Abstract
Impaired sphingolipid synthesis is linked genetically to childhood asthma and functionally to airway hyperreactivity (AHR). The objective was to investigate whether sphingolipid synthesis could be a target for asthma therapeutics. The effects of GlyH-101 and fenretinide via modulation of de novo sphingolipid synthesis on AHR was evaluated in mice deficient in SPT (serine palmitoyl-CoA transferase), the rate-limiting enzyme of sphingolipid synthesis. The drugs were also used directly in human airway smooth-muscle and epithelial cells to evaluate changes in de novo sphingolipid metabolites and calcium release. GlyH-101 and fenretinide increased sphinganine and dihydroceramides (de novo sphingolipid metabolites) in lung epithelial and airway smooth-muscle cells, decreased the intracellular calcium concentration in airway smooth-muscle cells, and decreased agonist-induced contraction in proximal and peripheral airways. GlyH-101 also decreased AHR in SPT-deficient mice in vivo. This study identifies the manipulation of sphingolipid synthesis as a novel metabolic therapeutic strategy to alleviate AHR.
Keywords: sphingolipid, de novo synthesis, asthma, smooth muscle
Clinical Relevance
This study uses sphingolipid synthesis, a novel metabolic pathway in asthma pathogenesis, as a therapeutic target. We provide evidence that the targeted increase of sphingolipid de novo synthesis can alleviate airway hyperreactivity. This not only enhances the understanding of asthma but could inform novel therapies.
Asthma is a heterogeneous chronic disease characterized by airway hyperreactivity (AHR) and a strong genetic predisposition. ORMDL biosynthesis regulator 3 (ORMDL3) in the genetic asthma locus located on chromosome 17q21 (1, 2). Asthma 17q21 risk alleles lead to increased expression of ORMDL3 (1, 3–5), a negative regulator of sphingolipid de novo synthesis (6–8). A functional role of decreased sphingolipid synthesis in asthma pathogenesis is supported by animal studies showing that decreased de novo sphingolipid synthesis results in AHR and that overexpression of ORMDL3 results in AHR and low sphingolipids (9, 10). There is increasing evidence that sphingolipid metabolism is altered in asthma (11–13) and that 17q21 asthma risk alleles are associated with decreased sphingolipid synthesis in children (14).
Sphingolipids are critical building blocks of cellular membranes and constitute key cellular-signaling mediators (15). The rate-limiting step for de novo sphingolipid synthesis is catalyzed by SPT (serine palmitoyl-CoA transferase) and comprises the condensation of an amino acid (usually serine) and a fatty acyl-CoA (usually palmitoyl-CoA) to produce 3-ketosphinganine that is immediately reduced to sphinganine. Sphinganine can be acylated to form dihydroceramides or phosphorylated to sphinganine-1 phosphate. Sphinganine, sphinganine-1 phosphate, and dihydroceramides constitute the sphingolipids that are exclusively generated through the de novo sphingolipid-synthesis pathway (see Figure E1 in the data supplement). This is in contrast to ceramides, sphingosine, sphingosine 1-phosphate (S1P), sphingomyelins, and complex glycosphingolipids that are generated in a recycling pathway and whose production does not solely depend on SPT (16) (Figure E1). Sphingolipids are known to affect skeletal-muscle, gastric, and vascular smooth-muscle contractility and proliferation (17–20), and S1P can contract murine tracheal smooth-muscle cells (21). S1P also contributes to asthma by increasing inflammatory mediators (22, 23).
Overexpression of ORMDL3 in murine airway smooth-muscle cells leads to increased contractility and calcium oscillations (24). Sphingolipid synthesis may thus be a therapeutic target to influence airway smooth-muscle contractility.
We evaluated the effects on airway reactivity by two modulators that increase sphingolipids within the de novo pathway: GlyH-101 and fenretinide (Figure E1). The glycinyl hydrazone compound GlyH-101 is a selective and reversible open-channel CFTR (cystic fibrosis transmembrane conductance regulator) chloride-channel blocker (25, 26). CFTR inhibition or malfunction stimulates de novo sphingolipid synthesis in human lung epithelial cells with increases in sphinganine and dihydroceramides in the de novo synthesis pathway but also with increases in sphingosine and long-chain saturated ceramides (27, 28). The exact mechanism for this activation is unknown but could be related to the ABC transporter feature of CFTR that helps shuttle S1P across the plasma membranes and activates sphingolipid synthesis (29). In contrast, the synthetic retinoid fenretinide inhibits DES1 (dihydroceramide desaturase) (30, 31). The resultant decrease in ceramides by DES1 inhibition results in a compensatory activation of SPT (32, 33).
Here, we show that GlyH-101 and fenretinide increase sphinganine and dihydroceramides in lung epithelial and airway smooth-muscle cells, decrease intracellular calcium concentration ([Ca2+]i) in airway smooth-muscle cells, and decrease agonist-induced contraction in proximal and peripheral airways. We further show that GlyH-101 decreases AHR in SPT-deficient mice. This strongly suggests that sphingolipid synthesis can serve as a metabolic therapeutic target for asthma.
Methods
Reagents
Myriocin (Calbiochem), a specific inhibitor of SPT that decreases sphingolipid content (34), GlyH-101 (Tocris Bioscience), and fenretinide [N-(4 hydroxyphenyl)retinamide; Millipore-Sigma] were solubilized in DMSO to prepare 10-mM stocks and diluted in physiological medium to their final concentration on the same day that the experiments were performed.
Cells
A human airway smooth-muscle cell line was originated from a healthy proximal airway and was immortalized by stable expression of hTERT (human telomerase reverse transcriptase). Moloney murine leukemia retrovirus was provided to us by W. Gerthoffer (University of Nevada) (35, 36). The human lung alveolar epithelial cell line A549 was obtained from the American Type Culture Collection.
Mice
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee of Weill Cornell Medicine. Female C57Bl/6 mice and heterozygous SPT-deficient mice (Sptlc2+/−) mice or homozygous wild type (WT) (Sptlc2+/+), were used at 10–14 weeks of age.
