Abstract
AAT (alpha-1 antitrypsin) deficiency (AATD), characterized by low levels of circulating serine protease inhibitor AAT, results in emphysematous destruction of the lung. Inherited serum deficiency disorders, such as hemophilia and AATD, have been considered ideal candidates for gene therapy. Although viral vector–meditated transduction of the liver has demonstrated utility in hemophilia, similar success has not been achieved for AATD. The challenge for AAT gene therapy is achieving protective levels of AAT locally in the lung and mitigating potential liver toxicities linked to systemically administered viral vectors. Current strategies with ongoing clinical trials involve different routes of adeno-associated virus administrations, such as intramuscular and intrapleural injections, to provide consistent therapeutic levels from nonhepatic organ sites. Nevertheless, exploration of alternative methods of nonhepatic sourcing of AAT has been of great interest in the field. In this regard, pulmonary endothelium–targeted adenovirus vector could be a key technical mandate to achieve local augmentation of AAT within the lower respiratory tract, with the potential benefit of circumventing liver toxicities. In addition, incorporation of the CRISPR/Cas9 (CRISPR-associated protein 9) nuclease system into gene-delivery technologies has provided adjunctive technologies that could fully realize a one-time treatment for sustained, lifelong expression of AAT in patients with AATD. This review will focus on the adeno-associated virus– and adenoviral vector–mediated gene therapy strategies for the pulmonary manifestations of AATD and show that endeavoring to use genome-editing techniques will advance the current strategy to one fully compatible with direct human translation.
Keywords: alpha-1 antitrypsin deficiency, pulmonary emphysema, gene therapy, adenovirus
Clinical Relevance
The topic seems to us timely for several reasons. First, the recent gene-therapy successes in hemophilia beg the question as to why similar success has not been achieved for AAT (alpha-1 antitrypsin) deficiency. In addition, the advent of new gene-editing technology raises new interventional possibilities. So, we believe this might be a timely and interesting review for the Journal readership.
AAT (alpha-1 antitrypsin) is a glycoprotein secreted primarily by hepatocytes but also by neutrophils, monocytes, and epithelial cells of the lung and gut. As the most common serine protease inhibitor in human plasma, AAT normally acts to inhibit neutrophil elastase and other related proteases. In addition, AAT has antiinflammatory and immunomodulatory properties (1).
AAT deficiency (AATD) is an autosomal recessive disorder that is caused by mutations in SERPINA1, the gene that encodes for AAT. Over 120 mutations have been reported at this locus, with alleles associated with AATD affecting 1 in 1,500 to 1 in 5,000 people with European ancestry (2, 3). Null mutation (Pi*Null) is very rare and caused by a premature stop codon in the SERPINA1 gene that results in early-onset and very severe pulmonary manifestations (4).The most common deficiency variants are the S and Z alleles, carried by more than 3.4 million individuals. AATD disease is undiagnosed or diagnosed with a delay of 5–10 years (5).
The S allele results in an unstable AAT protein with reduced secretion. Although individuals with the homozygous S genotype have 50% of the normal AAT serum levels and are asymptomatic, people with the heterozygous S/Z (Pi*SZ) genotype have only 15–20% of the normal levels. In the case of homozygosis of the Z variant (Pi*ZZ), the misfolded and polymerized AAT protein aggregates within the endoplasmic reticulum (ER) of the hepatocytes. The aggregation prevents the secretion of AAT into the blood and can lead to liver disease. As a pulmonary consequence, patients with Pi*ZZ and 20% of the patients with Pi*SZ genotypes have very low serum AAT levels (<11 μM), which predisposes them to AATD-associated lung disease (6). A recent study showed that people with heterozygous Pi*SZ genotype are less likely to develop emphysema, but the lung function decline can be similar to that of patients with homozygous Pi*ZZ. In Pi*SZ patients, AAT level and smoke exposure are the most critical factors for the severity of AATD-related lung disease (7).
Liver disease results from the accumulation of abnormally folded AAT molecules in the ER of hepatocytes. AATD is associated with slowly progressive cirrhosis, hepatitis, and liver failure that can occur at any age, and ∼13% of patients with the Pi*ZZ genotype die of liver disease (1). For AATD-associated liver disease, liver transplantation is the only available treatment. However, it can lead to complications and deaths resulting from infections, hemorrhage, thrombosis, severe graft dysfunction, and chronic rejection (8).
Severe lung destruction associated with the Pi*ZZ or Pi*SZ genotype develops at the age of 30–40. If left untreated, the insufficient inhibition of neutrophil elastase and other serine proteases causes progressive alveolar destruction and eventually emphysema and chronic obstructive pulmonary disease (see Figure 1A). AATD-associated emphysema, with symptoms such as breathlessness, hacking cough, wheezing, and a barrel-shaped chest, is the most common cause of death for patients with the Pi*ZZ genotype. Nevertheless, AATD can also present as bronchitis, bronchiectasis, asthma, and pneumonia (1, 9). Besides genetic predisposition, the presence of AATD-associated lung disease is dependent on environmental exposures, smoking being the greatest risk factor. Although active smoking in patients with severe AATD is associated with a reduced lifespan, never-smokers might never develop lung disease and might have a normal lifespan (1, 10).
Figure 1.

Pathogenesis of AAT (alpha-1 antitrypsin) deficiency–associated lung disease. (A) Null mutations and the Pi*ZZ allele in the serine protein inhibitor encoding the SERPINA1 locus leads to absent or misfolded AAT (Z-AAT) that creates a predisposition for liver and lung disease. Z-AAT aggregates in the endoplasmic reticulum (ER) of hepatocytes, leading to ER stress and potentially liver disease. Polymers of Z-AAT fail to reach the lung in sufficient levels, putting the lung parenchyma (alveoli, alveolar ducts, and bronchioles) at risk of injury because of unopposed NE (neutrophil elastase) activity. The alveolar endothelial and epithelial cell junctions restrict the diffusion of AAT (molecular weight, 52 kD) to the alveolar space, such that the AAT level in the epithelial lining fluid (ELF) is often bellow detection (<1–2%). Z polymers of AAT colocalize with neutrophils in alveoli and regulate neutrophil chemotaxis. Thus, in case of AAT deficiency, there is an increased neutrophil pool in the interstitium and in the alveolar airspace, which might be further activated by alveolar macrophages through secreted cytokines and ILs. The protease/antiprotease imbalance compromises the alveolar integrity and accelerates the pathogenesis of emphysema (24, 83–85). (B) Current AAT gene-therapy strategies advanced to human clinical trials (phase I/II) apply intravenous/intrapleural/intramuscular injection of rAAV vectors to provide sufficient amounts of AAT in the lung and the serum. hAAT = human AAT; Pi*ZZ = homozygous Z variant; rAAV = recombinant adeno-associated virus; rAAVrh-10-hAAT = hAAT–expressing rAAV, rhesus macaque serotype 10; rAAV1/2-hAAT = hAAT–expressing rAAV, human serotype 1 or 2. The illustrations were created with BioRender.com.
