Asthma is a highly prevalent chronic lung disease characterized by airway hyperresponsiveness (AHR), with excessive narrowing of the airways influenced by both inflammation and airway remodeling extending to the distal lung. Alternative therapeutic approaches to reduce AHR are required for severe uncontrolled asthma when the efficacy of current treatments is limited. There is increasing interest in the contribution of altered sphingolipid metabolism to multiple aspects of asthma pathophysiology (1–3), but whether this could be manipulated to reduce symptoms and improve outcomes for difficult-to-treat patients remains unclear.
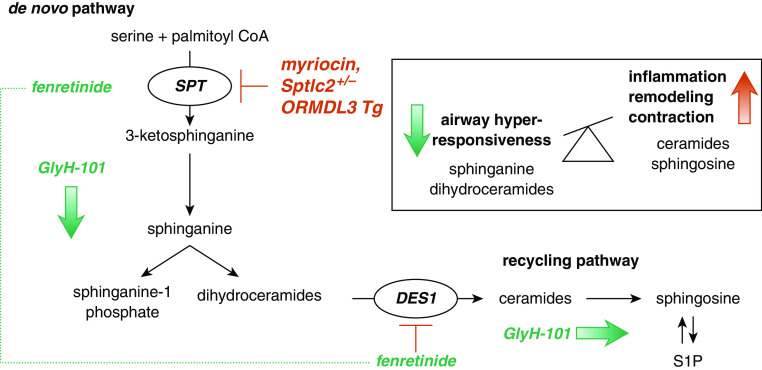
Sphingolipids were first identified in brain tissue in the 19th century by Johann Ludwig Wilhelm Thudichum and named after the mythological sphinx based on their enigmatic nature. Multiple bioactive lipids in this class are synthesized de novo, sharing a common chemical backbone of sphingosine or one of its derivatives. The rate-limiting first step is catalyzed by SPT (serine palmitoyl-CoA transferase) to produce sphinganine, with subsequent phosphorylation or acetylation steps generating sphinganine-1-phosphate or dihydroceramides, respectively. Further downstream, DES1 (dihydroceramide desaturase) can catalyze the formation of ceramides that may enter the recycling pathway to produce additional metabolites, including sphingosine and sphingosine 1-phosphate (S1P) (Figure 1).
Figure 1.
Modulation of de novo and recycling pathways of sphingolipid metabolism may reduce airway hyperresponsiveness. Condensation of serine and palmitoyl CoA by SPT (serine palmitoyl-CoA transferase) is the rate-limiting step in the production of 3-ketosphinganine, which is immediately reduced to sphinganine. This can be phosphorylated to sphinganine-1 phosphate or acylated to form dihydroceramides. These metabolites are converted to ceramides by DES1 (dihydroceramide desaturase 1) and subsequently recycled as sphingosine and sphingosine-1 phosphate. De novo synthesis can be inhibited by myriocin or manipulated in mice by deletion of SPT in Sptlc2+/− mice or overexpression of the SPT inhibitor ORMDL3 (orosomucoid-like protein isoform 3) in transgenic mice. Fenretinide inhibits DES1, resulting in the decreased synthesis of ceramides and compensatory SPT activation to increase selected sphingolipids upstream of DES1. The CFTR (cystic fibrosis transmembrane conductance regulator) chloride channel inhibitor GlyH-101 (glycinyl hydrazone-101) broadly increases sphingolipid production via both de novo and recycling pathways. Altering the balance of sphingolipid synthesis to increase sphinganine and dihydroceramides may reduce airway hyperresponsiveness and oppose airway inflammation, remodeling, and contraction. S1P = sphingosine-1 phosphate; Tg = transgenic.
In the context of asthma, specific sphingolipids may play protective or deleterious roles in regulating AHR. In healthy airways, sphingolipids contribute to the integrity of cell membranes and mediate multiple cellular processes and intracellular signaling. However, when the airways are inflamed in asthma, the enzymatic processes involved in sphingolipid synthesis and interconversion may become dysregulated, disturbing their homeostatic balance and potentially contributing to AHR (1, 2).
