Abstract
Previously, we showed that global knockout (KO) of the circadian clock transcription factor PER1 in male, but not female, mice fed a high salt diet plus mineralocorticoid treatment (HS/DOCP) resulted in non-dipping hypertension and decreased night/day ratio of sodium (Na) excretion. Additionally, we have shown that the endothelin-1 (ET-1) gene is targeted by both PER1 and aldosterone. We hypothesized that ET-1 would exhibit a sex-specific response to HS/DOCP treatment in PER1 KO. Here we show that male, but not female, global PER1 KO mice exhibit a decreased night/day ratio of urinary ET-1. Gene expression analysis revealed significant genotype differences in ET-1 and endothelin A receptor (ETA) expression in male, but not female, mice in response to HS/DOCP. Additionally, both WT and global PER1 KO male mice significantly increase endothelin B receptor (ETB) expression in response to HS/DOCP, but female mice do not. Finally, siRNA-mediated knockdown of PER1 in mouse cortical collecting duct cells (mpkCCDc14) resulted in increased ET-1 mRNA expression and peptide secretion in response to aldosterone treatment. These data suggest that PER1 is a negative regulator of ET-1 expression in response to HS/DOCP, revealing a novel mechanism for the regulation of renal Na handling in response to HS/DOCP treatment.
Keywords: kidney, aldosterone, endothelin, non-dipping hypertension, renal sodium handling
Introduction
Circadian clocks are physiological timekeepers that are built into nearly every living organism. These internal biological clocks regulate expression of thousands of genes in a tissue-specific manner, allowing for adaptation to a 24-hour cycle. At the molecular core of the circadian clock are a set of transcription factors. The transcription factors, BMAL1 and CLOCK, heterodimerize and bind to E-box response elements located in target gene promoters to promote expression of the genes. BMAL1 and CLOCK induce expression of the circadian clock proteins Period (PER) and Cryptochrome (CRY). PER and CRY heterodimerize and work in a negative feedback loop to inhibit the actions of BMAL1 and CLOCK, and in turn, decrease their own transcription. The cycle between BMAL1/CLOCK and PER/CRY actions resets approximately every 24hrs. This interplay of transcription activation and repression by the molecular clock creates fluctuations in physiological functions that can be observed at the whole organism level. Both blood pressure and urinary sodium (Na) excretion are physiological outputs that exhibit circadian rhythms.
Our interest in the clock mechanism began with the finding that the blood pressure regulating hormone, aldosterone, acutely induces expression of the PER homolog, PER1, in cultured renal collecting duct cells (Gumz et al., 2003). In that same study, we identified endothelin-1 (ET-1) as an early aldosterone target gene. ET-1 is a peptide hormone that has a variety of tissue- and cell type-specific functions. ET-1 is the most potent vasoconstrictor known, but in the distal tubule and collecting duct of the kidney, it functions in the regulation of sodium (Na) handling through its actions on the endothelin A (ETA) and B (ETB) receptors (Bugaj et al., 2012; Lynch et al., 2013; Speed et al., 2015). At the transcriptional level, ET-1 is regulated by microRNAs, epigenetic mechanisms, and mineralocorticoid action (Jacobs et al.,2013; Stow et al., 2009; Stow et al., 2011; Welch et al., 2013). Subsequently, we found that PER1 regulates ET-1 expression at the level of transcription in vitro and in vivo, and this regulation extends to the endothelin receptors in a tissue- and time-specific manner in 129/sv mice (Richards et al., 2014; Stow et al., 2012).
More recently, we have shown that global KO of PER1 in male C57BL/6J mice results in blunted blood pressure rhythms following high salt (HS) diet and treatment with the long-acting mineralocorticoid desoxycorticosterone pivalate (DOCP) (Solocinski et al., 2017). This non-dipping hypertension phenotype is associated with altered renal Na handling rhythms in the global PER1 KO male mice (Douma et al., 2018). Importantly, the non-dipping hypertension is absent in female global PER1 KO mice on the same HS/DOCP treatment (Douma et al., 2019). These data suggest that PER1 has sex-dependent effects on the expression of genes that regulate blood pressure rhythms.
Given our previous findings that the ET-1 gene is targeted by both PER1 and aldosterone, the goal of the present study was to measure changes in expression of ET-1 in response to an acute HS/DOCP treatment in male and female global PER1 KO mice and littermate controls on a C57Bl/6J background. We show that the changes in renal Na handling previously reported in male PER1 KO mice (Douma et al., 2018) are paralleled by changes in urinary ET-1 levels in the same male mice. Furthermore, these effects are associated with increased levels of aldosterone. Female global PER1 KO mice maintain their rhythms of Na handling and urinary ET-1 after the HS/DOCP treatment. On a molecular and cellular level, our additional in vitro data suggest that PER1 regulates ET-1 expression in response to aldosterone at the gene expression and peptide levels in renal cortical collecting duct cells.
Methods
Mice:
All experiments involving animals were approved by the University of Florida and the North Florida/South Georgia Veterans Administration Institutional Animal Care and Use Committees (IACUC) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Metabolic cage HS/DOCP studies:
Male and female C57BL/6J global PER1 KO and WT mice of ages 16–20 weeks were treated as described in (Solocinski et al., 2017). Male urine samples for the present study are from the same mice in our previously published paper (Douma et al., 2018) in order to directly correlate any changes in ET-1 production with the sodium handling phenotype observed in the male mice. Additionally, since we already had the samples required for this study, we wanted to observe the Three Rs outlined in the Principles of Humane Experimental Technique (Hubrecht & Carter, 2019).
Age-matched female mice ages 16–20 weeks underwent the same acute treatment regime that the males received in the 2018 study. The estrus cycle was not monitored in female mice for the present study. After acclimation to metabolic cages for 3 days, mice were given control diet (Envigo Teklad custom diet TD.99131) for 3 days baseline urine collections were made on the 3rd day. 12-hr urine collections were made throughout the experiment with the female mice. They were then given 4% NaCl (Envigo Teklad custom diet TD.170601) gel diet for a total of 6 days with ad libitum access. On the 3rd day of the high salt diet, right before the beginning of the mouse active period (6pm) the mice received intramuscular (I.M.) injection with 70 μg/g body weight (BW) DOCP (Novartis) as previously described (Douma et al., 2019; Solocinski et al., 2017). HS/DOCP urine collections were taken for 3 days. On the 4th day of the HS/DOCP treatment, tissues were harvested at noon.
BQ788 studies:
Male WT and global PER1 KO mice were acclimated to metabolic cages and to receiving I.P. injections. Male mice were given the same HS/DOCP regime as described above. On the 4th day of the HS/DOCP treatment, BQ788 (Sigma) was administered through an I.P. injection at 1.5mg/kg BW dosage at 6pm, the time of lights off and the beginning of the mouse active period. Urine was collected 3hrs later at 9pm. additionally, urine from 6pm-6pm the following day (24hrs post injection) was collected. The dosage of 1.5mg/kg was selected based on previous studies demonstrating 1mg/kg is the minimal effective dose (Vercauteren et al., 2017). Additionally, higher doses at 3mg/kg led to changes in blood pressure (Okada & Nishikibe, 2002), and to reduce this effect we lowered the dosage.