In Vitro Effects of GlyH-101 and Fenretinide
To assess the effects of GlyH-101 and fenretinide on cellular sphingolipid composition, almost confluent monolayers of human airway smooth-muscle and A549 cells were incubated with either GlyH-101 (2 μM), fenretinide (5 μM), myriocin (1 μM) or DMSO (0.1%) for 5 hours.
The effects of altered de novo sphingolipid synthesis on agonist-induced [Ca2+]i concentration, human airway smooth-muscle cells were incubated with GlyH (2 μM), fenretinide (5 μM), myriocin (1 μM), or DMSO (0.1% control) for 5 hours.
Quantitative Sphingolipid Determination
Sphingolipids were quantified in cultured cells and precision-cut lung slices (PCLS) by high-pressure liquid chromatography electrospray ionization–tandem mass spectrometry using a minor modification of a described method (37). The primary outcome parameter reflective of de novo synthesis was sphinganine.
Assessment of Airway Reactivity
Airway reactivity was assessed using three methods: 1) in PCLS to assess small-airway reactivity (38), myriocin (10 μM), GlyH-101 (2 μM) or fenretinide (1–10 μM) were added for the incubation of the PCLS for 15 hours; 2) in isolated bronchial rings by wire myography to assess large-airway reactivity (39); and 3) in anesthetized and tracheostomized mice to measure airway resistance by using a rodent lung-function testing system (flexiVent; SCIREQ) 4 hours after intratracheal administration of GlyH-101 (80 mg/kg).
See the data supplement for full details.
Statistics
The results are presented as the mean ± SEM. Nonparametric testing was performed when data were not normally distributed. A two-sample t test, Mann-Whitney test, or Wilcoxon test was used to compare two groups depending on the data type; comparisons of more than two groups were conducted by using the Kruskal-Wallis test corrected for multiple comparisons, as indicated in each assay. For all tests, differences were considered significant when P < 0.05, and the three significance levels are indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. GraphPad Prism version 8.2 was used for all statistical analyses.
Results
GlyH-101 and Fenretinide Increase Metabolites of Sphingolipid De Novo Synthesis In Vitro
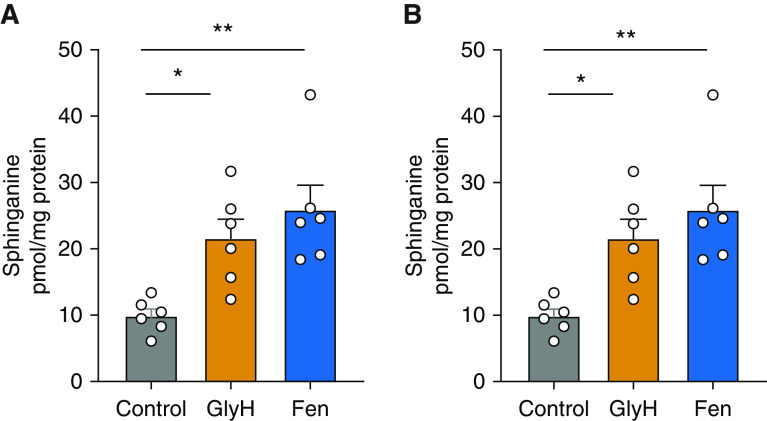
To evaluate whether GlyH-101 and fenretinide increase metabolites of sphingolipid synthesis in lung cells, human airway smooth-muscle and A549 cells were incubated with GlyH-101 (2 μM) or fenretinide (5 μM) for 5 hours, and sphingolipid masses were analyzed by high-pressure liquid chromatography electrospray ionization–tandem mass spectrometry. Both cell lines had been tested for the presence of SPT by responses to the specific SPT inhibitor myriocin (data not shown). In both cell lines, GlyH-101 increased sphinganine (Figures 1A and 1B), dihydroceramides, ceramides, and some sphingomyelins (see Figures E2A–E2C and E3A–E3C), whereas fenretinide increased mostly sphinganine (Figures 2A and 2B) and dihydroceramides but had no effect on ceramides or sphingomyelins (see Figures E2A–E2C and E3A–E3C). GlyH-101’s and fenretinide’s commonality is their increase in sphingolipids that are almost only solely generated through de novo synthesis, with some differences in the effects on ceramides and sphingomyelins, consistent with the inhibitory action of fenretinide on DES1, which generates ceramides from dihydroceramides.
Figure 1.
GlyH-101 (GlyH) and fenretinide (Fen) increase sphinganine de novo synthesis in human lung alveolar epithelial and airway smooth-muscle cells. Sphinganine synthesis in cultures of (A) the lung epithelial cell line A549 or (B) a human airway smooth-muscle cell line treated for 5 hours with 0.1% DMSO (control), 2 μM GlyH, and 5 μM Fen. Data are means ± SEMs (n = 6 biologically independent samples). White circles represent n. *P < 0.05 and **P < 0.01, Kruskal-Wallis test with multiple comparisons.
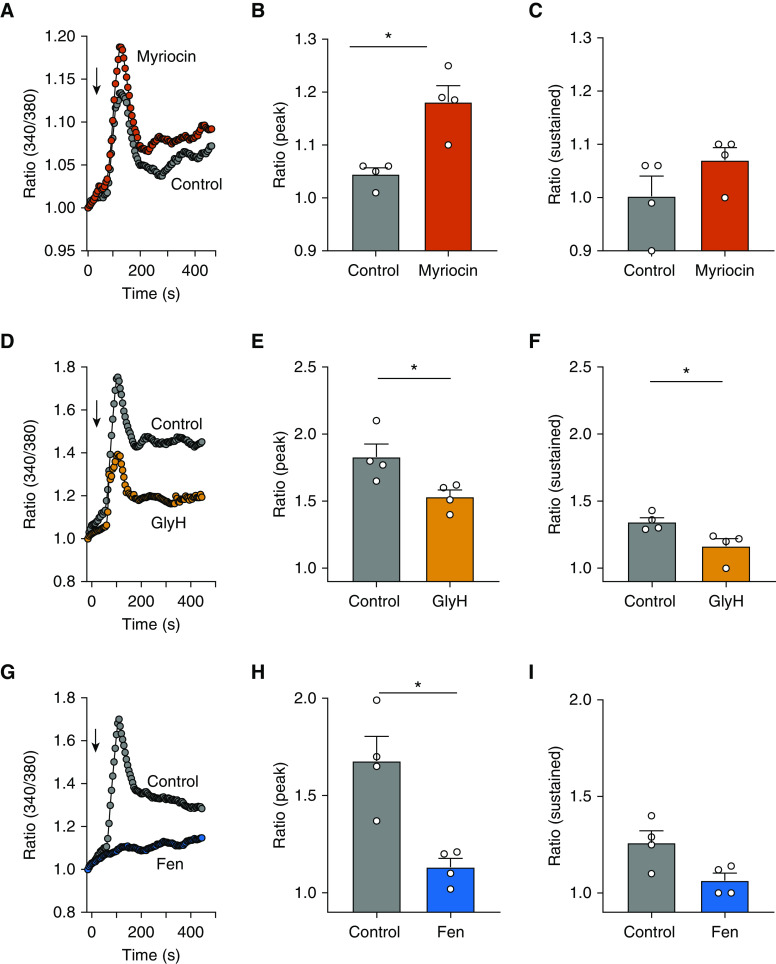
Figure 2.
