freely available (home-cage) food and water. Before surgery, rats were anesthetized with a ketamine/xylazine mixture (50 and 5 mg/kg, i.p., respectively) and were inserted with catheters into the jugular vein, as described previously [20]. Catheters were then attached to modified 22-gauge 5-UP Plastics One cannulas and mounted on top of rats’ skulls with dental cement. Each modified cannula was covered with fitted Plastics One dust caps. Rats were then injected with buprenorphine only once after surgery (0.1mg/kg, s.c.), individually housed, and allowed to recover for 5 days prior to any METH SA procedures. During the recovery phase, METH SA and punishment phases, catheters were flushed with gentamicin (Butler Schein; 4 mg/ml) every 2 days. All animal procedures were conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the NIDA Intramural Research Program.
2.2. Apparatus
Five days after surgery, rats were placed in SA chambers controlled by two Med Associates systems. Each chamber was housed inside separate sound-attenuating cabinets and equipped with two retractable levers located above a metal grid floor. Grid floor were individually connected to electric generators but were only activated during the shock phase of our experiment. Presses on the active SA lever resulted in METH infusions paired with a 5 second tone-light cue and followed by a 20 second time-out period. Presses on the inactive lever resulted in no scheduled reinforcements. The catheters of rats in both METH SA and saline groups were attached to intravenous lines consisting of polyethylene50 tubing, protected by a metal coil, and connected to a liquid swivel (Instech) allowing the rats free movement inside the SA chamber.
2.3. Methamphetamine self-administration
On the first day of the experiment, rats were re-located from our vivarium facilities, brought to a self-administration room, and chronically housed in Med Associates SA chambers. Rats were then randomly assigned to either the METH or saline condition. Each SA sessions began at the onset of the rat’s dark cycle, where the active and inactive levers were presented and a red house-light illuminated the chamber. Self-administration sessions consisted of three 3-hr daily sessions (total of 9-h a day), with the sessions being separated by 30-min intervals. At the end of each session, the house light was switched off, and both levers were automatically retracted. We trained the rats to self-administer METH (0.1 mg/kg/injection, i.v.) in four cycles, consisting of five days each, with a 2-day rest period between cycles. During each rest period, rats were disconnected from the intravenous lines and remained housed in the SA chambers. The 2-day rest period was used to prevent excessive weight loss known to occur when rats are given long access to the drug [22]. In order prevent drug overdose, we also limited the number of METH infusions to 35 per session, for a maximum total of 105 available METH infusions each day. As per our previous studies [20, 22], METH escalation and maintenance lasted a total of 22 SA sessions. Control rats self-administered saline under identical conditions.
2.3.1. Punishment Phase
Following the maintenance phase of METH SA, electric generators were activated and rats were subjected to contingent punishment procedures. During this phase, 50% of active lever-presses resulted in the concurrent delivery of a footshock lasting 0.5-seconds through metal grid floors. Throughout the punishment phase, rats continued METH SA every day (9-h /day) on an FR-1 reinforcement schedule, with tone-light cues available for an additional thirteen 9-hr METH SA sessions performed in a fashion identical to the training phase., and no 2-day rest periods. The initial footshock session was set at 0.18 mA and amplified by 0.06 mA increments to a final intensity of 0.42 mA over a period of 13 days. This contingent footshock regiment led to the separation of two distinct METH SA phenotypes [shock-resistant (SR) vs shock-sensitive (SS)] as previously described [20]. Animals were classified as SS if they reduced their intake by 70%. Rats that were assigned the saline SA condition were not subjected to any footshock punishments.
2.4. Withdrawal and incubation of METH craving
At the end of the punishment phase, rats were returned to the animal vivarium and individually housed with no access to METH. Intravenous catheters were covered using dust caps, and rats had access to home-cage food and water ad libitum. Cue-induced drug craving was then assessed at days 2 and 21 of withdrawal. During this phase, rats were brought back to their corresponding SA chambers on the morning of each test, but without any METH infusions available. Each test consisted of a single 1-h session during which presses on the drug-associated lever resulted in contingent presentations of the tone and light cues previously paired with METH. Cue-induced drug seeking behavior was assessed using a within-subject design such that all rats tested on day 2 were also tested on day 21 of withdrawal. Animals were euthanized after the second cue-induced drug craving test.