ET-1 ELISA:
Urinary ET-1 peptide was measured by ELISA from R&D Systems (Endothelin-1 QuantiGlo ELISA kit). ELISA was performed according to manufacturer’s instructions with undiluted urine samples after centrifugation to remove debris. This kit detects both full-length ET-1 and processed ET-1. The cross-reactivity for this kit is 51% for ET-2, 0.01% for full-length ET-2, and 9% for ET-3. The detectable range is from 0.064–250pg/mL.
Aldosterone ELISA:
Urinary Aldosterone peptide was measured by ELISA from Enzo (ADI-900–173) according to manufacturer’s instructions. The cross reactivity for this kit is 100% for Aldosterone, 0.3% for 11-Deoxycorticosterone, 0.19% for Corticosterone, 0.20% for Progesterone, and <0.001% for Cortisol, DHT, Estradiol, Testosterone. The detectable range is from 3.9–250pg/mL.
Flame Photometry:
Flame photometry (Cole-Parmer, Model 2655–00, Chicago, IL) was used to determine urine Na and K concentrations.
RNA isolation and real-time quantitative RT-PCR:
Kidneys were dissected and cortex was isolated. Total RNA was isolated using Trizol (Invitrogen). RNA was treated with DNaseI (Ambion). The resulting RNA samples were used along with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) to create cDNA. Applied Biosystems TaqMan probes were used for gene expression analysis as described in (Solocinski et al., 2015).
Cell culture experiments.
For cell culture experiments, an immortalized mouse principal cortical collecting duct cell line of male origin (mpkCCDc14) was utilized (Bens et al., 1999). mpkCCDc14 cells were grown and transfected with non-target or Per1-specific siRNA as described previously (Gumz et al., 2009). Transfection of the PER1-specific siRNA results in an approximate 80% knockdown of PER1 mRNA expression (Gumz et al., 2010).
Statistics:
Graphpad Prism was used to perform T-tests, 2-way ANOVA, and 2-way ANOVA with repeated measures. All values are presented as mean ± SEM.
Results
Reduced night/day urinary ET-1 in global PER1 KO mice.
In humans and rats, urinary ET-1 has been shown to negatively correlate with salt-sensitive hypertension (Hoffman et al., 1994; Speed et al., 2011). Our laboratory previously reported that male PER1 KO mice exhibit salt-sensitive non-dipping hypertension (Solocinski et al., 2017) and an altered night/day ratio of Na excretion in response to HS/DOCP treatment (7x in WT vs. <4x in KO) with no differences in the night/day ratio of food intake (Douma et al., 2018). To determine if the Na excretion phenotype is associated with altered night/day ratio of urinary ET-1 excretion, urine samples from the metabolic cage study reported in Douma et al. 2018 were analyzed for ET-1 peptide levels. This metabolic cage study included male WT and PER1 KO mice which were acclimated to metabolic cages and then administered the same treatment regimen that caused non-dipping hypertension in the male global PER1 KO mice. Briefly, this treatment consisted of a normal Na diet for 3 days, high Na diet for 3 days, followed by the HS/DOCP treatment for 3 days. This model is an acute treatment, meaning the mice are not in balance and we are measuring their short-term response to the HS/DOCP treatment. The HS/DOCP model mimics the low renin, high aldosterone state seen in salt-sensitive hypertension. Urine collections were made every 24hrs on the normal (NS) and high salt (HS) diet, followed by 12hr urine collections during the HS/DOCP treatment. Urinary ET-1 peptide levels were measured by ELISA analysis. Figure 1A shows the total urinary ET-1 peptide (pg) per 12hrs on the HS/DOCP treatment. The night/day ratio of urinary ET-1 was calculated for the 24hrs after DOCP administration and for the average of all 72hrs on the HS/DOCP treatment due to the significant changes seen in the 1st 24hrs. WT male mice had an average night/day ratio of ET-1 excretion of 3.2 for the first 24hrs of the HS/DOCP treatment and an average night/day ratio of 5.8 over the entire 3 days of HS/DOCP (Figure 1B–C). Male PER1 KO mice had a significantly reduced night/day ratio of ET-1 excretion compared to WT mice with an average night/day ratio of 1 on day 1 and an average of 2.2 over the 3 days of the HS/DOCP treatment.