Currently, the only U.S. Food and Drug Administration (FDA)-approved therapeutic solution for AATD lung disease is augmentation therapy, whereby AAT is purified from human plasma and delivered intravenously weekly (11). This dramatically raises the serum and alveolar epithelial lining fluid (ELF) levels, which remain at over 11 μM and 1.2 μM, respectively, for 4.5 days. These levels were defined as protective thresholds by the FDA and the European regulatory agencies. Although it was approved for marketing, the clinical benefits of augmentation therapy were confirmed only decades later (12–14). The high expenses, limited supply, and purity concerns associated with plasma-derived native AAT have led to preclinical development of recombinant AAT produced by bacteria, yeast, and transgenic sheep (15). However, recombinant AAT undergoes incorrect glycosylation that shortens its half-life and affects renal clearance, which is why it has not advanced to the stage of FDA approval (1, 16). Although augmentation therapy is capable of slowing the progression of emphysema in patients with AATD, the treatment is extremely expensive (>$100,000/yr in the United States) and requires repeated, lifelong intravenous infusions, and, moreover, has not been shown to improve patients’ quality of life (1).
Many alternative approaches are therefore currently being developed and tested in preclinical and/or clinical studies for their use in the treatment of AATD. There are orally bioavailable neutrophil elastase inhibitors (in a phase 2 clinical trial; NCT 03636347), liver-directed therapies that include gene-silencing molecules (17), protein-folding and chaperone modifiers (18), autophagy enhancers and polymerization blockers (19, 20), and potentially many more. Some of these products have advanced to clinical trials, but none have reached the U.S. marketplace to date.
Gene Therapy for AATD
Besides the fact that AATD is a monogenic disorder, there are a number of other reasons that it has been considered an ideal candidate for gene-therapy strategies. The earliest gene-replacement studies have shown that augmentation of AAT in the serum to the protective level (∼11 μM, 570 μg/ml) can protect the lung from proteolytic destruction; on the other hand, supraphysiological levels of AAT have no deleterious effects (11, 16, 21). On this basis, gene-therapy strategies have been designed to synthesize M-AAT (M-type AAT) in various tissues and have demonstrated that cellular origin of M-AAT is not critical for physiological action. Thus, the sourcing of AAT is nonrestrictive; a physiologically protective AAT level in the serum is sufficient to normalize the lung ELF (22).
Although the first in vivo gene therapy for AATD was conducted in animals almost 30 years ago, there is still no FDA-approved gene therapy for patients with AATD (23). Similar to attempts with other serum-deficiency disorders such as hemophilia B, initial attempts to reconstitute serum AAT levels focused on viral or nonviral vector–mediated targeting of the liver, wherein hepatocytes represent the normal physiological source of the deficient serum factor. Plasmid DNA vectors and retrovirus, adeno-associated virus (AAV), and adenovirus (Ad) vectors have been employed for liver transduction to achieve hepatic sourcing of the deficient serum factor (reviewed in Reference 24). The clear success of liver-directed gene therapy strategies has been limited by two challenges. First, most of these approaches have not been successful in achieving adequate magnitudes of AAT replacement in the ELF of the lung. Second, vector-associated liver toxicity makes consideration of nonhepatic sourcing of serum factors desirable. Hence, alternative approaches must be explored to overcome the issues related to efficacy and liver toxicity before bringing AAT gene therapy to the bedside.
Proteolytic destruction mediated by neutrophil elastase almost exclusively occurs in the lung parenchyma in the case of serum deficiency of AAT; this organ represents the front line of the disease (24). Local production of AAT, within the lower respiratory tract, may provide a viable pharmacologic strategy to correct the elastase/antielastase balance within the lung and would theoretically circumvent vector-related liver toxicities. The focus of this work is thus directed toward the numerous non–liver-based approaches that are being explored in the preclinical and clinical research of recent years for the pulmonary manifestations of AATD. First, we review the AAV-based gene-therapy strategies, as, to date, these have been moved furthest toward the “liver sparing” required for human clinical translation.
AAV-based AAT Gene Therapy
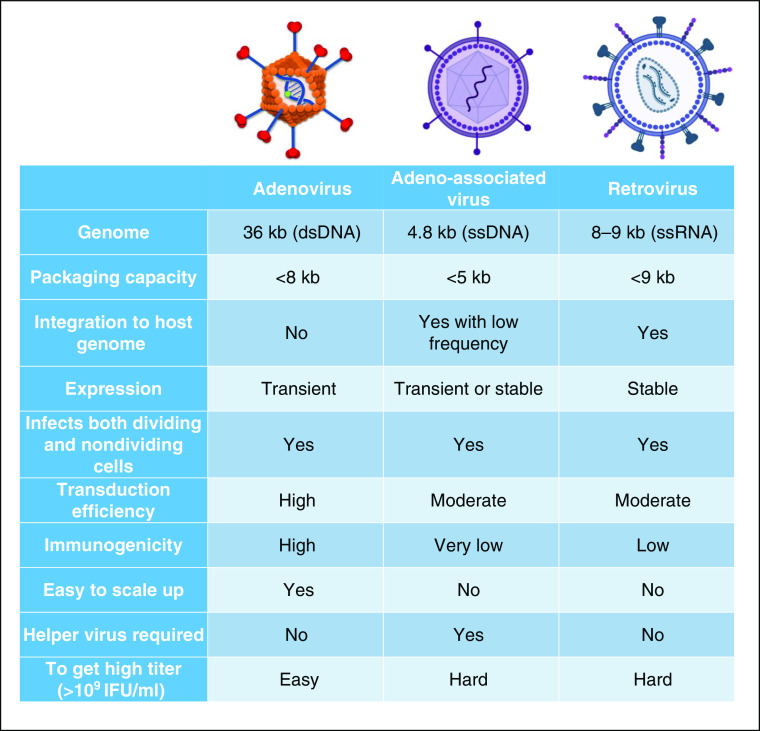
Currently, the most commonly employed gene-delivery vector for AAT gene therapy is the AAV, a nonpathogenic, single-stranded DNA virus that is able to infect both dividing and nondividing cells. AAV is relatively safe, with low immunogenicity and stable long-term expression; however, its replication is dependent on coinfection of other so-called helper viruses (Ad or herpesvirus), and contamination with these might lead to inflammation in the host. However, “helper-free” production systems are being developed (25). Approximately 0.1% of wild-type AAV genomes integrate in a site-specific manner in a region called the AAVS1 site on chromosome 19; nevertheless, studies have shown that it can also integrate at other sites of the human genome (e.g., chromosome 1) (26) (see Figure 2).
Figure 2.
Comparison of adenovirus (Ad), adeno-associated virus, and retrovirus as gene-therapy delivery systems. Illustration of the viral vectors was created using BioRender.com. dsDNA = double-stranded DNA; IFU = infectious units; ssDNA = single-stranded DNA; ssRNA = single-stranded RNA.