Altered sphingolipid synthesis affects bradykinin-induced calcium signaling in human airway smooth-muscle cells. (A, D, and G) Representative traces showing bradykinin (10 μM)–induced intracellular calcium responses (readout 340/380 nm) in a human airway smooth-muscle cell line incubated for 5 hours with myriocin (1 μM) (A), GlyH (2 μM) (D), or Fen (5 μM) (G). Arrows represent the addition of bradykinin. Summary data showing the effect of each treatment on the (B, E, and H) peak and (C, F, and I) sustained increases in the intracellular calcium concentration. Data are means ± SEMs from four independent experiments (represented by white circles) like those shown in the representative traces for each drug. *P < 0.05, Mann-Whitney test.
Altered Sphingolipid Synthesis Affects Agonist-induced [Ca2+]i Release
As agonist-induced [Ca2+]i release is associated with airway smooth-muscle cell contraction, we evaluated whether inhibition or activation of de novo sphingolipid synthesis affects bradykinin-induced [Ca2+]i release in a human airway smooth-muscle cell line treated for 5 hours with myriocin (1 μM) (Figure 2A), GlyH-101 (2 μM) (Figure 2D), or fenretinide (5 μM) (Figure 2G). Myriocin led to an increased peak [Ca2+]i release (Figure 2B) but had only a mild effect on sustained [Ca2+]i increase (Figure 2C). In contrast, both GlyH-101 and fenretinide decreased [Ca2+]i peaks (Figures 2E and 2H, respectively) and also sustained a [Ca2+]i decrease compared with control treatment (Figures 2F and 2I, respectively). Although not directly reflecting contractility, the decreased agonist-induced [Ca2+]i release may be an indicator of the effects of increased de novo sphingolipid synthesis on the cell-signaling mechanisms that regulate contractility.
Decreased SPT Activity Leads to Increased Methacholine-induced Small-Airway Contraction
Prior studies have shown that decreased SPT activity leads to AHR in mice (9, 40). These studies did not accurately assess small-airway reactivity, a key feature of asthma. To investigate the effect of decreased sphingolipid synthesis, we assessed small (peripheral) airway reactivity in PCLS in genetically SPT-deficient Sptlc2+/− mice and after inhibition of SPT with myriocin (10 μM) for 15 hours. Visualization (Figure 3A) and quantification (Figures 3B and 3C) of small-airway contractility from Sptlc2+/− mice, after stimulation with methacholine, showed enhanced contractility compared with the WT Sptlc2+/+ mice. Consistent with these results, SPT inhibition in PCLS from WT mice with myriocin enhanced methacholine-induced airway contractility compared with the control group (Figures 3D and 3E). This suggests that SPT inhibition leads to small-airway AHR and demonstrates that PCLS can be used to assess sphingolipid-associated changes in small-airway reactivity.