Figure 1: Male Global PER1 KO Mice have Increased Urinary ET-1 During Inactive Periods.
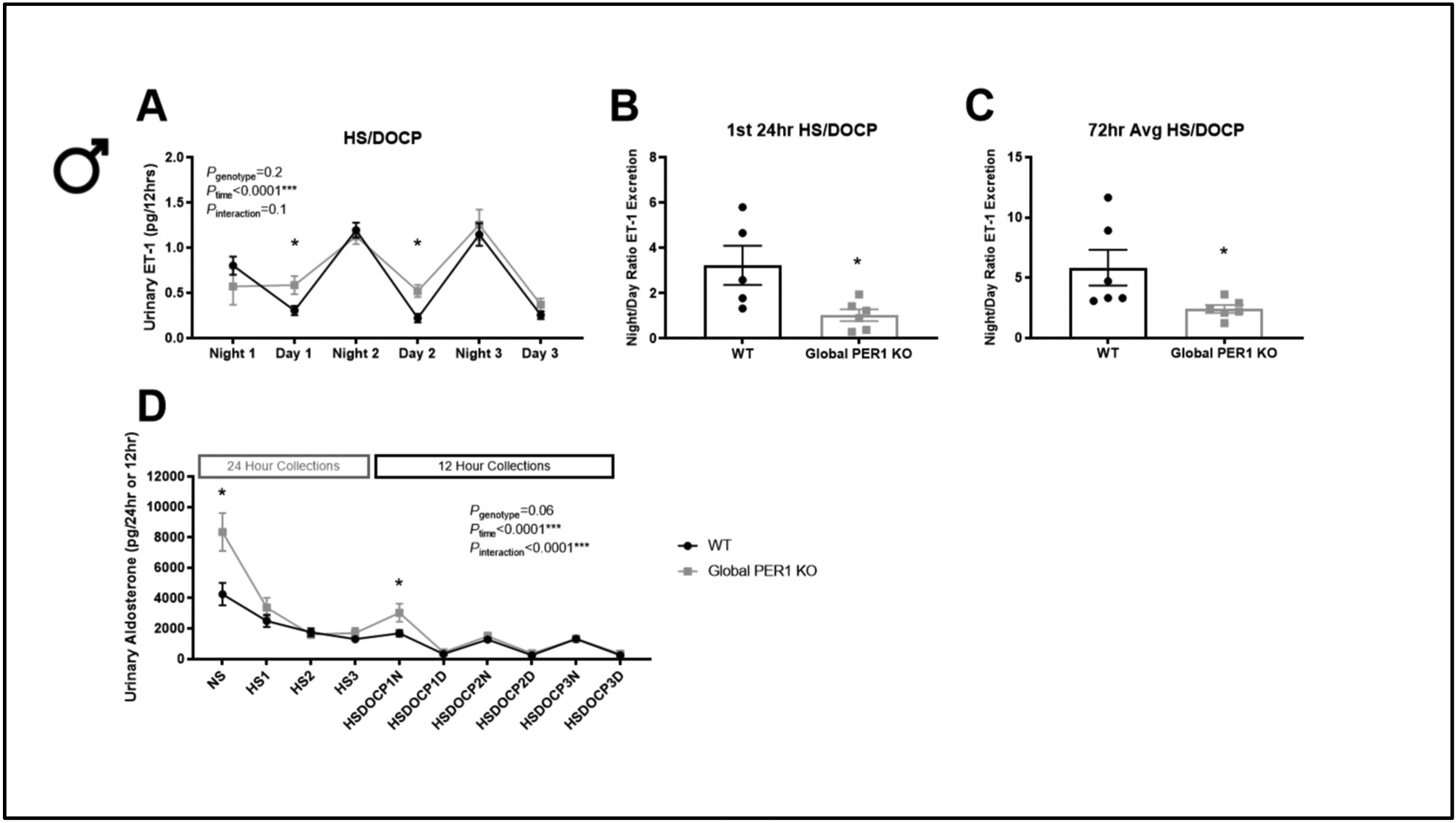
12-Hour urinary ET-1 peptide in male WT and global PER1 KO mice immediately following a HS/DOCP treatment (A). Night corresponds to 6pm-6am (lights off; mouse active period). Day corresponds to 6am-6pm (lights on; mouse inactive period). Night/Day ratio of urinary ET-1 excretion was calculated for the 1st 24-hrs on the HS/DOCP treatment (B) and for 72hrs on the treatment (C). Urinary Aldosterone (per 24- or 12-hr) was measured for male WT and global PER1 KO mice throughout the course of the metabolic cage experiment. N=5–6 per group, statistical significance measured by T-test WT vs KO within each collection (*P<0.05), overall genotype and time effects for all days of treatments were measured by 2-way ANOVA with repeated measures.
Because aldosterone is a known regulator of ET-1 production, urinary aldosterone was measured by ELISA. The ELISA kit used is highly specific for aldosterone and only has 0.3% cross reactivity with DOC (Enzo ADI-900–173). At baseline, male global PER1 KO mice had higher urinary aldosterone compared to WT mice (Figure 1D). The level of urinary aldosterone decreased following administration of a HS diet, as expected. Additionally, the PER1 KO mice had significantly higher urinary aldosterone during the first 12hrs after the DOCP injection was administered, which corresponded to their active period.
Female PER1 KO mice maintain rhythms of Na and ET-1 excretion.
Unlike the male global PER1 KO mice, female global PER1 KO mice do not develop non-dipping hypertension on the HS/DOCP treatment (Douma et al., 2019). A metabolic cage study was performed to determine if female global PER1 KO mice also maintain normal night/day ratios of Na excretion. Female WT and global PER1 KO mice were acclimated to metabolic cages for 3 days and then given the same treatment as described for the males. There was no significant difference in urine output or Na excretion throughout the metabolic cage study (Figure 2A). In contrast to what we previously observed in male global PER1 KO mice, there was no difference in the night/day rhythms of Na excretion in female global PER1 KO on the HS/DOCP treatment compared to WT mice (Figure 2B). There were no observed differences in night/day food intake (Figure 2C). Additionally, there was no significant difference in potassium (K) excretion, overall food intake, or urine output. There was a significant difference in body weight throughout the metabolic cage study between female WT and PER1 KO mice, but this difference was only approximately 7%, matching what we have previously reported in female PER1 KO mice (Douma et al., 2019) (Figure S1A–D).
Figure 2: Female Global PER1 KO Mice have Increased ET-1 during Active Period Following HS+DOCP.
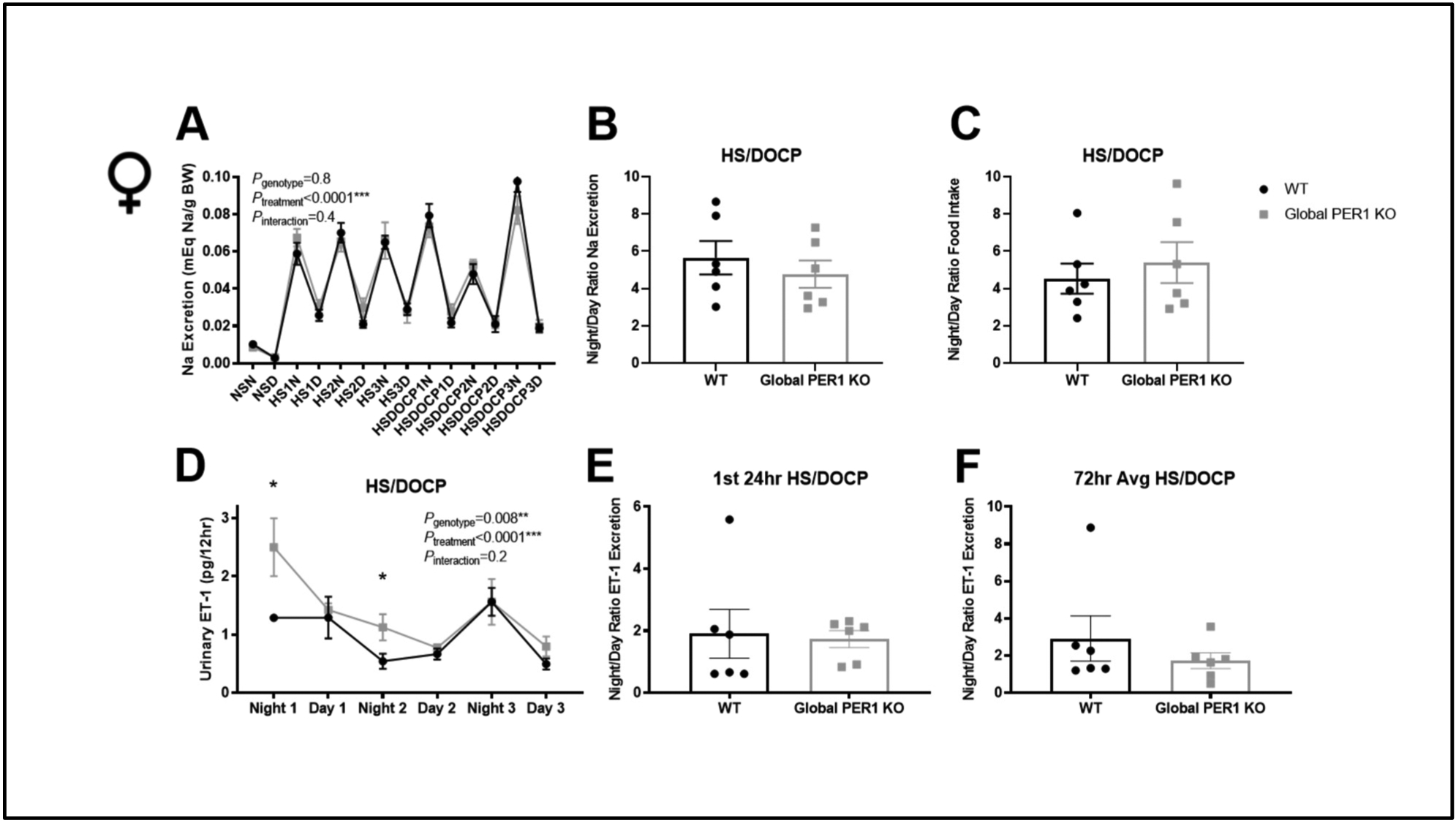