The rep and cap genes of the virus can be replaced by the AAT cDNA sequence (∼1.3 kb) under the control of a strong constitutive promoter that results in a sustained expression of AAT after injection into laboratory animals. These coding sequences are flanked by ITRs that are necessary for AAV replication and packaging (24). Different serotypes and routes of administration have been applied in preclinical settings, reviewed in References 24 and 27, and AAV1, AAV2, and AAV rhesus macaque (rh) 10 serotypes have been advanced into clinical trials (6, 28–30) (see Figure 1B). Of note in this regard, the host’s immune response to the AAV can be dependent on the AAV serotype, the dose, the route of administration, and the transgene itself, as well as on the species being studied (28, 31). In the first in-human phase I study, all of the enrolled AAT-deficient (Pi*ZZ) patients (serum AAT level < 11 μM) developed neutralizing antibodies against AAV1 capsid after 2 weeks of intramuscular injections of AAV1 vector expressing normal M-AAT. Even so, it did not cause complete elimination of vector-transduced myofibers for up to 1 year. Importantly, no subject developed antibodies to the AAT transgene. Although the achieved level was subtherapeutic (200-fold below the therapeutic target), the dose escalation continued in a phase IIa study (28, 29). The same vector, this time manufactured with a herpes simplex virus-1 helper system to increase the amount and potency, showed a linear dose response and reached a 10-fold-higher level compared with the first trial; however, this required 100 intramuscular injections in a single session (29). Because the applied AAV vector could not be concentrated within a smaller volume, the so-called “pincushion method” (referring to the multiple intramuscular injections) was necessary to achieve the desired total AAV-vector dose (29).
Over a 5-year follow-up period, the same AAT-deficient patients demonstrated sustained AAT transgene expression at 2.5–3% of the therapeutic target level. In addition, functional tolerance to the hAAT (human AAT)–expressing AAV-transduced cells, increased serum antielastase activity, and decreased neutrophil degranulation indicated biological activity of the exogenous AAT (31). Nevertheless, to continue the dose escalation, the route of administration had to be changed. Thus, current efforts focus on ways to increase efficient delivery, such as by isolated limb perfusion, that could potentiate greater amounts of AAT secretion from a larger mass of skeletal muscle (30).
Other routes of AAV delivery are also being investigated, such as intrapleural injection. In this context, pleural administration delivers the AAV gene-transfer vectors both to the lung and to the liver, thereby increasing the possibility of achieving the threshold levels of AAT protein both in the serum and in the alveolar ELF (6). The pleural surface is easily accessible by using a chest tube (for patients with emphysema or pleural effusion) or a blunt-tipped intrapleural needle and catheter (for patient without pleural effusion). Lungs are targeted by transduction of mesothelial cells lining the pleura, which is a pair of serous membranes between the chest wall and the lung parenchyma. In parallel, through the lymphatic system of the pleura, the AAV vector is also delivered to the liver (6). To explore this approach, De and colleagues (32) screened 25 human and nonhuman primate (NHP) serotypes of AAV vector and found that the rhesus AAVrh.10 serotype provides the highest AAT expression both in the serum and in the lung after intrapleural administration. Because the rhesus AAVrh.10 serotype also circumvents human immunity to AAV, this vector was chosen for further preclinical studies (32, 33). Furthermore, the combined data from the toxicology studies in two species supported a phase I/II clinical trial to assess safety and efficacy of intrapleural versus intravenous human AAVrh.10-hAAT for AATD in patients with Pi*ZZ or Pi*Null genotypes (NCT 02168686) (6). No results were posted at the time of writing this review.
Several liver-targeted AAV-mediated hAAT gene-delivery systems have been tested in preclinical studies, without advancing to clinical trials (34–37). Although liver-targeted or systemically administered AAV vectors seem to provide the best option to augment serum AAT levels by expressing high levels of M-AAT within the liver (major producer of endogenous AAT), there are many concerns regarding this route of administration, particularly in individuals with the Pi*ZZ genotype. The mutant, Z-AAT (misfolded AAT) protein accumulates in the ER of hepatocytes, leading to ER stress, and is associated with upregulation of proinflammatory genes (IL6, CXCL8). Therefore, patients with AATD require regular monitoring for development of liver disease (1). Intravenous injection of AAV8 for hemophilia B gene therapy showed liver transaminase elevation in many patients, probably due to the immune response to AAV-vector capsids (38). Corticosteroid (prednisone) therapy might help to rescue this effect; however, it is not clear whether this would be the case for patients with AATD, especially for patients with manifested liver disease (30, 38).
In addition to the issues related to liver transduction, studies suggest that the AAV genome can integrate nonspecifically to the host-cell genome, raising the possibility of insertional mutagenesis (39). A particular integration event within the Rian gene led to hepatocellular carcinoma in neonatal mice after AAV injection (40). Nevertheless, in NHPs and human samples, the integration rate of AAV2 or 5 genomes was very low, especially in genes associated with liver-cancer development (41). Taken together, the oncogenic potential of AAV vectors is still controversial; however, there is increasing evidence of AAV-vector integration. The latter has been confirmed in AAV-treated dogs with hemophilia after 10 years of follow-up as well as in murine samples in which AAV-vector sequences have been unintentionally introduced at nuclease-induced breaks of therapeutically relevant genes (42, 43).
Adenoviral AAT Gene Therapy
Ad is considered the most effective vector for delivering genes in vivo because of its many desired features, such as its large, exogenous-DNA-insertion capacity (>7.5 kb); its nonintegrative, stable viral genome; its episomal transduction ability for both dividing and nondividing cells; and the possibility of high titer production (44, 45). The fiber knob of the Ad binds to the CAR (coxsackie-Ad receptor) that is involved in the formation of junctions between epithelial cells and is expressed in most human and animal cells, owing to the high transduction efficacy of these vectors (44, 46). The binding triggers intracellular signaling through cellular integrins that results in virion internalization, a key proinflammatory activation event, and, as a consequence, high immunogenicity (46) (see Figure 2).
Modification of adenoviral double-stranded DNA by deleting its E1 and E3 genes gave the biomedical research community a large-packaging-capacity (>7.5 kb) gene-transfer vector that has become the most common vector used in clinical trials (44). Although the high immunogenicity limits the time of transgene expression to 1–2 weeks, it makes Ad vectors ideal for clinical applications in which the aim is to initiate a biological process (e.g., angiogenesis) or for anticancer therapies in which conditionally replicative Ads could target and kill cancer stem cells (47, 48). Furthermore, by expressing antigen in the capsid, Ad5-based vectors induce rapid cellular and humoral immune responses against pathogens, which is the basis of vaccine development (49, 50). Nonhuman Ad serotypes are able to prevent preformed anti-Ad5 immunity in humans, leading to important advances in vaccine technology (49, 51).
Nonetheless, the transient gene-expression profile has largely limited consideration of Ad for AATD gene-therapy applications. Recently, reconsideration of Ad for this application has been undertaken. Earlier strategies have applied the technical mandate to genetically correct hepatocytes to restore AAT serum levels by retroviral (52, 53), AAV (34, 36), and Ad vectors (54, 55); however, none of these studies have proven to be effective in achieving protective AAT levels in the lung ELF, nor have they mitigated liver toxicity. Clinical trials of AAV8- and AAV10-driven hemophilia B gene therapies have been discontinued because of safety concerns about hepatotoxicity, in addition to the loss of Factor IX expression associated with capsid-related immunity responses (56).