Figure 3.
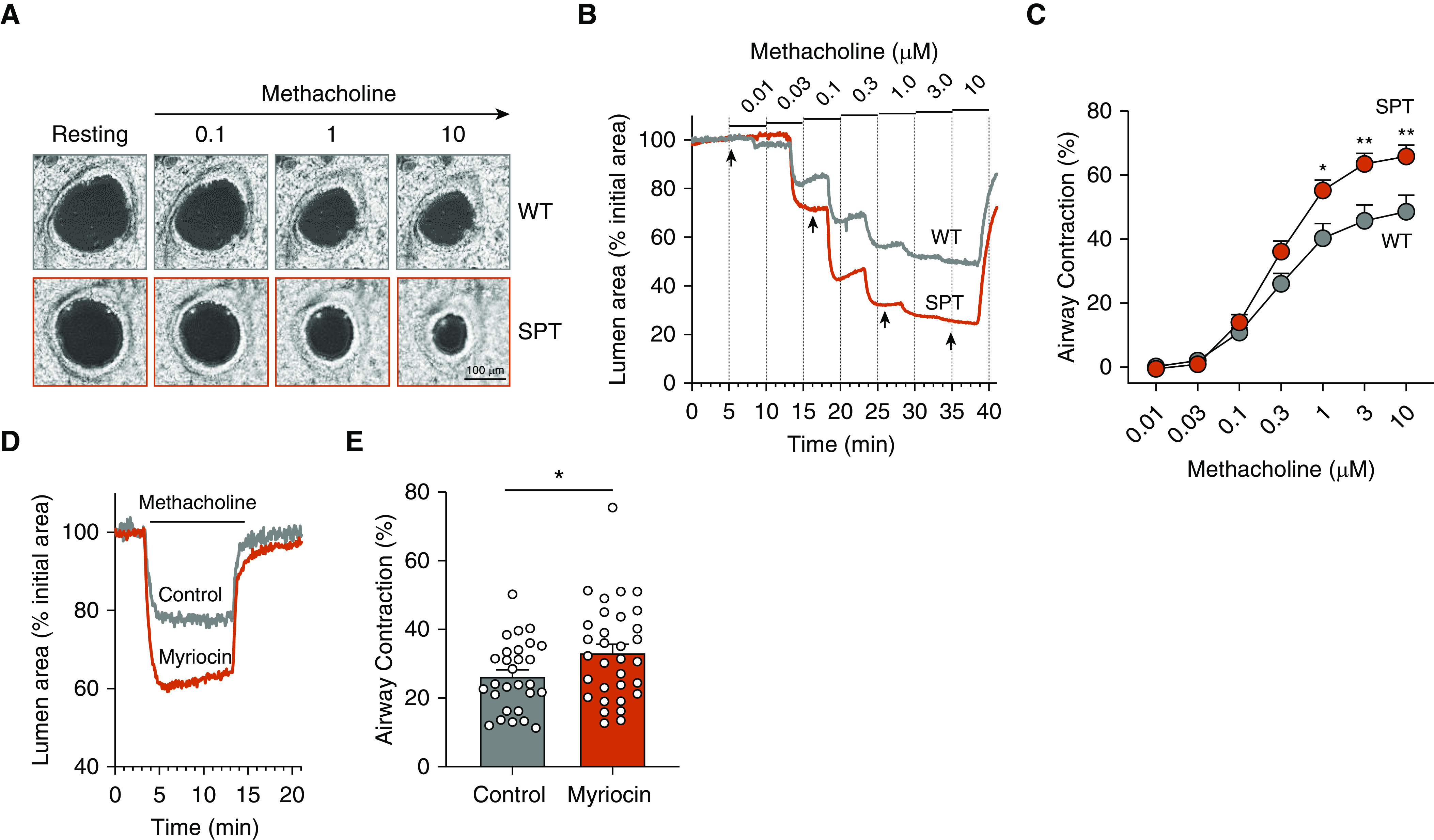
Decreased SPT (serine palmitoyl-CoA transferase) activity leads to increased airway hyperresponsiveness. (A) Series of phase-contrast images, showing a small airway in one of the precision-cut lung slices (PCLS) from a wild-type (WT; gray) and Sptlc2+/− (SPT; red) mouse before (resting) and after exposure to increasing concentrations of methacholine (MCh) and recorded at the times indicated by arrows in B. (B) Representative experiments showing the cross-sectional luminal-area changes of airways from WT and SPT mice with respect to time in response to increasing MCh concentration. (C) Summary of the MCh concentration–dependent contraction of the airways from WT and SPT mice obtained from experiments like those shown in B. Data are means ± SEMs of 18–24 airways from four biologically independent mice in each group and were fitted with a logistic function. *P < 0.05 and **P < 0.01, Wilcoxon test. (D) Representative experiments showing the contractile responses of airways to 0.3 μM MCh and its subsequent washout in PCLS from WT mice that were incubated with 10 μM myriocin or vehicle (0.1% DMSO, control) for 15 hours. (E) Summary data of MCh-induced airway contraction in PCLS incubated with myriocin or vehicle obtained from experiments shown in D. Data are means ± SEMs of 28–32 airways (represented by white circles) from four biologically independent mice in each group. *P < 0.05, unpaired t test. Scale bar, 100 μm.
Activation of De Novo Sphingolipid Synthesis Decreases Proximal and Peripheral Airway Reactivity to Methacholine
To assess whether activation of de novo sphingolipid synthesis by GlyH-101 and fenretinide affects airway reactivity to agonists, we used three models. First, we incubated PCLS from SPT-deficient mice with GlyH-101 (2 μM) or DMSO (control) for 15 hours and found that GlyH-101 treatment increased sphinganine (Figure 4A) and other sphingolipids, foremost among these being dihydroceramides and ceramides (see Figures E4A and E4B). Subsequently, we studied methacholine-induced contraction of small airways within these PCLS and found that in PCLS treated with GlyH-101 (2 μM) for 15 hours, airway contraction was reduced compared to the DMSO control group (Figures 4B and 4C). Second, we found that in vivo airway hyperactivity to methacholine was decreased in SPT mice 4 hours after intratracheal administration of GlyH-101 (80 μg/kg) (Figure 4D). The GlyH-101 effect was detectable, even with a lower absolute airway resistance in the SPT control compared with previous data (9). Third, bronchial rings isolated from SPT mice incubated with (2 μM) GlyH-101 for 3 hours showed reduced methacholine-induced force generation as compared with those treated with DMSO (control) (Figure 4E).
Figure 4.