Na excretion was measure in WT and female global PER1 KO mice 12-hr collections under a normal salt diet (NS), high salt diet (HS), and HS+DOCP treatment (A). Night (N) corresponds to 6pm-6am (lights off; mouse active period). Day (D) corresponds to 6am-6pm (lights on; mouse inactive period). Night/day ratio of Na excretion (B) and food intake (C) on the HS/DOCP treatment was calculated as previously done for male mice (REF DOUMA 2018). 12-Hour urinary ET-1 peptide in female WT and global PER1 KO mice immediately following a HS/DOCP treatment (D). Night/Day ratio of urinary ET-1 excretion was calculated for the 1st 24-hrs on the HS/DOCP treatment (E) and for 72hrs on the treatment (F). N=6 per group, statistical significance measured by T-test WT vs. KO (*P<0.05).
Urinary ET-1 peptide excretion was measured by ELISA to determine if the female KO mice had changes in ET-1 production. As seen in Figure 2D, female global PER1 KO mice have significantly increased ET-1 production compared to WT mice during their active periods following the DOCP injections. This is in contrast to male PER1 KO mice, in which an increase in ET-1 peptide was observed during their inactive periods. There was no significant difference in the night/day ratio of urinary ET-1 between female PER1 KO mice and WT mice (Figure 2E–F).
Urinary aldosterone of female WT and PER1 KO mice was measured during a normal salt diet and during the 1st 24hrs of the HS/DOCP treatment, where we saw significant differences in urine aldosterone in the male mice. At baseline there is no significant different in urine aldosterone between female WT and global PER1 KO mice (Figure S2A). Additionally, there was no significant difference in the urine aldosterone levels during the 1st 24hrs of the HS/DOCP treatment (Figure S2B).
Gene expression of ET-1 and ET receptors.
The alterations in ET-1 urinary peptide levels in the male global PER1 KO mice prompted a gene expression analysis of the ET-1 axis. The goal was to compare the response to HS/DOCP between genotypes, as we have done previously (Douma et al., 2018). On the 4th day of the acute HS/DOCP treatment, male and female WT and PER1 KO kidneys were harvested at noon, the midpoint of the mouse inactive period. The midpoint of the inactive period also correlated to the period in which non-dipping hypertension was observed in male, but not female, PER1 KO mice (Solocinski et al., 2017) (Douma et al., 2019). In response to the HS/DOCP treatment, male PER1 KO mice significantly increased ET-1 expression in the renal cortex by ~50% (Figure 3A). There was no change in cortex ET-1 mRNA in the male WT mice. In response to HS/DOCP, expression of the endothelin A receptor (ETA) was upregulated by 25% in the male WT mice, but not in the PER1 KO mice (Figure 3B). Both the WT and PER1 KO male mice upregulated expression of the endothelin B receptor (ETB) by 60% in response to the HS/DOCP treatment (Figure 3C). Female WT and global PER1 KO mice upregulated ET-1 expression in response to HS/DOCP (Figure 3D). There were no significant changes in the ETA or ETB receptor in the WT or KO female mice in response to the HS/DOCP treatment (Figure 3E&F).
Figure 3: Gene Expression Analysis of the ET-1 Axis in Response to HS+DOCP Treatment.
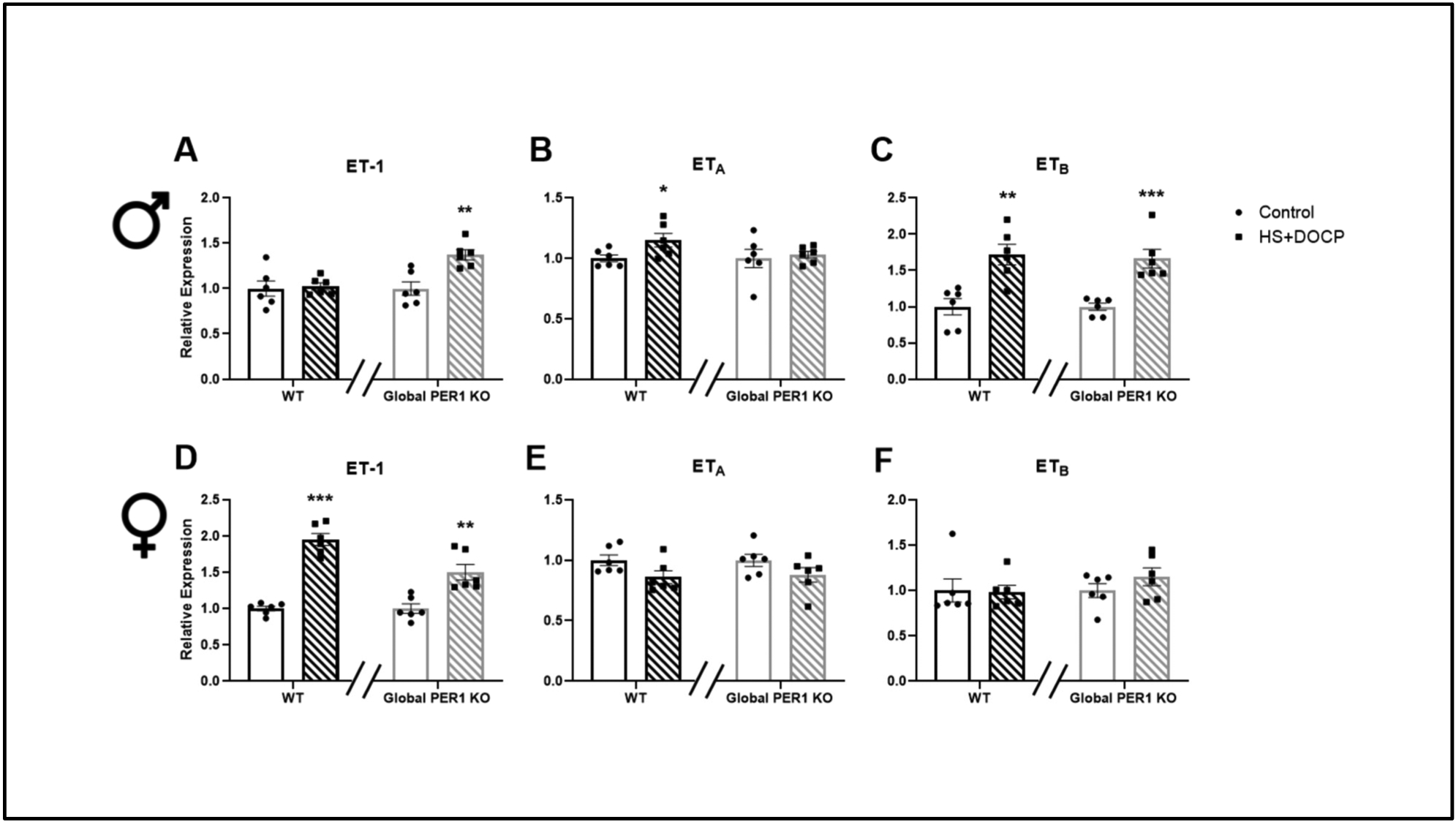
Kidney cortex samples were isolated at noon, the midpoint of the mouse rest period, from male (A-C) and female (D-F) WT and global PER1 KO mice on NS or HS/DOCP for 3 days. Total RNA was isolated from the dissected renal cortex samples and measured by quantitative real time RT-PCR. To look at the gene expression changes in response to the HS/DOCP treatment, expression was relativized to NS expression within each genotype. N=6 mice per group, statistically significant effect of HS/DOCP treatment on gene expression was determined by T-test (*P<0.05, **P<0.01, ***P<0.001).