Genetic capsid modifications of Ad vectors, such as incorporation of a myeloid binding peptide (MBP) into the Ad5 fiber knob (Ad5.MBP) (see Figure 3A), have resulted in altered tropism with significantly enhanced transduction efficiency of the pulmonary endothelium, together with reduced hepatotropism (57). MBP, identified by phage-display biopanning, binds to CD11b+ leukocytes/B cells through the WTLXXGY sequence. Its binding specificity is maintained when MBP is genetically incorporated into knob-deleted Ad5 virions (58). The systemically administered targeted Ad5.MBP vector successfully accomplished AAT gene delivery to the lung endothelial cells (ECs) and resulted in protective AAT levels, both in the serum (>20 μM) and in the lung ELF (>5 μM) (59) (see Figures 3A and 3B). This was the first study that showed augmentation of the ELF AAT level to the therapeutic level with a systemically administered gene-therapy vector without any limiting inflammatory stress response (6, 59).
Figure 3.
Targeted Ad via genetic engineering. Ad vector tropism can be altered by genetic capsid modification. (A) A myeloid binding peptide (MBP)-targeting ligand has been incorporated into the fiber knob domain of Ad, which resulted in a highly efficient and selective gene-delivery vector to the pulmonary endothelial cells (57, 58, 86). In addition to altering tropism, capsid modifications can provide the means to mitigate liver toxicity. Genetic modification of the major capsid protein, hexon, by either hexon swap with a hexon from a different Ad serotype or a hypervariable-region swap only, leads to ablated liver sequestration of Ad vectors (45, 62, 63). Deletion of the E1 and E3 genes of the adenoviral dsDNA enables a large-packaging-capacity (>7.5 kb) vector suitable for delivery of large therapeutic genes driven by a constitutively active promoter. (B) Serum and urea normalized ELF AAT levels in mice injected with either 1 × 1010 virus particles (VP) of Ad5–cytomegalovirus (CMV) promoter–hAAT or 1 × 1010 VP of Ad5 fiber knob incorporating MBP (Ad5.MBP)–CMV–hAAT. The mice were killed 8, 24, or 72 hours later, and their serum and ELF were collected to determine hAAT by ELISA (n = 5 mice in each group at each time point). There was no significant difference in serum hAAT concentration at any time point, but mice injected with Ad5.MBP–CMV–hAAT had significantly higher hAAT concentrations in their ELF compared with mice injected with Ad5–CMV–hAAT at 24 and 72 hours after injection (B). *Significant differences with P < 0.05 between equivalent groups, whereas an absence of asterisk represents no significant difference. The illustration was created with BioRender.com. B is adapted by permission from Reference 59.
In addition to altering tropism, capsid modifications can provide the means to mitigate toxicity. In this regard, the humoral immune response induced by Ad5 is mostly directed at antigen epitopes located in the major capsid protein, the hexon. Studies have determined that there is a direct interaction between hypervariable regions 5 and 7 of the hexon and blood coagulation FX (Factor X). Consequently, the high-affinity binding between the hexon and FX has been identified as the key mediator of Ad5 liver transduction after intravenous injection (60, 61). Genetic modification of the hexon, either a hexon swap with a hexon from a different Ad serotype or a hypervariable region swap only, leads to ablated liver sequestration of Ad vectors (45, 62, 63). These advances in the field have provided several opportunities to mitigate liver toxicity.
Taken together, to reach Ad’s full therapeutic efficacy for AAT gene therapy, both liver-avoiding strategies (hexon modification) and retargeting strategies (capsid engineering via MBP incorporation) must be employed (45) (see Figure 3A). Therefore, the translational application of Ad vectors requires a combination of nonhepatic sourcing of AAT to mitigate liver toxicity and pulmonary-endothelium targeting for local AAT augmentation.
Despite the fact that even tropism-modified Ad vectors can assure highly efficient gene transfer, the high immunogenicity of Ad vectors limits the duration of transgene expression, which necessitates the development of delivery systems with less robust immune responses (44, 59). In this regard, the helper-dependent Ads, lacking all viral coding sequences, could provide a means to achieve long-term gene expression via Ad (64). These vectors, known to elicit a negligible cytotoxic T-cell response triggered by the immunogenic viral proteins, have been shown to drive much longer AAT expression in baboons (up to 1 yr) and to achieve therapeutic serum levels. Nevertheless, being liver-targeted approaches, they raised safety concerns and thus never advanced to human trials (21).
Besides helper-dependent Ads, application of NHP serotypes could offer a novel means to avoid preexisting immunity. Recent studies have explored novel primate Ad systems that exhibit very low seroprevalence in human populations and are not neutralized efficiently by antibodies of human sera (65). Lu and his colleagues (66) have evaluated one of these vectors (gorilla Ad type 9, isolate 46 [GAd]) as a gene-transfer vector in mice and compared it with human Ad serotype 5. GAd had robust pulmonary EC tropism and a lack of hepatotropism with undetectable liver toxicity and inflammatory response. This work demonstrated a novel lung-targeted gene-therapy vehicle with an excellent safety profile in mice, providing a novel technology to accomplish gene-based interventions at the pulmonary endothelium.
These studies, in the aggregate, highlight the therapeutic potentials embodied in targeting AAT production at the site of disease pathobiology in the lower respiratory tract. For the full success of retargeted Ad/GAd-driven AAT gene therapy, gene-editing technologies such as CRISPR/Cas (CRISPR-associated protein) nucleases may hold great promise for facilitating the achievement of long-term corrective gene expression critical to overall gene-therapy success for AATD. In the next section, we focus on how the integration of gene-delivery and gene-editing technologies revolutionized the entire vector-transfer field by providing a generalizable approach applicable to several inherited diseases, including AATD.
Delivery of Genome Editors for Lifelong Correction
Integrating vector systems (lentiviral, retroviral vectors) can deliver transgenes with high efficiency, enabling long-term expression (67). In this regard, earlier studies showed promising results for producing sustained and high levels of serum AAT with in vivo retroviral vectors, but the main disadvantage of these delivery systems is their potential risk of causing insertional mutagenesis and cytotoxicity (52, 53, 67). Therefore, targetable genomic integration tools such as CRISPR/CRISPR-Cas nucleases could be exceptionally useful for nonintegrative gene-therapy vectors, with the potential to traverse the issue of the loss of gene expression without the high risk of genotoxic effects.
AAV and Ad vectors persist as separate extrachromosomal elements in the infected cells, as long as the host immune responses against viruses and/or transgenes eliminate the transduced cells, and are considered to be nonintegrative vectors (67). The CRISPR/Cas9 system, which was developed by the acquired immune system in microbes, introduces a DNA double-strand break at a targeted locus site guided by highly specific RNA molecules (guide RNA [gRNA]). The DNA double-strand breaks are repaired by either the nonhomologous end-joining (NHEJ) pathway or the more precise homology-director repair (HDR) pathway (68). Since CRISPR’s creation as a genome-editing technique in all eukaryote cells, it has quickly become a widely used tool in many fields of biological science, with the goal of facilitating precise genetic manipulation (68, 69). Application of the CRISPR/Cas9 tool has been introduced to treatment of preclinical disease models of several genetically inherited diseases, such as cystic fibrosis, sickle cell anemia, hemophilia, thalassemia, Huntington’s disease, and Duchenne muscular dystrophy, reviewed in Reference 69.