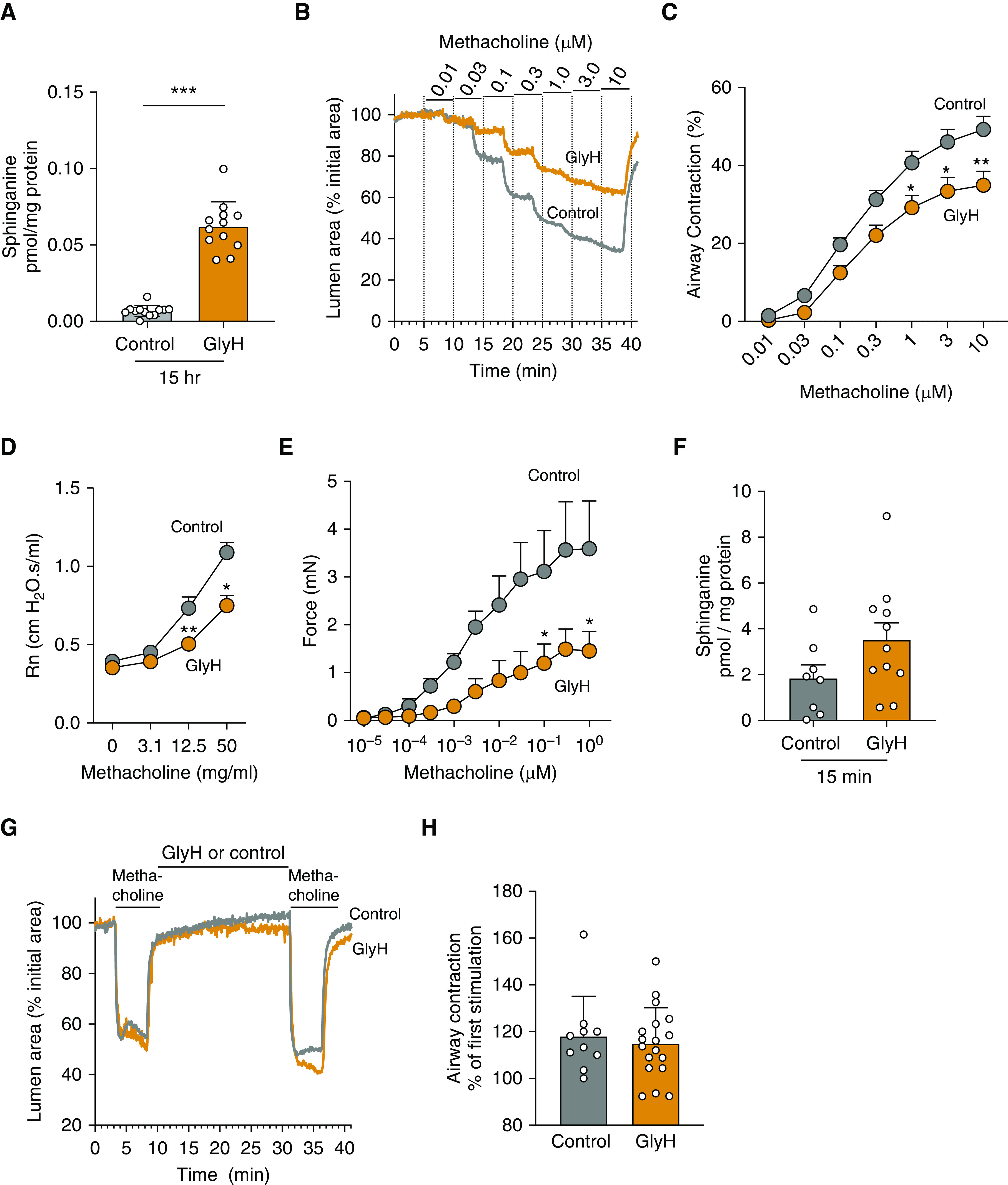
Activation of de novo sphingolipid synthesis by GlyH decreases MCh-induced constriction in proximal and peripheral airways in vitro and airway hyperresponsiveness in vivo. (A and F) Sphinganine in PCLS from SPT mice that were incubated with 2 μM GlyH or 0.1% DMSO (control) for (A) 15 hours or (F) 15 minutes. Data are means ± SEMs of 52–58 PCLS from four biologically independent mice in each group (represented by white circles). ***P < 0.001, Mann-Whitney test. (B) Representative experiments showing MCh-induced changes in the airway lumen area in PCLS from SPT mice that were exposed to 2 μM GlyH or 0.1% DMSO (control) for 15 hours. (C) Summary of the MCh concentration–dependent airway contraction in PCLS from SPT mice, obtained from experiments like those shown in B. Data are means ± SEMs of 36–39 airways from four biologically independent mice in each group *P < 0.05 and **P < 0.01, Wilcoxon test. (D) In vivo airway resistance (Rn) in response to MCh in SPT mice exposed to 0.1% DMSO (control) or GlyH (80 μg/kg) intratracheally 4 hours before testing. There were three biologically independent mice in each group. *P < 0.05 and **P < 0.01, Wilcoxon test. (E) MCh-induced isometric-force generation by bronchial rings from SPT mice that were exposed to 0.1% DMSO (control) or GlyH (2 μM) for 3 hours. Data are means ± SEMs of eight rings in each group. *P < 0.05, Wilcoxon test. (G) Representative experiment showing the changes in the airway lumen area in response to MCh (3 μM) before and after exposure to GlyH (2 μM) or 0.1% DMSO (control) for 15 minutes. (H) Summary of the MCh-induced airway contraction in PCLS exposed to DMSO (control) or GlyH. Data are the percentage of the second MCh-induced airway contraction with respect to that obtained in response to the first contraction before the exposure of the PCLS to DMSO or GlyH, as shown in the representative traces in G. Data are means ± SEMs of 10–19 airways (represented by white circles) from two biological independent SPT mice. The Mann-Whitney test was used.
Interestingly, the GlyH-101–induced decrease in airway reactivity was not seen in PCLS prepared from WT (Sptlc2+/+) mice and equally treated with GlyH-101(2 μM) for 15 hours (see Figure E5A). These findings suggest that GlyH-101 reverses the small- and large-airway AHR associated with SPT deficiency. Finally, we tested whether the effect of GlyH-101 on airway contractility was caused by sphingolipid-independent effects of GlyH-101, such as blockage of cellular chloride channels, by incubating PCLS from SPT mice with GlyH-101 (2 μM) for only 15 minutes. This short incubation time is sufficient to affect airway contractility mediated by signaling mechanisms but insufficient for the synthesis and accumulation of sphingolipids through activation of sphingolipid de novo synthesis. Accordingly, we found that sphinganine (Figure 4F) and most other sphingolipids (see Figures E6A–E6C) concentrations in the PCLS were not different compared with those treated with DMSO (controls). Importantly, the reactivity of small airways within these PCLS was not affected by exposure to GlyH-101 (2 μM) for only 15 minutes (Figures 4G and 4H). This strongly suggests that the decreased airway responsiveness to methacholine seen after 3 or 15 hours of treatment with GlyH-101 of rings or PCLS is associated with the observed drug-induced increased accumulation of cellular sphingolipids in the SPT-deficient airways.