BQ788, ETB antagonist, results in increased Na retention in male PER1 KO mice.
BQ788, an ETB receptor antagonist, has been previously shown to decrease urine volume and urinary excretion of Na (Girchev et al., 2004). In the current study, BQ788 was used to determine if the sensitivity of the known BQ788 effect was altered in male PER1 KO mice on the HS/DOCP treatment. ET-1 action on the ETB receptor has been shown to promote Na excretion through inhibition of the epithelial Na channel (ENaC) (Bugaj et al., 2012; Hyndman et al., 2015; Lynch et al., 2013). Because we did not observe a Na excretion phenotype in the female PER1 KO mice, we decided to focus on the effect of BQ788 in the male PER1 KO mice. WT and male PER1 KO mice were given an I.P. injection of BQ788 at the beginning of their active period (6pm) and urine was collected 3hrs post-injection (6pm-9pm) and 24hrs post-injection (6pm-9pm the following day) to examine genotype effects on electrolyte excretion. No significant genotype effects in urine output, Na excretion, or potassium excretion, were observed 3hrs post-administration of BQ788 (Figure 4A). After 24hrs, male PER1 KO mice had a significant reduction in Na excretion and K excretion compared to WT mice (Figure 4B–C), indicating increased sensitivity to ETB inhibition. No significant differences in urinary aldosterone were observed (Figure 4D).
Figure 4: BQ788 Treatment Effects in Male WT and Global PER1 KO Mice.
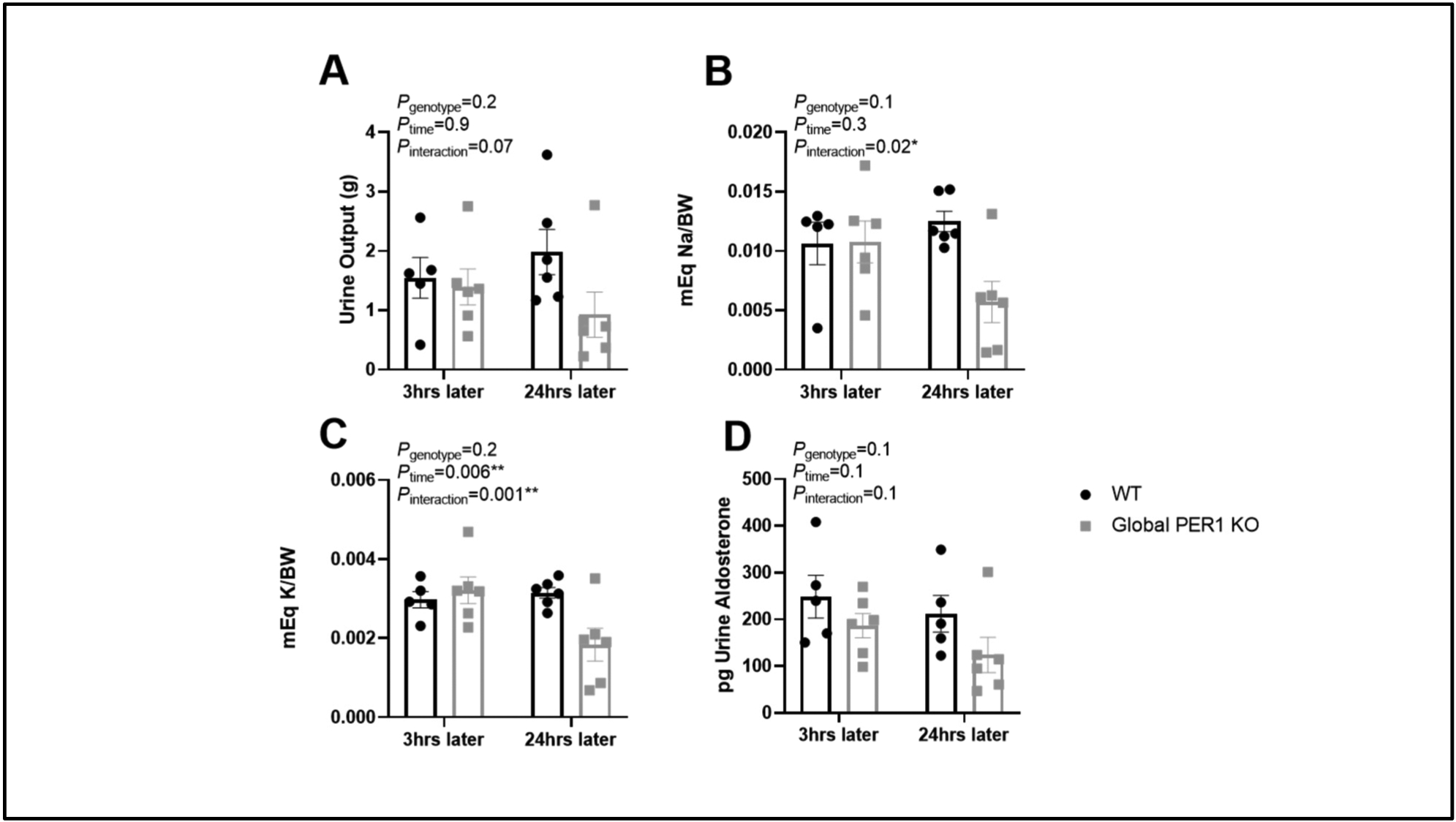
After acclimation to both metabolic cages and receiving I.P. injections, male WT and global PER1 KO mice were given a single I.P. injection (1.5mg/kgBW) of the ETB antagonist, BQ788 around 6pm (time of lights off and the beginning of the mouse active period). Urine was then collected at 9pm (3hrs post BQ788) and urine from 6pm-9pm the next day was collected (24hrs post BQ788). Urine output is reported (A). Urine Na content (B), K content (C), and Aldosterone (D) was determined. N=5–6 per group, statistical significance measured by 2-way ANOVA with repeated measures to determine effects of genotype and time on HS/DOCP with BQ788 (*P<0.05,**P<0.01).