2.5. Statistical Analysis
Statistical analysis of SA data was conducted separately for the escalation, maintenance, and foot-shock phases of our experiment. Repeated measures ANOVAs were used and followed by post hoc tests (Newman-Keuls) for between-subjects comparisons where appropriate (SPSS 20, IBM, Armonk, NY). The dependent variables were the number of METH or saline infusions across SA sessions. Student’s t-test were used to examine the infusion patterns for every 3-h interval on sessions 1, 6, 11, and 22. Student t-tests were also used to analyze cue-induced drug seeking behaviors on extinction tests performed 2 and 21 days after withdrawal. The dependent variables for both extinction tests were total (non-METH-reinforced) active lever presses. All data are presented as means ± SEM and considered statistically significant when p ≤ 0.05. Herein, we only report interaction or main effects from ANOVA’s critical for data interpretation.
3. Results
3.1. METH SA caused rapid escalation and maintenance of METH intake
Figure 1A illustrates the timeline of our study. Figure 1B, shows the number of METH infusions taken during the training period of 22 days. The statistical analysis for infusions included the between-subject factor of group (SR, SS, and controls) and the within-subject factor of SA session (days 1–22). All rats significantly escalated their METH intake during the first 9 days of METH SA training [F (16, 144) = 5.85, p < 0.01] (Figure 1B). Newman-Keuls post-hoc tests revealed significant increases in active lever presses between the SR (N = 7) and SS (N = 9) rats compared to control rats (N = 5) (p < 0.05). Figure 1B also shows that, during the 13-day maintenance phase of METH SA, SR and SS rats took similar amounts of METH [F (24, 216) = 0.70, p = 0.84], with both SR and SS rats reaching an average of 90 infusions per day during the 9-h daily training sessions. During the escalation and maintenance phases, both SR and SS rats took most of the METH infusions within the first hour of each 3-h interval during SA sessions 6, 11, and 22 (Figure 1C), suggesting a binge pattern of drug taking for both phenotypes. The groups were then separated post-facto based on their responses to contingent footshocks on METH intake.
Figure 1.

(A) Experimental time-line of METH SA, footshocks and withdrawal tests. (B) METH infusions during the training period of 22 days (9-h /day). Rats escalate their METH intake during the training phase of the experiment. METH SA rats were segregated into two distinct phenotypes post facto based on their responses to footshocks, namely rats that continue to take METH despite negative outcomes (shock-resistant, SR) and rats that significantly reduce their METH intake (shock-sensitive, SS). Both SR (n=7) and SS (n=9) rats significantly increased their METH intake during escalation phase of SA training. During the maintenance phase of SA training, both SR and SS rats reached similar plateaus of METH intake. SR rats are denoted in black circles, SS rats are denoted in white circles while saline controls (n=5) rats are shown in white squares. Data are represented as means ± SEM of number of METH infusions (0.1 mg/kg/infusion). Key to statistics: *p<0.05. (C) Shock-resistant (SR) and shock-sensitive (SS) phenotypes take most METH infusions during the first hour of the three daily 3-hour sub-interval on sessions 6, 11, and 22 of METH SA. Rats did not take much METH at the beginning of METH SA training. Key to statistics: *P<0.05, **P<0.01, ***P<0.001, in comparison to first day of training.
3.2. Mild footshocks segregate METH self-administering rats into animals with distinct addiction profiles.
During the punishment phase, footshock intensity was increased gradually from 0.18 mA to 0.42 mA over a period of 13 days (Figure 2A). This response-contingent punishment led to significant overall decreases in METH infusions [F (24, 216) = 1.90, p < 0.05], with the SR rats continuing to lever press to a high level and the SS rats markedly reducing their METH intake. Like our earlier report, we classified 7 out of the 16 METH self-administering rats as shock-resistant because they showed less than a 30% decrease in the number of METH infusions from pre-shock level during the last three sessions of the punishment phase. In contrast, we classified 9 animals as shock-sensitive because they showed more than 70% suppression of drug infusions relative to pre-shock levels [20, 21]. Newman-Keuls tests revealed significant differences in METH infusions during the punishment phase between SR and SS rats (p < 0.05). Moreover, significant punishment-induced separation of the two groups could be observed on SA day 28 [F (2, 18) = 28.9, p < 0.01] (Figure 2A). Figure 2B shows average METH infusions during the last session of the maintenance phase (white bars) and last session of the punishment phase (black bars) for SR and SS rats. Although both groups decreased their METH intake, the SR rats continued to lever press at a significantly higher rate than the SS group (p<0.001).
Figure 2.