One single dose of a genome-editing tool (Cas9 and gRNA targeting human hSERPINA1) delivered by an Ad vector has completely reverted the pathologic liver phenotype of the transgenic Pi*ZZ mouse model. This animal model develops liver disease because of the introduction of the ∼14 kb human Z-AAT transgene into the germline, which results in artificially high levels of the human mutant Z-AAT protein in the hepatocytes (70, 71). Disruption of hSERPINA1 transcription by the Ad-delivered CRISPR/Cas9 genome-editing tool reduced the circulating transaminases and mutant Z-AAT levels and improved liver health in Pi*ZZ mice (71). Although this study opened up the door for potential use of this gene-editing technique for the treatment of liver disease in individuals with AATD, achieving a protective level of serum M-AAT is essential to prevent damage of the lung in patients with AATD.
The same year that the liver disease of Pi*ZZ mice was corrected with CRISPR/Cas9, several other groups published viral vector–mediated CRISPR delivery for targeted in vivo genome editing of hAAT. Song and colleagues (72) coinjected two AAVs to deliver a Cas9 and sgRNA/HDR donor template, which led to correction of the common Z-AAT mutation at the hAAT locus region of the liver and partial restoration of the serum M-AAT in both neonatal and adult Pi*ZZ mice.
Shen and colleagues (73) managed to rescue the liver disease phenotype of the Pi*ZZ mice by NHEJ-induced disruption of hSERPINA1 transcription. In addition, using a dual-AAV vector approach, a small number of the hepatocytes could be edited from the Z to the M form of hAAT. CRISPR induced both indel (insertion and deletion) mutations by NHEJ and precise gene correction by HDR at the hSERPINA1 locus of the Pi*ZZ mouse liver, although the latter occurred with much lower frequency. Two percent of the total liver DNA could be precisely corrected to the normal M-AAT level in the study from Song and colleagues (72), and 4–5%, a slightly higher percentage, could be corrected to the normal level using the other dual-AAV vector system injected into Pi*ZZ mice, whereas indel rates were 15–22% and 8–20%, respectively (72, 73).
For such a strategy to be applicable to humans, HDR efficiency for precise gene editing must be increased and, in parallel, the NHEJ pathway–mediated indel frequencies must be reduced to prevent loss of function of the targeted gene, despite the consideration that indels might be capable of inactivating the Pi*ZZ allele, and thus protecting the liver (72). Accurate gene correction at a rate of 15–20% is suggested to be sufficient to reach the protective AAT level, which is still a 5- to 10-fold-greater correction compared with what the previous studies reported. However, the required correction might be lower, considering the fact that the therapeutic threshold was determined in asymptomatic Pi*SZ patients, and the S/Z forms of AAT protein are enzymatically less active than the normal M-AAT protein (73).
Alternatively, gene-editing approaches targeting a neutral locus for full cDNA knockin could be more clinically applicable with the potential to treat patients with Pi*Null mutations. Several new human safe-harbor loci (SHS231, 233) and several “old ones” (hROSA26, CCR5, AAVS1) have been recently well characterized as enabling safe translational human gene-editing applications (74).
Our group reported the first successful knockin of the full hAAT cDNA sequence at the safe-harbor mROSA26 loci of wild-type mice with CRISPR/Cas9-encoding Ad vector, and this was efficient enough to persist over 200 days (75) (see Figures 4A and 4B). The HDR-mediated knockin rate has been estimated at 13.7% of the total mROSA26 alleles from genomic liver DNA, and the NHEJ-mediated indel rate ranged between 0.5% and 13.5% (75). The high insertional rate was explained by the permissibility of mROSA26 for integration, the high transduction efficiency, the immature a1ge of the mice, and the adenoviral protein’s ability (E4orf6, E3 ubiquitin ligase) to inhibit the NHEJ pathway, thereby promoting HDR (75–77).
Figure 4.

Adenovirally mediated delivery of CRISPR/Cas9 (CRISPR-associated protein 9) for targeted in vivo knockin of hAAT cDNA. (A) Ad5–Cas9–guide RNA (gRNA) expresses both the Cas9 gene and a murine mROSA26–targeting gRNA sequence. Ad5–Ef1α–hAAT expresses the hAAT gene driven by Ef1α promoter and contains homology arms to the mROSA26 locus surrounding the expression cassette. Coinjection of the Ad5–Cas9–gRNA and Ad5–Ef1α–hAAT vectors in wild-type mice results in knockin of the hAAT gene at the mROSA26 locus of the hepatocytes. (B) Somatic integration of hAAT maintains stable long-term gene expression over 210 days. Nonintegrative mouse groups were injected with either PBS (black triangles, n = 6), 7.5 × 1010 VP of hAAT donor vector (Ad5–EF1α–hAAT) and 2.5 × 1010 VP of sham vector (Ad5–CMV–EGFP; red, n = 6) or 5 × 1010 VP of each (purple, n = 4). Integrative mouse groups were injected with 7.5 × 1010 VP of hAAT donor vector (Ad5–EF1α–hAAT) and 2.5 × 1010 VP of CRISPR-containing vector (Ad5–Cas9–gRNA; dark blue, n = 5), 5 × 1010 VP of each (green, n = 6), or 2.5 × 1010 VP of hAAT donor vector (Ad5–EF1α–hAAT) and 7.5 × 1010 VP of CRISPR-containing vector (solid blue). Plasma levels of hAAT were determined intermittently via ELISA. Error bars are SDs of the mean. (C) Schematic representation of “all-in-one” targeted Ad5 vector with fiber knob–incorporated MBP and hexon capsid modifications expressing the CRISPR/Cas9 genome editing tool and the hAAT donor template. A single injection of this novel pulmonary epithelium–targeted Ad vector, encoding both CRISPR/Cas9 and the hAAT donor template, for functional knockin of AAT locally within the lower respiratory tract may accomplish gene therapy for AAT lung disease. The illustrations were created with Biorender.com. *Significant differences with P < 0.05 between equivalent groups, whereas an absence of asterisk represents no significant difference. B is reprinted by permission from Reference 75. HA = homology arm.
To sum up, CRISPR/Cas9-mediated correction of hSERPINA1 point mutation or knockin of wild-type M-AAT in safe-harbor loci could shed a light on long-term treatment of patients with AATD lung disease. To address the toxic gain-of-function disease in the liver in individuals with the Pi*ZZ genotype, associated with abnormally folded Z-polymer accumulation and related inflammation, additional approaches are required to ablate the transcription of the mutant Z-AAT gene. Besides CRISPR/Cas9-mediated disruption of the hSERPINA1 transcription, other liver-directed therapies (gene-silencing molecules, protein-folding modifiers, polymerization blockers, etc.) are being developed to slow the progression of AATD liver disease. These approaches are beyond the scope of this review.