Knockdown of PER1 in cortical collecting duct cells results in increased ET-1 expression.
ET-1 expression was increased in the cortex of male PER1 KO mice on HS/DOCP treatment, so mouse cortical collecting duct cells (mpkCCDc14) were used to assess whether KO of PER1 increases ET-1 production in vitro. Cells transfected with a non-target siRNA or a PER1-specific siRNA were treated with vehicle or aldosterone and expression of ET-1 was measured after 4hrs. Aldosterone slightly increased ET-1 expression in mpkCCDc14 cells treated with a non-target siRNA by 1.5-fold (Figure 5A). When cells were treated with PER1 siRNA, ET-1 expression was upregulated by 3.5-fold compared to non-target siRNA vehicle. Aldosterone further increased ET-1 expression in cells treated with PER1 siRNA 4.5-fold compared to non-target siRNA vehicle.
Figure 5: KO of PER1 in Mouse Cortical Collection Duct Cells Results in Increased ET-1 mRNA and Secreted Peptide.
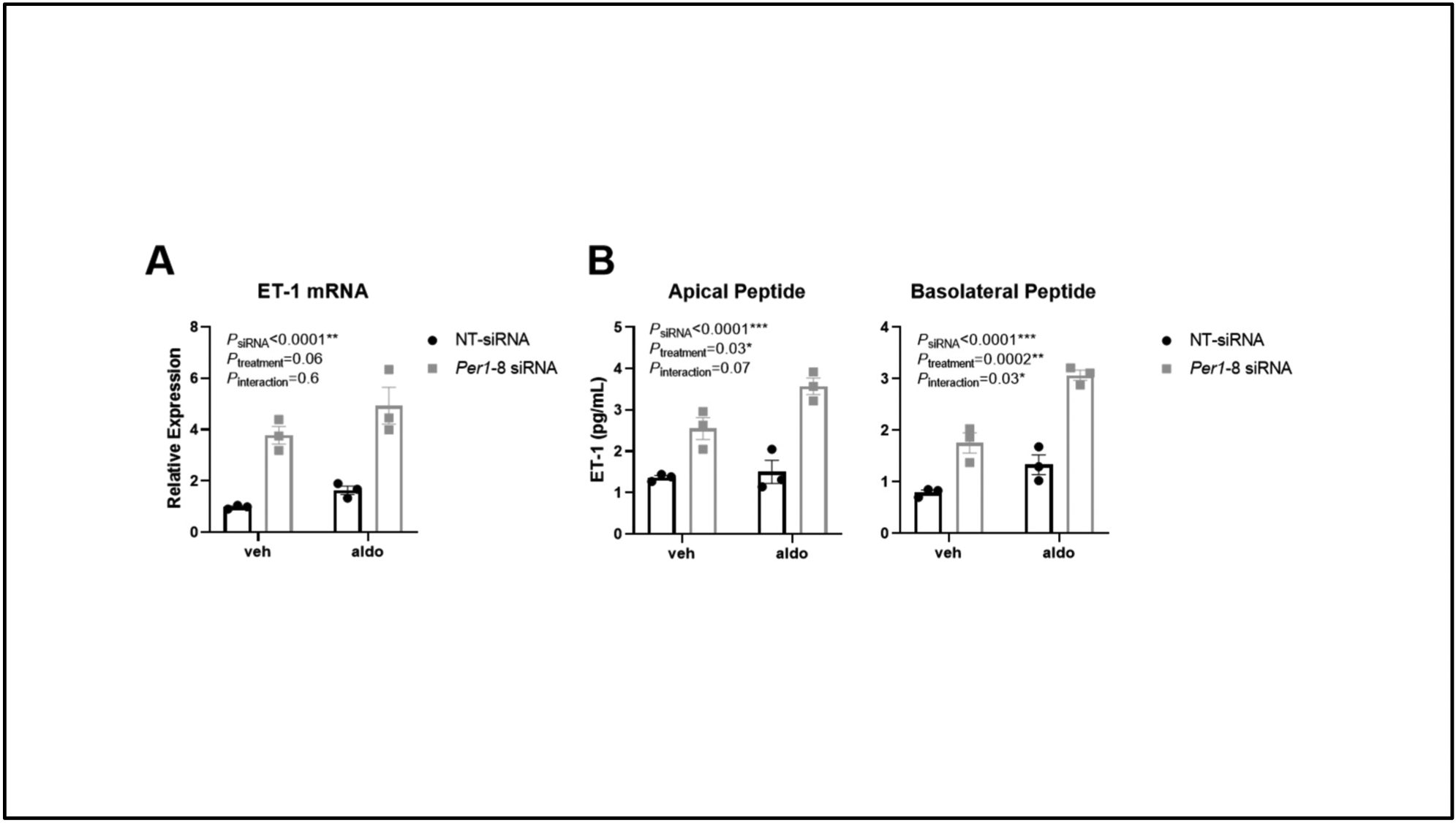
Mouse cortical collecting duct cells (mpkCCD14) in 6-well transwell plates were treated with a non-target siRNA (NT-siRNA) or a Per1 specific siRNA (Per1-8 siRNA). Vehicle (ethanol) and Aldosterone (1uM) treatments were performed for 4hrs. Total RNA was isolated and ET-1 expression was measured by quantitative real time RT-PCR (A). Media from the apical and basolateral side of the transwells was collected to measure secreted ET-1 peptide levels by ELISA (B). N=3 biological replicates per group and treatment, 2-way ANOVA was used to determine effects of siRNA and Aldosterone treatment (*P<0.05, **P<0.01, ***P<0.0001).