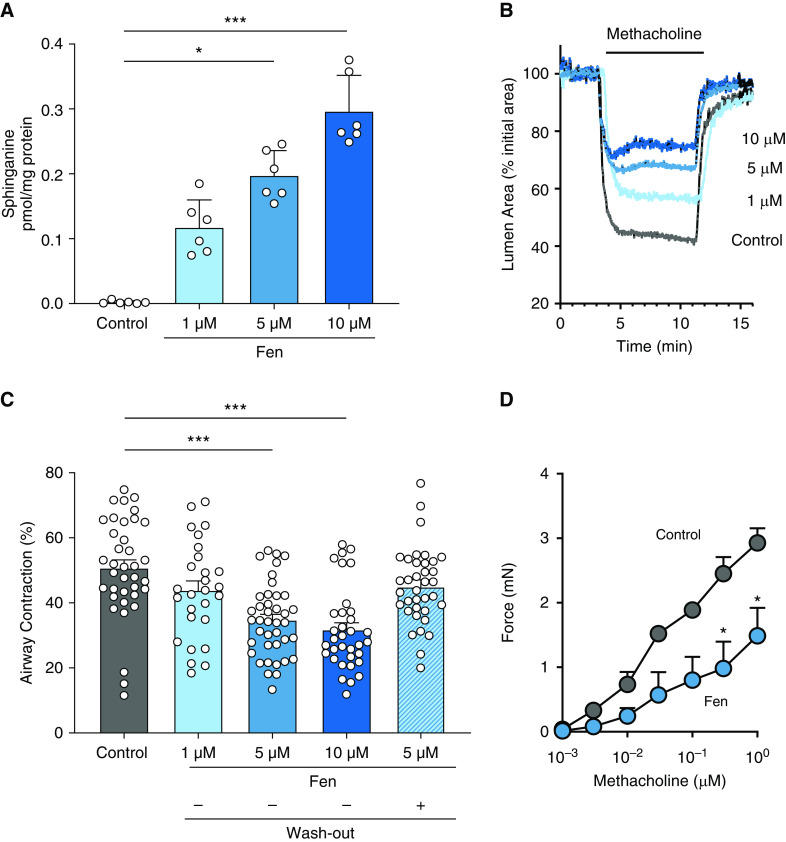
To assess the effects of another drug that activates sphingolipid de novo synthesis on airway reactivity, PCLS from SPT mice were treated for 15 hours with 1, 5, and 10 μM of fenretinide. There was a dose-dependent increase of sphinganine (Figure 5A) and dihydroceramides, but there were no significant changes in ceramides or sphingomyelins in fenretinide-treated PCLS compared with vehicle-treated control group (see Figure E7). Peripheral airway contractility was decreased in the fenretinide-treated PCLS compared with PCLS of controls (Figures 5B and 5C) in a dose-dependent manner. To exclude an effect of fenretinide toxicity on decreased airway reactivity, PCLS exposed to fenretinide for 15 hours were placed in medium for 6 hours to wash out the drug. Airways in these PCLS contracted in a manner similar to those of untreated controls (Figure 5C). The reappearance of contraction after washout indicates that fenretinide treatment was not toxic to the smooth-muscle cells. Fenretinide (5 μM) also decreased large-airway reactivity to methacholine in bronchial rings prepared from SPT-deficient mice (Figure 5D). Together, these findings suggest that specific targeting of the de novo sphingolipid-synthesis pathway can alleviate AHR.
Figure 5.
Activation of de novo sphingolipid synthesis by Fen decreases MCh-induced contraction in proximal and peripheral airways. (A) Sphinganine in PCLS from SPT mice that were incubated with 0.1% DMSO (control) or 1, 5, and 10 μM Fen for 15 hours. Data are means ± SEMs of 48 PCLS from six biologically independent mice in each group (represented by white circles). *P < 0.05 and ***P < 0.001, Kruskal-Wallis test. (B) Representative experiment showing the contractile responses of airways exposed to 0.3 μM MCh in PCLS from SPT mice incubated with DMSO or Fen as described in A. (C) Summary of the MCh-induced airway contraction in PCLS from experiments like those shown in B. A subset of the PCLS treated with 5 μM Fen for 15 hours was subsequently incubated for 6 hours in medium without Fen (washout). Data are means ± SEMs of 30–40 airways (represented by white circles) from four biologically independent mice in each group. ***P < 0.001, Kruskal-Wallis test. (D) Isometric-force generation of bronchial rings isolated from SPT mice exposed to 5 μM Fen or DMSO (control) for 3 hours. Data are means ± SEMs of 12–16 rings from four biologically independent mice. *P < 0.05, Wilcoxon test.
Discussion
The present study shows that the increase of metabolites that are almost exclusively generated through the de novo sphingolipid-synthesis pathway can reverse AHR induced by decreased SPT activity. This is relevant, as a genetic predisposition for asthma through ORMDL3 in the 17q21 locus is associated with decreased sphingolipid synthesis (1, 2, 8). Sphingolipid blood and tissue concentrations are altered in experimental models with genetically increased or decreased ORMDL3 levels (10–12) and, most importantly, in human asthma (13, 14). As ORMDL3 inhibits sphingolipid synthesis primarily through interaction with SPT, SPT-deficient mice or airways serve as models to screen for interventions against this asthma genotype. We had previously shown that murine large bronchial airways or lungs from genetically SPT-haplodeficient Sptlc2−/+ mice, as well as large human or murine bronchial airways treated with the SPT-specific inhibitor myriocin, result in AHR (9). Here, we confirmed that SPT deficiency also leads to hyperreactivity of small airways and showed that pharmacological approaches aimed at restoring or increasing sphingolipid synthesis in this phenotype resulted in reduced AHR.
Both, GlyH-101 and fenretinide increased sphinganine and dihydroceramides, robust measures of de novo sphingolipid synthesis, in two human cells lines and, most relevant to this study, in murine bronchial rings and PCLS containing small airways and in lungs from SPT-deficient mice within a few hours after ex vivo or in vivo administration of GlyH-101 or fenretinide. The increased sphinganine and dihydroceramides were associated with decreased airway responsiveness in tissues and in mice treated with these drugs. In addition to sphinganine and dihydroceramides, GlyH-101 also increased ceramides, sphingomyelins, sphingosine, and S1P upstream of DES1, which could have also contributed to the decreased airway responsiveness induced by GlyH-101 in the SPT-deficient airways. These metabolites did not increase with fenretinide, suggesting that changes in sphinganine and dihydroceramides could play a role for AHR associated with decreased sphingolipid synthesis. Nevertheless, the inhibition of DES1 by fenretinide affects cellular ceramide homeostasis and alters the regulation of the synthesis of complex sphingolipids and S1P that may have also contributed to AHR. In human airway smooth-muscle cells, S1P can lead to β2-adrenoreceptor desensitization (23), and sphingosine analogs as well as sphingosine kinase 2 substrates can reverse smooth-muscle cell thickening (41).