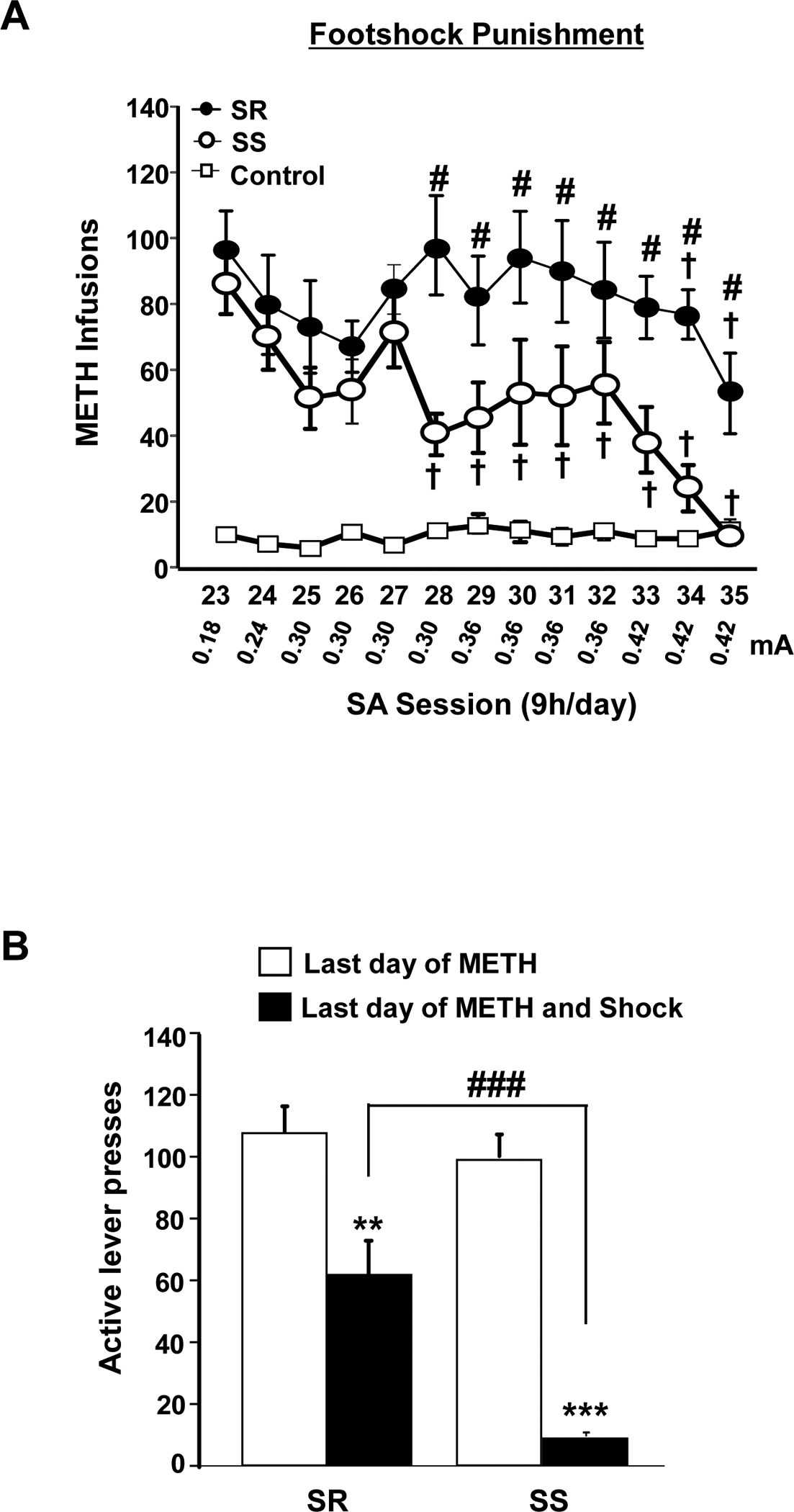
(A) Mild footshocks segregate METH self-administering rats into two distinct phenotypes. The figure shows suppression of METH infusions by punishment in shock-sensitive (SS) rats and continued METH intake in shock-resistant (SR) phenotype rats. During this phase, footshock intensity was increased from 0.18 to 0.42mA with 50% of drug-associated lever presses resulting in active footshocks punishments. Significant difference in METH infusion the between SR and SS phenotypes were observed. Data are represented as means ± SEM of number of METH infusions (0.1 mg/kg/infusion). Key to statistics: †p<0.05, relative to the first punishment session; #p<0.05 denotes a significant difference between SR and SS rats. (B) The figure shows active lever presses during the last day of maintenance phase (white bars) and last day of foot-shock punishment (black bars). Key to statistics: **P<0.01, ***P<0.001, significantly different from the last day of METH SA maintenance, ###=P<0.001 significant difference between SR and SS rats on the last punishment session.