Adenoviral delivery of CRISPR/Cas9 may have several potential advantages over AAV-mediated delivery. The large packaging capacity could overcome one of the existing challenges with AAVs (64). Dual-AAV vector systems have been developed to deliver Cas9, gRNA, and the HDR template in separate constructs; however, targeting the same cell with two vectors adds more complexity and likely results in less efficiency than a single one (72, 73). Nevertheless, smaller versions of Cas9 that have approximately 70% of the size of SpCas9 (Streptococcus pyogenes Cas9; 4,104 bp), such as the ones derived from Staphylococcus aureus (SaCas9, 3,159 bp) or from Campylobacter jejuni (CjCas9, 2,952 bp), are available. SaCas9 and CjCas9 could fit into one AAV particle but would not provide enough space for the full coding sequence of AAT (1,255 bp), driven by the target T cell–specific promoter (78, 79).
Moreover, Ad-mediated delivery of CRISPR/Cas9 might pose less risk for random integration. Recently, high-integration frequencies of AAV sequences at the CRISPR cut sites of target genes have raised genotoxic concerns in vivo, which has to be considered when AAV vectors are used for genome editing (42). In contrast, recombination events between Ad vector DNA and the host chromosome are very low compared with the spontaneous mutation frequency calculated in the liver (80). In addition, the finding that Ad vector delivery of the donor template leads to “scarless” homology-directed genome editing, after genomic cleavage by TALENs or CRISPR-Cas9 nucleases, promotes the safe use of Ads as CRISPR-delivery vehicles (81).
Taken together, targetable genome editing as a therapeutic tool is moving closer to reality, with ongoing clinical trials assessing the safety and efficacy of Zinc Finger, TALEN, and CRISPR/Cas9 nucleases for several interventions. However, there are several challenges that need to be addressed before extending virally based gene-editing approaches to broad clinical applications. The major drawbacks that could confound the use of this system as an FDA-approved therapeutic agent are the off-target cleavages, Cas9’s evocation of the immune response in vivo, and genotoxic effects, all of which require more detailed understanding. Nevertheless, the evolution of gene delivery and gene-editing technologies has revolutionized the way physicians and scientists can approach treating even the most debilitating diseases, including AATD.
Conclusions
Currently, most gene-editing and gene-augmentation approaches to treat AATD are focused on reaching a circulating serum level of 572 μg/ml. This serum concentration, defined by the FDA as a protective threshold, is capable of counterbalancing the protease/antiprotease imbalance in patients with AATD. However, a physiologically more relevant endpoint such as the AAT level in the lower respiratory tract (at the site of the action) might be more relevant for the advancement of new therapeutics for AATD lung disease. Maximization of local protein production in the pulmonary endothelium may be equally or more beneficial for achieving this mandate. In parallel with this goal, there has been a growing interest in alternative methods for circumventing vector-associated liver toxicities linked to systemic virus administration. The lung endothelium–targeted Ad approach that we propose has the potential to provide a liver-sparing strategy aside from the local enhancement of AAT production. To this end, nonhuman Ad vectors such as GAd could traverse the barrier associated with the humoral and cellular immune responses against human serotype Ad vectors. Incorporation of CRISPR/Cas9 genome-editing tools in GAd or lung EC–targeted Ad vector might embody an excellent AAT gene-therapy platform that would circumvent preformed immunity, achieve protective local AAT production, mitigate liver toxicity, and provide long-term gene correction (Figure 4C).
New studies using the novel mSERPINA1a-e–knockout animal model may allow determination of alternative endpoints of human clinical trials addressing AATD lung disease (e.g., the AAT level in the alveolar ELF instead of in the serum). Only recently has the AATD field gained an animal model that recapitulates the loss of function in AATD. Borel and colleagues (82) used CRISPR/Cas9-mediated genome editing to delete all five copies of the complex mSERPINA gene. This null mouse has undetectable levels of circulating mouse AAT and, as a consequence, has reduced antineutrophil elastase capacity that results in spontaneous development of emphysema. Lung mechanics and morphometry measurements observed in the mSERPINA1a-e–knockout mice are very comparable with that of patients with emphysema. This model is highly relevant for the ongoing preclinical development of gene-editing therapeutics for AATD lung disease. Moreover, it provides an ideal genetic background for generation of animal models carrying the pathogenic variants that are needed to investigate the novel therapeutic approaches addressing both lung and liver symptoms in patients with AATD.
Taken together, the new technologies including capsid engineering, NHP gene-transfer vectors, and genome editing and emerging animal models provide the basis to transition the AAT gene-therapy strategy to a viable technology that has clinical potential.
Acknowledgments
Acknowledgment
The authors thank Amanda Baker for help in preparing the Ad illustration.
Footnotes
Supported by U.S. National Institutes of Health grants UG3 TR002851 and R01 EB026468 (D.T.C.).
Author Contributions: R.L. reviewed the literature and drafted the review paper. R.L. and D.T.C. wrote the different sections. D.T.C. critically reviewed and edited the paper.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0159PS on July, 15, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, Lomas DA, et al. α1-Antitrypsin deficiency. Nat Rev Dis Primers. 2016;2:16051. doi: 10.1038/nrdp.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.de Serres FJ, Blanco I. Prevalence of α1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6:277–295. doi: 10.1177/1753465812457113. [DOI] [PubMed] [Google Scholar]
- 3.Blanco I, Bueno P, Diego I, Pérez-Holanda S, Casas-Maldonado F, Esquinas C, et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561–569. doi: 10.2147/COPD.S125389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouhadi Z, Odou MF, Zerimech F, Bousfiha AA, Mikou N, Porchet N, et al. Alpha1 antitrypsin deficiency due to an homozygous PI*Null Q0Cairo mutation: early onset of pulmonary manifestations and variability of clinical expression. Respir Med Case Rep. 2018;24:58–62. doi: 10.1016/j.rmcr.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres-Durán M, Lopez-Campos JL, Barrecheguren M, Miravitlles M, Martinez-Delgado B, Castillo S, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis. 2018;13:114. doi: 10.1186/s13023-018-0856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiles KM, Sondhi D, Kaminsky SM, De BP, Rosenberg JB, Crystal RG. Intrapleural gene therapy for alpha-1 antitrypsin deficiency-related lung disease. Chronic Obstr Pulm Dis (Miami) 2018;5:244–257. doi: 10.15326/jcopdf.5.4.2017.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CE, Vayalapra S, Hampson JA, Mukherjee D, Stockley RA, Turner AM. PiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax. 2015;70:939–945. doi: 10.1136/thoraxjnl-2015-206906. [DOI] [PubMed] [Google Scholar]