Reduced sphingolipid synthesis has been implicated in increased asthma susceptibility. ORMDL3 (Orosomucoid-like protein isoform 3) is an endogenous endoplasmic reticulum–localized protein that inhibits SPT. Genome-wide association studies have identified polymorphisms in the ORMLD3 gene, located in chromosome 17q21, associated with increased risk of asthma both in children and adults (4). Overexpression of ORMDL3 in mice to mimic this risk factor reduced sphingolipids and increased peribronchial smooth muscle and fibrosis, resulting in AHR in the absence of inflammation (5).
In contrast, increased levels of some sphingolipids, particularly ceramides and S1P, may contribute to the pathophysiology of asthma. S1P is elevated in the airways of patients with asthma after segmental allergen challenge (6). In a house dust mite model of allergic airways disease, AHR was associated with increased lung ceramides (7). In support of these correlative associations, both ceramide and S1P have numerous proasthmatic functions, including direct contraction of airway smooth muscle (ASM) (6, 8).
These potentially conflicting findings lead to the riddle of the sphingolipids and AHR—should the therapeutic goal be to increase levels to restore the homeostatic balance of metabolites that are lacking in asthma or to decrease the synthesis of metabolites that are in apparent excess? In a study reported in this issue of the Journal, Heras and colleagues (pp. 690–698) address the first option, describing well-designed studies using two novel compounds to promote the synthesis of SPT-dependent sphingolipids and assessing the consequences for airway contraction (9).
The authors tested fenretinide, an inhibitor of DES1 that reduces ceramide synthesis but also indirectly activates de novo synthesis of other sphingolipids (via positive feedback on SPT activity) (10), and GlyH-101, a CFTR (cystic fibrosis transmembrane conductance regulator) chloride channel blocker that increases levels of multiple sphingolipids by a mechanism that is yet to be fully defined (11) (Figure 1). Both fenretinide and GlyH-101 stimulated endogenous sphinganine and dihydroceramide synthesis by human ASM and epithelial cells. Prolonged treatment of ASM with either drug also reduced bradykinin-induced calcium signaling, whereas the selective SPT inhibitor myriocin had the predicted opposite effect, suggesting a protective role for these particular SPT-dependent sphingolipids to oppose airway contraction.
Heterozygous SPT-deficient mice were then used to test the authors’ hypothesis that increasing de novo sphingolipid synthesis would limit AHR. Like the ORMLD3 transgenic mice, Sptlc2+/− mice lack an inflammatory phenotype but are hyperresponsive to methacholine both in vivo and in vitro, even in the absence of allergen challenge (9, 12). Heras and colleagues showed that in vitro treatment of tracheal rings or precision cut lung slices with either fenretinide or GlyH-101 over several hours increased tissue sphinganine and dihydroceramides. GlyH-101, but not fenretinide, also increased ceramides, sphingmyelins, sphingosine, and S1P, with some of these additional metabolites generated via the SPT-independent recycling pathway. Methacholine-induced contraction was reduced by treatment of proximal and distal airways from Sptlc2+/− mice treated with fenritinide or GlyH-101, implicating the increase in SPT-dependent sphingolipids by both drugs in this protective effect. Though the therapeutic potential of fenretinide was not assessed following in vivo treatment, GlyH-101 was shown to retain its in vitro efficacy in reducing the inherent AHR in Sptlc2+/− mice (9).
Both fenretinide and GlyH-101 appear to be promising candidates to oppose AHR by increasing SPT-dependent sphinganine and dihydroceramides. Further work is needed to progress the positive findings with these novel drugs toward clinical translation. Of note, the potential proasthmatic consequences of GlyH-101–mediated increases in ceramides and S1P should be assessed. Because S1P also induces β2-adrenoceptor desensitization (13), the impact of drug treatment on dilator responses in Sptlc2+/− mice should also be evaluated. Testing the direct effects of individual sphingolipid metabolites on airway reactivity will also be an important step in defining the mechanisms of action underlying the relative benefits of fenretinide and GlyH-101.
These studies were performed in mice with SPT deficiency as the sole driver of AHR. The positive findings are therefore of particular relevance to the limited subset of patients with asthma with reduced activity of SPT, as may occur with ORMLD3 polymorphisms. Fenretinide also inhibited AHR in an ovalbumin challenge model of asthma, but this was attributed to antiinflammatory actions rather than regulation of sphingolipid synthesis (14). Comparisons with GlyH-101, including detailed analysis of the sphingolipid profiles with each treatment, are still to be performed to support broader clinical translation for asthma.