3.3. Shock-resistant rats show higher cue-induced drug craving than SS rats at 21 days after withdrawal from METH SA.
Figure 3A shows cue-induced METH seeking during the first (white bars) and second (black bars) extinction tests for SR and SS rats. As previously reported for multiple drugs of abuse [23], we found time-dependent increases in cue-induced reward seeking behavior during the extinction tests (our operational measure of craving) for both groups of methamphetamine-trained rats. Specifically, SR and SS rats did not significantly press the METH-associated lever during the first 1-h test but increased lever presses during the second extinction test. Interestingly, the SR rats preformed more drug-associated lever presses compared to SS rats on withdrawal day 21 (p<0.01). These findings suggest that compulsive drug intake might be associated with increased cue-induced drug craving. A correlational analysis also documented that individual SS and SR rats clustered within their respective subgroups, with SS rats showing fewer lever presses than SR rats on withdrawal day 21 (Figure 3B).
Figure 3.

(A) Time-dependent cue-induced METH seeking in SR and SS rats. The figure also shows drug-associated lever presses on day 2 (white bars) and 21 (black bars) of METH withdrawal. Both SR and SS phenotypes, respectively, show minimal cue-induced drug seeking behavior on the first incubation test. However, 21 days after withdrawal from METH both groups of rats displayed significant cue-induced drug seeking behavior, with SR rats showing higher craving than SS rats. Data are represented as means ± SEM of number of active lever presses. Key to statistics: ***p<0.001, significantly different from withdrawal day 2, ##p<0.01 significant difference between SR and SS rats on withdrawal day 21. (B) The figure shows a correlational analysis between average infusions during the last 3 days of punishment and active lever presses on withdrawal day 21. Data are presented as the average of METH infusions (0.1 mg/kg/infusion) on the x axis and average active level presses during withdrawal on the y axis. Key to statistics: **P<0.01.
4. Discussion
The major findings of this study include: [i] demonstration that adverse consequences can help to segregate rats into distinct phenotypes of METH intake based on their resistance or sensitivity to contingent footshock punishments during SA sessions; and [ii] cue-induced drug seeking behavior after suppression of METH SA by contingent mild punishment was more prominent in a subpopulation of animals that had continued to lever press for METH despite incremental footshock punishments.
The major hallmark of substance use disorder is loss of control over drug consumption despite adverse consequences [18]. Hence, we set out to examine compulsive drug seeking behavior in an animal model of METH addiction. Our results are consistent with those of recent publications using the SA paradigm to identify rats with persistent drug taking behaviors despite contingent punishment to METH [20, 21] or alcohol [24] intake. Previous studies have also noted that persistent cocaine SA in rodents can also occur in a subpopulation of rats that also show resistance to punishment [25, 26]. This discussion is significant from a clinical perspective given that not all drug users meet the criteria for drug dependence [19, 27, 28]. Thus, the shock-induced segregation of METH SA rats into two distinct subpopulations may not only provide a more clinically relevant model to investigate the transition from recreational drug use to addiction, but might also help to better identify molecular substrates of compulsive drug use. This statement is consistent with our recent observations that patterns of DNA hydroxymethylated regions and increased expression of potassium-gated channels in the nucleus accumbens distinguish between SR from SS phenotypes after a short period of shock-induced abstinence [20]. However, further studies are warranted to gain a better insight into the biological basis of addictive phenotypes. It will also be of interest to expand this model to include adverse consequences that occur after each or several days after a day or two of METH SA because adverse consequences in human addicts can also occur not only during but also following variable delays after drug use. In the future, models that incorporate clinically relevant adverse occurrences should help to better clarify the underlying neurobiology of drug addiction.
Cue-induced reinstatement of METH seeking in rats has been well established [12, 13, 29–32]. Specifically, our previous work has shown that incubation of METH craving is observed after suppression of METH intake by footshock punishment [14]. However, the separation of abstinent and addicted-like phenotypes in rats may provide a better animal model to study the propensity to relapse. Indeed, a major finding of our report is that SR rats show increased cue-induced METH seeking after 21 days of abstinence relative to SS rats. These observations indicate that compulsive drug use in animals is associated with increased likelihood of relapse, an idea put forward by previous studies using cocaine [33]. These notions are consistent with the findings that humans who suffer from more intense addiction are also more likely to suffer from intense craving over time [16]. In addition, the growing interest to more closely mimic the human condition of drug-addiction in animals is highlighted by recent reports in which investigators used drug priming or drug-associated cues to re-establish opioid SA [34], resume responding for heroin [35], cocaine [25, 36–39], or alcohol seeking [40] in rats after various punishment regiments.