Specifically, we speculate that changes in one or more metabolites within the de novo sphingolipid synthesis pathway are linked to airway contractility. These sphingolipids may thus contribute to asthma pathogenesis or could be useful as biomarkers.
The decreased AHR with GlyH-101 required treatment of the tissues or the mice for hours and was not seen when the tissues were treated for only 15 minutes. This suggests that the decreased airway responsiveness induced by GlyH-101 is independent of its chloride channel–blocking activity or any acute action on other signaling mechanisms regulating smooth-muscle contraction. Interestingly, the effect of GlyH-101 on small-airway reactivity was not observed in PCLS from control (WT) mice, suggesting that global activation of sphingolipid synthesis by GlyH-101 in an unperturbed state is not effective. Similarly, the positive effect of fenretinide on AHR was concentration-dependent in SPT-deficient airways, but a decrease in methacholine-induced airway contraction was only significant at the highest fenretinide dose in PCLS from WT mice. Although approved for use in humans, the use of GlyH-101 and fenretinide for asthma might be limited by their narrow dose range and off-target effects. These studies may stimulate the development of other stimulators of de novo synthesis.
We found that GlyH-101 and fenretinide decreased bradykinin-induced [Ca2+]i release in human airway smooth-muscle cells, whereas myriocin increased this activity. We acknowledge the limitations of the cell models for human asthma. However, these results suggest that alteration of sphingolipid synthesis affects intracellular calcium signaling in airway smooth muscle. Because [Ca2+]i release is a cell-signaling mechanism that regulates contractility, these findings can provide the basis for future mechanistic studies, such as measuring the effects of specific sphingolipids on intracellular calcium oscillations in airway smooth-muscle cells in PCLS (38), which would also require assessing agonists other than bradykinin. Such studies could provide a mechanistic link between sphingolipid synthesis and airway contraction. Future studies also need to define the role of specific airway cell types and smooth muscle, ideally in primary cells or human airway models, and to identify whether specific metabolite(s) within the de novo synthesis pathway can affect contraction.
Inflammation is not a feature of the SPT deficiency associated with AHR (9) but is a critical factor in asthma. SPT inhibition by myriocin after allergic sensitization also leads to AHR in mice, suggesting that altered sphingolipid metabolism in immune cells might contribute to asthma pathogenesis (40). Furthermore, fenretinide reduced airway reactivity in ovalbumin-sensitized mice by reducing airway inflammation (42). Interestingly, the lower sphingolipid concentrations in children with asthma and asthma-associated 17q21 genotypes seem more pronounced in nonallergic children (14), and the link of 17q21 genotypes to ORMDL3 was initially described for nonallergic childhood asthma (1). Future studies need to assess the effects of activation of sphingolipid synthesis in the presence of allergic inflammation.
In summary, we here provide proof-of-principle evidence that decreased de novo sphingolipid synthesis also increases small-airway reactivity and that pharmacological activation of this metabolic pathway in airways with an SPT-deficient phenotype improves airway hyperresponsiveness. We propose that the manipulation of the sphingolipid de novo synthesis pathway could be a novel metabolic strategy for asthma.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Xian-Cheng Jiang, The State University of New York–Downstate, for providing the Sptlc2+/− mice and thank Biin Sung, Wenzhu Wu, and Benjamin I. Kim for technical help.
Footnotes
Supported by R21 AI140724 (S.W.) and by generous support from Nancy and Dan Paduano, Christine and Pasco Alfaro, and Casey and Noah Weis.
Author Contributions: A.F.H., A.V., T.S.W., J.P.-Z., and S.W. designed and performed the research. A.F.H., R.B.S., C.W.E., T.S.W., and J.P.-Z. analyzed and interpreted the data. A.F.H., T.S.W., J.P.-Z., and S.W. wrote, revised and approved the final version to be published.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0194OC on July 24, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 2.Das S, Miller M, Broide DH. Chromosome 17q21 genes ORMDL3 and GSDMB in asthma and immune diseases. Adv Immunol. 2017;135:1–52. doi: 10.1016/bs.ai.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Acevedo N, Reinius LE, Greco D, Gref A, Orsmark-Pietras C, Persson H, et al. Risk of childhood asthma is associated with CpG-site polymorphisms, regional DNA methylation and mRNA levels at the GSDMB/ORMDL3 locus. Hum Mol Genet. 2015;24:875–890. doi: 10.1093/hmg/ddu479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verlaan DJ, Berlivet S, Hunninghake GM, Madore AM, Larivière M, Moussette S, et al. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet. 2009;85:377–393. doi: 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, et al. A decade of research on the 17q12-21 asthma locus: piecing together the puzzle. J Allergy Clin Immunol. 2018;142:749–764, e3. doi: 10.1016/j.jaci.2017.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiefer K, Carreras-Sureda A, García-López R, Rubio-Moscardó F, Casas J, Fabriàs G, et al. Coordinated regulation of the orosomucoid-like gene family expression controls de novo ceramide synthesis in mammalian cells. J Biol Chem. 2015;290:2822–2830. doi: 10.1074/jbc.M114.595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siow D, Sunkara M, Morris A, Wattenberg B. Regulation of de novo sphingolipid biosynthesis by the ORMDL proteins and sphingosine kinase-1. Adv Biol Regul. 2015;57:42–54. doi: 10.1016/j.jbior.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, et al. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med. 2013;5:186ra67. doi: 10.1126/scitranslmed.3005765. [DOI] [PubMed] [Google Scholar]