- 8.Goss JA, Stribling R, Martin P. Adult liver transplantation for metabolic liver disease. Clin Liver Dis. 1998;2:187–210. doi: 10.1016/s1089-3261(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 9.Brode SK, Ling SC, Chapman KR. Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease. CMAJ. 2012;184:1365–1371. doi: 10.1503/cmaj.111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204:345–351. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 11.Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli AR, Rouhani F, Li N, Schreck P, Brantly ML. Alpha-1-antitrypsin augmentation therapy in deficient individuals enrolled in the Alpha-1 Foundation DNA and tissue bank. Int J Chron Obstruct Pulmon Dis. 2009;4:443–452. doi: 10.2147/copd.s8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 14.Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in alpha1-antitrypsin deficiency. Thorax. 2003;58:1020–1026. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung H-S, Kim J-S, Lee SM, Park SJ. Additional N-glycosylation in the N-terminal region of recombinant human alpha-1 antitrypsin enhances the circulatory half-life in Sprague-Dawley rats. Glycoconj J. 2016;33:201–208. doi: 10.1007/s10719-016-9657-3. [DOI] [PubMed] [Google Scholar]
- 16.Casolaro MA, Fells G, Wewers M, Pierce JE, Ogushi F, Hubbard R, et al. Augmentation of lung antineutrophil elastase capacity with recombinant human alpha-1-antitrypsin. J Appl Physiol (1985) 1987;63:2015–2023. doi: 10.1152/jappl.1987.63.5.2015. [DOI] [PubMed] [Google Scholar]
- 17.Guo S, Booten SL, Aghajan M, Hung G, Zhao C, Blomenkamp K, et al. Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice. J Clin Invest. 2014;124:251–261. doi: 10.1172/JCI67968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee A, Hidvegi T, Araya P, Ewing M, Stolz DB, Perlmutter DH. NFκB mitigates the pathological effects of misfolded α1-antitrypsin by activating autophagy and an integrated program of proteostasis mechanisms. Cell Death Differ. 2019;26:455–469. doi: 10.1038/s41418-018-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parfrey H, Dafforn TR, Belorgey D, Lomas DA, Mahadeva R. Inhibiting polymerization: new therapeutic strategies for Z alpha1-antitrypsin-related emphysema. Am J Respir Cell Mol Biol. 2004;31:133–139. doi: 10.1165/rcmb.2003-0276OC. [DOI] [PubMed] [Google Scholar]
- 21.Morral N, Parks RJ, Zhou H, Langston C, Schiedner G, Quinones J, et al. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 22.Gadek JE, Fells GA, Zimmerman RL, Rennard SI, Crystal RG. Antielastases of the human alveolar structures: implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981;68:889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfeld M, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 24.Chiuchiolo MJ, Crystal RG. Gene therapy for alpha-1 antitrypsin deficiency lung disease. Ann Am Thorac Soc. 2016;13:S352–S369. doi: 10.1513/AnnalsATS.201506-344KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura T, Ferran B, Tsukahara Y, Shang Q, Desai S, Fedoce A, et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci Rep. 2019;9:13601. doi: 10.1038/s41598-019-49624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11:442–447. [PMC free article] [PubMed] [Google Scholar]
- 27.Sondhi D, Stiles KM, De BP, Crystal RG. Genetic modification of the lung directed toward treatment of human disease. Hum Gene Ther. 2017;28:3–84. doi: 10.1089/hum.2016.152. [DOI] [PubMed] [Google Scholar]
- 28.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller C, Gernoux G, Gruntman AM, Borel F, Reeves EP, Calcedo R, et al. 5 year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol Ther. 2017;25:1387–1394. doi: 10.1016/j.ymthe.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gernoux G, Gruntman AM, Blackwood M, Zieger M, Flotte TR, Mueller C. Muscle-directed delivery of an AAV1 vector leads to capsid-specific T cell exhaustion in nonhuman primates and humans. Mol Ther. 2020;28:747–757. doi: 10.1016/j.ymthe.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Chiuchiolo MJ, Kaminsky SM, Sondhi D, Hackett NR, Rosenberg JB, Frenk EZ, et al. Intrapleural administration of an AAVrh.10 vector coding for human α1-antitrypsin for the treatment of α1-antitrypsin deficiency. Hum Gene Ther Clin Dev. 2013;24:161–173. doi: 10.1089/humc.2013.168. [DOI] [PubMed] [Google Scholar]
- 34.Conlon TJ, Cossette T, Erger K, Choi YK, Clarke T, Scott-Jorgensen M, et al. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped alpha1-antitrypsin vector. Mol Ther. 2005;12:867–875. doi: 10.1016/j.ymthe.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Mueller C, Tang Q, Gruntman A, Blomenkamp K, Teckman J, Song L, et al. Sustained miRNA–mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol Ther. 2012;20:590–600. doi: 10.1038/mt.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song S, Embury J, Laipis PJ, Berns KI, Crawford JM, Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Zhang B, Lu Y, Jorgensen M, Petersen B, Song S. Adipose tissue-derived mesenchymal stem cell-based liver gene delivery. J Hepatol. 2011;54:930–938. doi: 10.1016/j.jhep.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doshi BS, Arruda VR. Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol. 2018;9:273–293. doi: 10.1177/2040620718791933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi A, Salvetti A. [Integration of AAV vectors and insertional mutagenesis] [in French] Med Sci (Paris) 2016;32:167–174. doi: 10.1051/medsci/20163202010. [DOI] [PubMed] [Google Scholar]
- 40.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 41.Gil-Farina I, Fronza R, Kaeppel C, Lopez-Franco E, Ferreira V, D’Avola D, et al. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol Ther. 2016;24:1100–1105. doi: 10.1038/mt.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanlon KS, Kleinstiver BP, Garcia SP, Zaborowski MP, Volak A, Spirig SE, et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen GN, Everett JK, Raymond H, Kafle S, Merricks EP, Kazazian HH, et al. Long-term AAV-mediated factor VIII expression in nine hemophilia A dogs: a 10 year follow-up analysis on durability, safety and vector integration. Blood. 2019;134:611. [Google Scholar]
- 44.Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. 2014;25:3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beatty MS, Curiel DT. Chapter two: adenovirus strategies for tissue-specific targeting. Adv Cancer Res. 2012;115:39–67. doi: 10.1016/B978-0-12-398342-8.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfrum N, Greber UF. Adenovirus signalling in entry. Cell Microbiol. 2013;15:53–62. doi: 10.1111/cmi.12053. [DOI] [PubMed] [Google Scholar]
- 47.Rosengart TK, Bishawi MM, Halbreiner MS, Fakhoury M, Finnin E, Hollmann C, et al. Long-term follow-up assessment of a phase 1 trial of angiogenic gene therapy using direct intramyocardial administration of an adenoviral vector expressing the VEGF121 cDNA for the treatment of diffuse coronary artery disease. Hum Gene Ther. 2013;24:203–208. doi: 10.1089/hum.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Short JJ, Curiel DT. Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther. 2009;8:2096–2102. doi: 10.1158/1535-7163.MCT-09-0367. [DOI] [PubMed] [Google Scholar]