Our adverse consequence model expands upon previous work by also identifying animals which are less prone to manifest compulsive drug taking and display resistance to incubation of METH craving. Indeed, our results demonstrate that individual SS rats clustered within their own respective subgroup and showed suppression of cue-induced drug seeking behavior after withdrawal from METH. These observations are interesting from a clinical perspective given that in humans, decreased drug use is also associated with lower rates of relapse to METH and other drugs of abuse [16, 41]. Furthermore, our observations are supported by clinical literature showing that in humans, drug use per se is not by definition equivalent to addiction [18] and that only a subset of humans develop addiction after repeated use of these substances [19]. Interestingly, other studies have also shown intensity-dependent suppression of cocaine SA in rats [42] and monkeys [43]. Thus, the grouping of all animals which show escalation of METH SA into a single cohort might limit our understanding of the mechanisms which limit drug-seeking behaviors following abstinence.
5. Conclusions
In conclusion, we demonstrate that mild footshocks can cause the segregation of rats with distinct addiction-like phenotypes, with some rats becoming compulsive METH takers and other rats developing significant reduction of their METH intake in the presence of adverse consequences. We found, in addition, that cue-induced drug craving is more prominent in rats with the compulsive drug taking phenotype. In contrast, rats with reduced intake showed decreased incubation of METH craving. Taken together, this punishment-based model highlights the possibility of identifying rats with differential profiles of drug intake and post-drug withdrawal behaviors. The use of similar clinically relevant models should help in the development of beneficial anti-addiction therapeutic modalities and uncover mechanism that promote susceptibility to drug relapse.
Acknowledgments
This research was supported by funds of the Intramural Research Program of the DHHS/NIH/NIDA.
Footnotes
Disclosure / Conflict of Interest
All the authors declare no competing financial interests or conflicts of interest.
References
- [1].Rusyniak DE, Neurologic manifestations of chronic methamphetamine abuse, Psychiatr. Clin. North Am 36 (2013) 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. , Neurocognitive effects of methamphetamine: a critical review and meta-analysis, Neuropsychol. Rev 17 (2007) 275–297. [DOI] [PubMed] [Google Scholar]
- [3].Brecht ML, Herbeck D, Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors, Drug Alcohol Depend 139 (2014) 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gowin JL, Ball TM, Wittmann M, Tapert SF, Paulus MP, Individualized relapse prediction: Personality measures and striatal and insular activity during reward-processing robustly predict relapse, Drug Alcohol Depend 152 (2015) 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. , Bupropion for the treatment of methamphetamine dependence, Neuropsychopharmacology 33 (2008) 1162–1170. [DOI] [PubMed] [Google Scholar]
- [6].Farabee D, McCann M, Brecht ML, Cousins SJ, Antonini VP, Lee AB, et al. , An analysis of relapse prevention factors and their ability to predict sustained abstinence following treatment completion, Am. J. Addict 22 (2013) 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gawin FH, Kleber HD, Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations, Arch.Gen. Psychiatry 43 (1986) 107–113. [DOI] [PubMed] [Google Scholar]
- [8].Milton AL, Everitt BJ, The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments, Neurosci. Biobehav. Rev 36 (2012) 1119–1139. [DOI] [PubMed] [Google Scholar]
- [9].Grimm JW, Hope BT, Wise RA, Shaham Y, Neuroadaptation. Incubation of cocaine craving after withdrawal, Nature 412 (2001) 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y, Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving, J. Neurosci 23 (2003) 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. , Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons, J. Neurosci 35 (2015) 8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shepard JD, Bossert JM, Liu SY, Shaham Y, The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse, Biol. Psychiatry 55 (2004) 1082–1089. [DOI] [PubMed] [Google Scholar]
- [13].Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM, Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine, Addict Biol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, et al. , Incubation of methamphetamine and palatable food craving after punishment-induced abstinence, Neuropsychopharmacol 39 (2014) 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF, Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment, J. Neurosci 20 (2000) 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Herbeck DM, Brecht ML, Christou D, Lovinger K, A qualitative study of methamphetamine users’ perspectives on barriers and facilitators of drug abstinence, J. Psychoactive Drugs 46 (2014) 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DSM5. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition ed: American Psychiatric Association; (2013). [Google Scholar]