To determine if the upregulation of ET-1 expression led to changes at the protein level, non-target or PER1 siRNA treated cells were placed into transwell dishes and allowed to grow past confluence. The cells were treated with vehicle or aldosterone for 4 hrs. ET-1 mRNA was induced following PER1 siRNA treatment (Figure 5A). Media from the apical and basolateral side of the transwell insert was collected to measure total ET-1 peptide levels. In both the apical and basolateral samples, KO of PER1 using siRNA resulted in increased ET-1 peptide levels compared to non-target siRNA treated mpkCCDc14 cells (Figure 5B). Additionally, treatment with aldosterone further increased ET-1 levels in the PER1 siRNA treated cells.
Discussion
Male global PER1 KO mice develop non-dipping hypertension and have altered rhythms of Na handling in response to HS/DOCP (Douma et al., 2018; Solocinski et al., 2017). In the present study, we show that the same male global PER1 KO mice have altered rhythms of ET-1 production with an increase in ET-1 production during their inactive period following HS/DOCP. Additionally, female global PER1 KO mice, which do not develop the non-dipping phenotype, do not have altered ET-1 excretion rhythms compared to WT female mice. The global PER1 KO female mice also have an increase in ET-1 production, but unlike the male KO mice, this increase occurs during their active period. These changes in ET-1 production are associated with changes in ET-1 receptor expression in the male PER1 KO mice, but not in the female mice. Consistent with the effects observed in vivo, our in vitro data using a cortical collecting duct cell culture model show that knockdown of PER1 via siRNA resulted in increased ET-1 production at both the mRNA and peptide level in the presence of aldosterone treatment. Importantly, the mpkCCDc14 cells are of male origin (Duong Van Huyen et al., 1998).
The results of the present study demonstrate that ET-1 levels are increased in global male and female PER1 KO mice in response to HS/DOCP. However, only the male PER1 KO mice exhibit a decreased night/day ratio of ET-1 peptide excretion that correlates with the decreased ratio of night/day Na excretion that we previously observed in male PER1 KO mice on HS/DOCP (Douma et al., 2018). These findings extend our previous work that showed a connection between PER1 and ET-1 in cell culture models of the renal collecting duct (Stow et al., 2012). Additionally, the circadian clock protein BMAL1 has been shown to be modulated by the ET-1 pathway through the actions of the ETB receptor (Speed et al., 2018). The connection between aldosterone and ET-1 has been confirmed in other tissues as well. ET-1 expression was increased by aldosterone in pancreatic islet endothelium (Wang et al., 2019). Jaffe and colleagues also found that PER1 and ET-1 were induced by aldosterone in ex vivo aortic rings (Newfell et al., 2011). PER1 is induced by aldosterone in cardiomyocytes as well (Fletcher et al., 2019). The present study is the first to demonstrate that there is a sex-dependent interaction between PER1, ET-1, and aldosterone.
We observed that urinary aldosterone was significantly increased in the male global PER1 KO mice following the DOCP injection, during their active period. Following this period, male KO mice had significant increase in urinary ET-1 during their inactive period. It is interesting to note that primary hyperaldosteronism patients had higher levels of ET-1 mRNA expression prior to adrenalectomy (Burrello et al., 2019). In humans, circulating ET-1 has been observed to have a circadian rhythm with an increase in the morning and peaking in the afternoon (Thosar et al., 2019). Consistent with our results demonstrating an association between ET-1 and blood pressure rhythms, Dhaun et al. showed that ETA blockade in CKD patients resulted in improved blood pressure rhythms (Dhaun et al., 2014). This association between ET-1 and RAAS may shed light on clinical observations that dual blockade of ET-1 and the RAAS may be beneficial in certain CKD cases (Komers & Plotkin, 2016).
CircaDB, a database of clock-controlled gene expression in mice, revealed that both PER1 and ET-1 had circadian rhythms of mRNA expression peaking at different times in the kidney (Pizarro, Hayer, Lahens, & Hogenesch, 2013). PER1 mRNA expression in the kidney peaks during the daytime, the mouse inactive period, around 16:00. ET-1 expression in the kidney peaks during the nighttime, the mouse active period, around midnight. It is interesting to note that PER1 and ET-1 have offset expression times. Although the CircaDB database is comprised from data collected at the mRNA level, these data are consistent with the peptide data we observe in the PER1 KO mice. PER1 might be important in repression of ET-1 during the mouse inactive period.
In the present study, we observe sex-specific responses in the ET-1 axis response to the HS/DOCP treatment. Sex differences in ET-1 action have been previously reported (Kittikulsuth et al., 2013). Female ETB deficient rats were better able to maintain natriuresis rhythms than male ETB deficient loss following a salt load (Johnston et al., 2016). Inhibition of the ETA receptor in these rats improved natriuresis patterns in both sexes. Ovarian hormones have been shown to regulate expression of the ENaC subunits (Chang et al., 2007; Heo et al., 2013; Zhang et al., 2019). As ET-1 is known to regulate ENaC expression through its actions on the ETB receptor (Bugaj et al., 2012; Hyndman et al., 2015; Lynch et al., 2013), ovarian hormones could be contributing to the sex difference in the global PER1 KO mice. Further studies will need to be performed to determine if the effects of ET-1 on ENaC differ between males and females. Our study, in combination with the previous studies mentioned above, also demonstrates that mice should be studied both during their active and inactive periods. The sex differences in ET-1 axis activation by the HS/DOCP treatment may have been missed if we only studied the mice during their inactive period, the daytime.
Together with our previous findings that genetic knockdown or knockout of PER1 results in increased ET-1 expression in renal tissue or cells (Richards et al., 2014; Stow et al., 2012) the current results demonstrate that loss of PER1 is associated with increased ET-1 and this effect is magnified in the presence of mineralocorticoid. There are some limitations to the present study. While these data suggest PER1 regulates the ET-1 response to aldosterone, this association is currently correlative. Future intervention studies are needed to determine if ET-1 action in this setting contributes to or antagonizes the non-dipping hypertension observed in male PER1 KO mice on HS/DOCP. Our results show for the first time that the circadian clock protein PER1 modulates the ET-1 axis in response to aldosterone in a sex-specific manner. These sex-dependent responses of ET-1 may contribute to the sex-differences observed in blood pressure rhythms in the setting of a salt-sensitive hypertension model (Douma et al., 2019; Solocinski et al., 2017).
Supplementary Material
Funding
This work was supported by National Institutes of Health (NIH) Grants R01DK109570, R21AG052861, American Heart Association Postdoctoral Fellowship Grants 18POST34030210 (L. Douma) and 19POST34450134 (G.R. Crislip), NIH Grant T32-DK-104721 awarded to the University of Florida (UF) Division of Nephrology (L. Douma).