- 10.Miller M, Rosenthal P, Beppu A, Gordillo R, Broide DH. Oroscomucoid like protein 3 (ORMDL3) transgenic mice have reduced levels of sphingolipids including sphingosine-1-phosphate and ceramide. J Allergy Clin Immunol. 2017;139:1373–1376, e4. doi: 10.1016/j.jaci.2016.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowal K, Żebrowska E, Chabowski A. Altered sphingolipid metabolism is associated with asthma phenotype in house dust mite-allergic patients. Allergy Asthma Immunol Res. 2019;11:330–342. doi: 10.4168/aair.2019.11.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J Allergy Clin Immunol. 2015;136:1035–1046, e6. doi: 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perzanowski MS, Ono JG, Acosta LM, Kim BI, Divjan A, Miller R, et al. Distinct serum sphingolipid profiles among school-aged children with exercise-induced wheeze and asthma persistence. Am J Respir Crit Care Med. 2017;195:1068–1070. doi: 10.1164/rccm.201609-1884LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono JG, Kim BI, Zhao Y, Christos PJ, Tesfaigzi Y, Worgall TS, et al. Decreased sphingolipid synthesis in children with 17q21 asthma-risk genotypes. J Clin Invest. 2020;130:921–926. doi: 10.1172/JCI130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 16.Merrill AH., Jr Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal. 2011;15:2501–2517. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S, Kim JA, Kim TH, Li HY, Shin KO, Lee YM, et al. Altering sphingolipid composition with aging induces contractile dysfunction of gastric smooth muscle via K(Ca) 1.1 upregulation. Aging Cell. 2015;14:982–994. doi: 10.1111/acel.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs LS, Kester M. Sphingolipids as mediators of effects of platelet-derived growth factor in vascular smooth muscle cells. Am J Physiol. 1993;265:C740–C747. doi: 10.1152/ajpcell.1993.265.3.C740. [DOI] [PubMed] [Google Scholar]
- 20.Sabbadini RA, Betto R, Teresi A, Fachechi-Cassano G, Salviati G. The effects of sphingosine on sarcoplasmic reticulum membrane calcium release. J Biol Chem. 1992;267:15475–15484. [PubMed] [Google Scholar]
- 21.Miller M, Tam AB, Mueller JL, Rosenthal P, Beppu A, Gordillo R, et al. Cutting edge: targeting epithelial ORMDL3 increases, rather than reduces, airway responsiveness and is associated with increased sphingosine-1-phosphate. J Immunol. 2017;198:3017–3022. doi: 10.4049/jimmunol.1601848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roviezzo F, D’Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, et al. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol. 2010;42:572–577. doi: 10.1165/rcmb.2009-0108OC. [DOI] [PubMed] [Google Scholar]
- 23.Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ. Effect of sphingosine 1-phosphate on cyclo-oxygenase-2 expression, prostaglandin E2 secretion, and β2-adrenergic receptor desensitization. Am J Respir Cell Mol Biol. 2016;54:128–135. doi: 10.1165/rcmb.2014-0443OC. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Miller M, Unno H, Rosenthal P, Sanderson MJ, Broide DH. Orosomucoid-like 3 (ORMDL3) upregulates airway smooth muscle proliferation, contraction, and Ca2+ oscillations in asthma. J Allergy Clin Immunol. 2018;142:207–218, e6. doi: 10.1016/j.jaci.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melis N, Tauc M, Cougnon M, Bendahhou S, Giuliano S, Rubera I, et al. Revisiting CFTR inhibition: a comparative study of CFTRinh-172 and GlyH-101 inhibitors. Br J Pharmacol. 2014;171:3716–3727. doi: 10.1111/bph.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective CFTR increases synthesis and mass of sphingolipids that modulate membrane composition and lipid signaling. J Lipid Res. 2009;50:1101–1108. doi: 10.1194/jlr.M800427-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teichgräber V, Ulrich M, Endlich N, Riethmüller J, Wilker B, De Oliveira-Munding CC, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 29.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, et al. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 30.Rahmaniyan M, Curley RW, Jr, Obeid LM, Hannun YA, Kraveka JM. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286:24754–24764. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poliakov E, Samuel W, Duncan T, Gutierrez DB, Mata NL, Redmond TM. Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Δ4-desaturase 1. PLoS One. 2017;12:e0176487. doi: 10.1371/journal.pone.0176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Maurer BJ, Reynolds CP, Cabot MCN. N-(4-hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61:5102–5105. [PubMed] [Google Scholar]
- 33.Fabrias G, Muñoz-Olaya J, Cingolani F, Signorelli P, Casas J, Gagliostro V, et al. Dihydroceramide desaturase and dihydrosphingolipids: debutant players in the sphingolipid arena. Prog Lipid Res. 2012;51:82–94. doi: 10.1016/j.plipres.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 35.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, et al. Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L248–L256. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, et al. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–L534. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 37.Bui HH, Leohr JK, Kuo MS. Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2012;423:187–194. doi: 10.1016/j.ab.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee S, Trice J, Shinde P, Willis RE, Pressley TA, Perez-Zoghbi JF. Ca2+ oscillations, Ca2+ sensitization, and contraction activated by protein kinase C in small airway smooth muscle. J Gen Physiol. 2013;141:165–178. doi: 10.1085/jgp.201210876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Densupsoontorn N, Worgall TS, Seo T, Hamai H, Deckelbaum RJ. Fatty acid supplied as triglyceride regulates SRE-mediated gene expression as efficiently as free fatty acids. Lipids. 2007;42:885–891. doi: 10.1007/s11745-007-3093-x. [DOI] [PubMed] [Google Scholar]
- 40.Edukulla R, Rehn KL, Liu B, McAlees JW, Hershey GK, Wang YH, et al. Intratracheal myriocin enhances allergen-induced Th2 inflammation and airway hyper-responsiveness. Immun Inflamm Dis. 2016;4:248–262. doi: 10.1002/iid3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blais-Lecours P, Laouafa S, Arias-Reyes C, Santos WL, Joseph V, Burgess JK, et al. Metabolic adaptation of airway smooth muscle cells to an SPHK2 substrate precedes cytostasis. Am J Respir Cell Mol Biol. 2020;62:35–42. doi: 10.1165/rcmb.2018-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanagaratham C, Kalivodová A, Najdekr L, Friedecký D, Adam T, Hajduch M, et al. Fenretinide prevents inflammation and airway hyperresponsiveness in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2014;51:783–792. doi: 10.1165/rcmb.2014-0121OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.