- [18].Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. , DSM-5 criteria for substance use disorders: recommendations and rationale, Am. J. Psychiatry 170 (2013) 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wagner FA, Anthony JC, From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol, Neuropsychopharmacology 26 (2002) 479–488. [DOI] [PubMed] [Google Scholar]
- [20].Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, et al. , Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence, Mol. Psychiatry (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, et al. , Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats, Sci. Rep 6 (2016) 37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. , Methamphetamine Self-Administration Is Associated with Persistent Biochemical Alterations in Striatal and Cortical Dopaminergic Terminals in the Rat, PLoS ONE 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y, Neurobiology of the incubation of drug craving, Trends Neurosci 34 (2011) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, et al. , Role of Ventral Subiculum in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence, J. Neurosci 36 (2016) 3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ, High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence, Biol. Psychiatry 65 (2009) 851–856. [DOI] [PubMed] [Google Scholar]
- [26].Pelloux Y, Murray JE, Everitt BJ, Differential vulnerability to the punishment of cocaine related behaviours: effects of locus of punishment, cocaine taking history and alternative reinforcer availability, Psychopharmacol 232 (2015) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hasin DS, Introduction to DSM-5 criteria linked papers in drug and alcohol dependence, Drug Alcohol Depend 122 (2012) 20–21. [DOI] [PubMed] [Google Scholar]
- [28].Vsevolozhskaya OA, Anthony JC, Estimated probability of becoming a case of drug dependence in relation to duration of drug-taking experience: a functional analysis approach, Int. J. Methods Psychiatr. Res (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T, Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward, Brain Res 1021 (2004) 272–276. [DOI] [PubMed] [Google Scholar]
- [30].Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. , Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence, J. Neurosci (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kufahl PR, Olive MF, Investigating Methamphetamine Craving Using the Extinction-Reinstatement Model in the Rat, J. Addict. Res. Ther S1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Venniro M, Zhang M, Shaham Y, Caprioli D, Incubation of Methamphetamine but not Heroin Craving after Voluntary Abstinence in Male and Female Rats, Neuropsychopharmacology (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Deroche-Gamonet V, Belin D, Piazza PV, Evidence for addiction-like behavior in the rat, Science 305 (2004) 1014–1017. [DOI] [PubMed] [Google Scholar]
- [34].Panlilio LV, Thorndike EB, Schindler CW, Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse, Psychopharmacology (Berl) 168 (2003) 229–235. [DOI] [PubMed] [Google Scholar]
- [35].Peck JA, Wercberger R, Kariyeva E, Ranaldi R, Cue-induced resumption of heroin and cocaine seeking in rats using a conflict model of abstinence and relapse, Psychopharmacology (Berl) 228 (2013) 651–658. [DOI] [PubMed] [Google Scholar]
- [36].Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. , Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking, Nature 496 (2013) 359–362. [DOI] [PubMed] [Google Scholar]
- [37].Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A, A conflict rat model of cue-induced relapse to cocaine seeking, Psychopharmacology (Berl) 194 (2007) 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pelloux Y, Everitt BJ, Dickinson A, Compulsive drug seeking by rats under punishment: effects of drug taking history, Psychopharmacology (Berl) 194 (2007) 127–137. [DOI] [PubMed] [Google Scholar]
- [39].Vanderschuren LJ, Everitt BJ, Drug seeking becomes compulsive after prolonged cocaine self-administration, Science 305 (2004) 1017–1019. [DOI] [PubMed] [Google Scholar]
- [40].Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y, Context-induced relapse to alcohol seeking after punishment in a rat model, Biol. Psychiatry 73 (2013) 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCabe SE, Cranford JA, Boyd CJ, Stressful Events and Other Predictors of Remission from Drug Dependence in the United States: Longitudinal Results from a National Survey, J. Subst. Abuse Treat 71 (2016) 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Limpens JH, Schut EH, Voorn P, Vanderschuren LJ, Using conditioned suppression to investigate compulsive drug seeking in rats, Drug Alcohol Depend 142 (2014) 314–324. [DOI] [PubMed] [Google Scholar]
- [43].Grove RN, Schuster CR, Suppression of cocaine self-administration by extinction and punishment, Pharmacol. Biochem. Behav 2 (1974) 199–208. [DOI] [PubMed] [Google Scholar]