It may also be informative to extend allergen challenge models to include Sptlc2+/− mice to determine whether inflammation, remodeling, and AHR are further exacerbated by SPT deficiency. The therapeutic consequences of the stimulatory effects of GlyH-101 on a wider range of sphingolipid metabolites could be defined. As previously described, S1P levels are elevated in allergic asthma, so further increases with GlyH-101 treatment may be problematic, as they could increase allergen-induced cytokine production and ASM hyperplasia or directly cause contraction (6).
A recent report has characterized serum sphingolipid profiles from subjects with uncontrolled asthma and correlated these metabolic signatures with their inflammatory phenotypes (15). Notably, the balance of sphingolipid metabolites favored S1P over ceramides in eosinophilic asthma, but the balance was reversed in neutrophilic asthma. This suggests that relative S1P and ceramide levels could be used as biomarkers to inform selectively targeted treatment. The studies by Heras and colleagues using fenretinide and GlyH-101 in human lung cells and Sptlc2+/− mice implicate a protective effect of increases in sphinganine and dihydroceramides to limit excessive airway contraction (9). Nevertheless, the complex riddle of how best to manipulate sphingolipid levels in asthma is yet to be answered. Emerging evidence predicts that using drugs to restore the overall sphingolipid balance—increasing the levels of those in deficit as well as reducing any deleterious effects of those in excess—may offer the greatest potential to oppose AHR in asthma.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2020-0324ED on August 21, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Clarke DL, Dakshinamurti S, Larsson AK, Ward JE, Yamasaki A. Lipid metabolites as regulators of airway smooth muscle function. Pulm Pharmacol Ther. 2009;22:426–435. doi: 10.1016/j.pupt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 2. Sturgill JL. Sphingolipids and their enigmatic role in asthma. Adv Biol Regul. 2018;70:74–81. doi: 10.1016/j.jbior.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James B, Milstien S, Spiegel S. ORMDL3 and allergic asthma: from physiology to pathology. J Allergy Clin Immunol. 2019;144:634–640. doi: 10.1016/j.jaci.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 5. Miller M, Rosenthal P, Beppu A, Mueller JL, Hoffman HM, Tam AB, et al. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014;192:3475–3487. doi: 10.4049/jimmunol.1303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 7. Oyeniran C, Sturgill JL, Hait NC, Huang WC, Avni D, Maceyka M, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J Allergy Clin Immunol. 2015;136:1035–1046.e6. doi: 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 9. Heras AF, Veerappan A, Silver RB, Emala CW, Worgall TS, Perez-Zoghbi J, et al. Increasing sphingolipid synthesis alleviates airway hyperreactivity. Am J Respir Cell Mol Biol. 2020;63 doi: 10.1165/rcmb.2020-0194OC. 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poliakov E, Samuel W, Duncan T, Gutierrez DB, Mata NL, Redmond TM. Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Δ4-desaturase 1. PLoS One. 2017;12:e0176487. doi: 10.1371/journal.pone.0176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, et al. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 12. Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, et al. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med. 2013;5:186ra67. doi: 10.1126/scitranslmed.3005765. [DOI] [PubMed] [Google Scholar]
- 13. Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ. Effect of sphingosine 1-phosphate on cyclo-oxygenase-2 expression, prostaglandin e2 secretion, and β2-adrenergic receptor desensitization. Am J Respir Cell Mol Biol. 2016;54:128–135. doi: 10.1165/rcmb.2014-0443OC. [DOI] [PubMed] [Google Scholar]
- 14. Kanagaratham C, Kalivodová A, Najdekr L, Friedecký D, Adam T, Hajduch M, et al. Fenretinide prevents inflammation and airway hyperresponsiveness in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2014;51:783–792. doi: 10.1165/rcmb.2014-0121OC. [DOI] [PubMed] [Google Scholar]
- 15. Kim SH, Jung HW, Kim M, Moon JY, Ban GY, Kim SJ, et al. Ceramide/sphingosine-1-phosphate imbalance is associated with distinct inflammatory phenotypes of uncontrolled asthma. Allergy. 2020;75:1987–2000. doi: 10.1111/all.14236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.