- 49.Fonseca JA, McCaffery JN, Kashentseva E, Singh B, Dmitriev IP, Curiel DT, et al. A prime-boost immunization regimen based on a simian adenovirus 36 vectored multi-stage malaria vaccine induces protective immunity in mice. Vaccine. 2017;35:3239–3248. doi: 10.1016/j.vaccine.2017.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews QL. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol Pharm. 2011;8:3–11. doi: 10.1021/mp100214b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayton EJ, Rose A, Ibrahimsa U, Del Sorbo M, Capone S, Crook A, et al. Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One. 2014;9:e101591. doi: 10.1371/journal.pone.0101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolodka TM, Finegold M, Kay MA, Woo SL. Hepatic gene therapy: efficient retroviral-mediated gene transfer into rat hepatocytes in vivo. Somat Cell Mol Genet. 1993;19:491–497. doi: 10.1007/BF01233254. [DOI] [PubMed] [Google Scholar]
- 53.Saylors RL, III, Wall DA. Expression of human alpha 1 antitrypsin in murine hematopoietic cells in vivo after retrovirus-mediated gene transfer. Mol Genet Metab. 1998;63:198–204. doi: 10.1006/mgme.1997.2665. [DOI] [PubMed] [Google Scholar]
- 54.Jaffe HA, Danel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld MA, et al. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 55.Kay MA, Graham F, Leland F, Woo SL. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 56.Pipe S, Leebeek FWG, Ferreira V, Sawyer EK, Pasi J. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev. 2019;15:170–178. doi: 10.1016/j.omtm.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu ZH, Kaliberov S, Zhang J, Muz B, Azab AK, Sohn RE, et al. The myeloid-binding peptide adenoviral vector enables multi-organ vascular endothelial gene targeting. Lab Invest. 2014;94:881–892. doi: 10.1038/labinvest.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alberti MO, Roth JC, Ismail M, Tsuruta Y, Abraham E, Pereboeva L, et al. Derivation of a myeloid cell-binding adenovirus for gene therapy of inflammation. PLoS One. 2012;7:e37812. doi: 10.1371/journal.pone.0037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buggio M, Towe C, Annan A, Kaliberov S, Lu ZH, Stephens C, et al. Pulmonary vasculature directed adenovirus increases epithelial lining fluid alpha-1 antitrypsin levels. J Gene Med. 2016;18:38–44. doi: 10.1002/jgm.2874. [DOI] [PubMed] [Google Scholar]
- 60.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, Waddington SN, et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood. 2010;116:2656–2664. doi: 10.1182/blood-2009-12-260026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Short JJ, Rivera AA, Wu H, Walter MR, Yamamoto M, Mathis JM, et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther. 2010;9:2536–2544. doi: 10.1158/1535-7163.MCT-10-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kochanek S, Schiedner G, Volpers C. High-capacity ‘gutless’ adenoviral vectors. Curr Opin Mol Ther. 2001;3:454–463. [PubMed] [Google Scholar]
- 65.Limbach K, Stefaniak M, Chen P, Patterson NB, Liao G, Weng S, et al. New gorilla adenovirus vaccine vectors induce potent immune responses and protection in a mouse malaria model. Malar J. 2017;16:263. doi: 10.1186/s12936-017-1911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu ZH, Dmitriev IP, Brough DE, Kashentseva EA, Li J, Curiel DT. A new gorilla adenoviral vector with natural lung tropism avoids liver toxicity and is amenable to capsid engineering and vector retargeting. J Virol. 2020;94:e00265-20. doi: 10.1128/JVI.00265-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehrhardt A, Haase R, Schepers A, Deutsch MJ, Lipps HJ, Baiker A. Episomal vectors for gene therapy. Curr Gene Ther. 2008;8:147–161. doi: 10.2174/156652308784746440. [DOI] [PubMed] [Google Scholar]
- 68.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotagama OW, Jayasinghe CD, Abeysinghe T. Era of genomic medicine: a narrative review on CRISPR technology as a potential therapeutic tool for human diseases. Biomed Res Int. 2019;2019:1369682. doi: 10.1155/2019/1369682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlson JA, Rogers BB, Sifers RN, Finegold MJ, Clift SM, DeMayo FJ, et al. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjursell M, Porritt MJ, Ericson E, Taheri-Ghahfarokhi A, Clausen M, Magnusson L, et al. Therapeutic genome editing with CRISPR/Cas9 in a humanized mouse model ameliorates α1-antitrypsin deficiency phenotype. EBioMedicine. 2018;29:104–111. doi: 10.1016/j.ebiom.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song CQ, Wang D, Jiang T, O’Connor K, Tang Q, Cai L, et al. In Vivo genome editing partially restores alpha1-antitrypsin in a murine model of AAT deficiency. Hum Gene Ther. 2018;29:853–860. doi: 10.1089/hum.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen S, Sanchez ME, Blomenkamp K, Corcoran EM, Marco E, Yudkoff CJ, et al. Amelioration of alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum Gene Ther. 2018;29:861–873. doi: 10.1089/hum.2017.227. [DOI] [PubMed] [Google Scholar]
- 74.Pellenz S, Phelps M, Tang W, Hovde BT, Sinit RB, Fu W, et al. New human chromosomal sites with “safe harbor” potential for targeted transgene insertion. Hum Gene Ther. 2019;30:814–828. doi: 10.1089/hum.2018.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephens CJ, Kashentseva E, Everett W, Kaliberova L, Curiel DT. Targeted in vivo knock-in of human alpha-1-antitrypsin cDNA using adenoviral delivery of CRISPR/Cas9. Gene Ther. 2018;25:139–156. doi: 10.1038/s41434-018-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- 77.Gwiazda KS, Grier AE, Sahni J, Burleigh SM, Martin U, Yang JG, et al. High efficiency CRISPR/Cas9-mediated gene editing in primary human T-cells using mutant adenoviral E4orf6/E1b55k “helper” proteins. Mol Ther. 2016;24:1570–1580. doi: 10.1038/mt.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E, Koo T, Park SW, Kim D, Kim K, Cho HY, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun. 2017;8:14500. doi: 10.1038/ncomms14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu CL, Ruan MZC, Mahajan VB, Tsang SH. Viral delivery systems for CRISPR. Viruses. 2019;11:28. doi: 10.3390/v11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, et al. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holkers M, Maggio I, Henriques SF, Janssen JM, Cathomen T, Gonçalves MA. Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat Methods. 2014;11:1051–1057. doi: 10.1038/nmeth.3075. [DOI] [PubMed] [Google Scholar]
- 82.Borel F, Sun H, Zieger M, Cox A, Cardozo B, Li W, et al. Editing out five Serpina1 paralogs to create a mouse model of genetic emphysema. Proc Natl Acad Sci USA. 2018;115:2788–2793. doi: 10.1073/pnas.1713689115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest. 2002;110:1603–1605. doi: 10.1172/JCI17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gooptu B, Ekeowa UI, Lomas DA. Mechanisms of emphysema in alpha1-antitrypsin deficiency: molecular and cellular insights. Eur Respir J. 2009;34:475–488. doi: 10.1183/09031936.00096508. [DOI] [PubMed] [Google Scholar]
- 85.Strnad P, McElvaney NG, Lomas DA. Alpha 1-antitrypsin deficiency. N Engl J Med. 2020;382:1443–1455. doi: 10.1056/NEJMra1910234. [DOI] [PubMed] [Google Scholar]
- 86.Alberti MO, Deshane JS, Chaplin DD, Pereboeva L, Curiel DT, Roth JC. A myeloid cell-binding adenovirus efficiently targets gene transfer to the lung and escapes liver tropism. Gene Ther. 2013;20:733–741. doi: 10.1038/gt.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]