References:
- Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, … Vandewalle A (1999). Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. Journal of the American Society of Nephrology, 10(5), 923–934. [DOI] [PubMed] [Google Scholar]
- Bugaj V, Mironova E, Kohan DE, & Stockand JD (2012). Collecting duct-specific endothelin b receptor knockout increases enac activity. American Journal of Physiology - Cell Physiology, 302(1). 10.1152/ajpcell.00301.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrello J, Gai C, Tetti M, Lopatina T, Deregibus MC, Veglio F, … Monticone S (2019). Characterization and Gene Expression Analysis of Serum-Derived Extracellular Vesicles in Primary Aldosteronism. Hypertension, 74(2), 359–367. 10.1161/HYPERTENSIONAHA.119.12944 [DOI] [PubMed] [Google Scholar]
- Chang C-T, Sun C-Y, Pong C-Y, Chen Y-C, Lin G-P, Chang T-C, & Wu M-S (2007). Interaction of Estrogen and Progesterone in the Regulation of Sodium Channels in Collecting Tubular Cells. In Chang Gung Med J (Vol. 30). [PubMed] [Google Scholar]
- Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, Kimmitt RA, … Webb DJ (2014). Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension (Dallas, Tex. : 1979), 64(2), 296–304. 10.1161/HYPERTENSIONAHA.114.03533 [DOI] [PubMed] [Google Scholar]
- Douma LG, Holzworth MR, Solocinski K, Masten SH, Miller AH, Cheng K-Y, … Gumz ML (2018). Renal Na-handling defect associated with PER1-dependent nondipping hypertension in male mice. American Journal of Physiology - Renal Physiology, 314(6). 10.1152/ajprenal.00546.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma Lauren G., Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, … Gumz ML (2019). Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 316(1), R50–R58. 10.1152/ajpregu.00381.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong Van Huyen J, Bens M, & Vandewalle A (1998). Differential effects of aldosterone and vasopressin on chloride fluxes in transimmortalized mouse cortical collecting duct cells. The Journal of Membrane Biology, 164(1), 79–90. 10.1007/s002329900395 [DOI] [PubMed] [Google Scholar]
- Fletcher El. K., Kanki M, Morgan J, Ray DW, Delbridge L, Fuller PJ, … Young MJ (2019). Cardiomyocyte transcription is controlled by combined MR and circadian clock signalling. The Journal of Endocrinology. 10.1530/JOE-18-0584 [DOI] [PubMed] [Google Scholar]
- Girchev R, Bäcker A, Markova P, & Kramer HJ (2004). Impaired response of the denervated kidney to endothelin receptor blockade in normotensive and spontaneously hypertensive rats. Kidney International, 65(3), 982–989. 10.1111/j.1523-1755.2004.00483.x [DOI] [PubMed] [Google Scholar]
- Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, & Wingo CS (2010). Regulation of αENaC expression by the circadian clock protein Period 1 in mpkCCDc14 cells. Biochimica et Biophysica Acta - Gene Regulatory Mechanisms, 1799(9), 622–629. 10.1016/j.bbagrm.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumz ML, Popp MP, Wingo CS, & Cain BD (2003). Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. American Journal of Physiology - Renal Physiology, 285(4 54–4). 10.1152/ajprenal.00353.2002 [DOI] [PubMed] [Google Scholar]
- Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, … Wingo CS (2009). The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. Journal of Clinical Investigation, 119(8), 2423–2434. 10.1172/JCI36908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo NJ, Son MJ, Lee JW, Jung JY, Kim S, Oh YK, … Han JS (2013). Effect of estradiol on the expression of renal sodium transporters in rats. Climacteric, 16(2), 265–273. 10.3109/13697137.2012.672494 [DOI] [PubMed] [Google Scholar]
- Hoffman A, Grossman E, Goldstein DS, Gill JR, & Keiser HR (1994). Urinary excretion rate of endothelin-1 in patients with essential hypertension and salt sensitivity. Kidney International, 45(2), 556–560. 10.1038/ki.1994.72 [DOI] [PubMed] [Google Scholar]
- Hubrecht RC, & Carter E (2019). The 3Rs and humane experimental technique: Implementing change. Animals, 9(10). 10.3390/ani9100754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman KA, Bugaj V, Mironova E, Stockand JD, & Pollock JS (2015). NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. American Journal of Physiology - Renal Physiology, 308(3), F244–F251. 10.1152/ajprenal.00596.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ME, Wingo CS, & Cain BD (2013). An emerging role for microRNA in the regulation of endothelin-1. Frontiers in Physiology, 4, 22 10.3389/fphys.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JG, Speed JS, Jin C, & Pollock DM (2016). Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. American Journal of Physiology - Renal Physiology, 311(5), F991–F998. 10.1152/ajprenal.00103.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittikulsuth W, Sullivan JC, & Pollock DM (2013, January). ET-1 actions in the kidney: Evidence for sex differences. British Journal of Pharmacology, Vol. 168, pp. 318–326. 10.1111/j.1476-5381.2012.01922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komers R, & Plotkin H (2016). Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310(10), R877–84. 10.1152/ajpregu.00425.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch IJ, Welch AK, Kohan DE, Cain BD, & Wingo CS (2013). Endothelin-1 inhibits sodium reabsorption by ETA and ETB receptors in the mouse cortical collecting duct. American Journal of Physiology - Renal Physiology, 305(4). 10.1152/ajprenal.00613.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, … Jaffe IZ (2011). Aldosterone regulates vascular gene transcription via oxidative stress-dependent and-independent pathways. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(8), 1871–1880. 10.1161/ATVBAHA.111.229070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, & Nishikibe M (2002, June 7). BQ-788, a selective endothelin ETB receptor antagonist. Cardiovascular Drug Reviews, Vol. 20, pp. 53–66. 10.1111/j.1527-3466.2002.tb00082.x [DOI] [PubMed] [Google Scholar]
- Pizarro A, Hayer K, Lahens NF, & Hogenesch JB (2013). CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Research, 41(Database issue), D1009–13. 10.1093/nar/gks1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Welch AK, Barilovits SJ, All S, Cheng KY, Wingo CS, … Gumz ML (2014). Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sciences, 118(2), 255–262. 10.1016/j.lfs.2014.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, … Gumz ML (2017). Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiologica, 220(1), 72–82. 10.1111/apha.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solocinski Kristen, Richards J, All S, Cheng KY, Khundmiri SJ, & Gumz ML (2015). Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein per1 in proximal tubule cells. American Journal of Physiology - Renal Physiology, 309(11), F933–F942. 10.1152/ajprenal.00197.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed JS, Fox BM, Johnston JG, & Pollock DM (2015, March 1). Endothelin and Renal Ion and Water Transport. Seminars in Nephrology, Vol. 35, pp. 137–144. 10.1016/j.semnephrol.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, … Pollock DM (2018). High dietary sodium causes dyssynchrony of the renal molecular clock in rats. American Journal of Physiology-Renal Physiology, 314(1), F89–F98. 10.1152/ajprenal.00028.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed JS, LaMarca B, Berry H, Cockrell K, George EM, & Granger JP (2011). Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 301(2), R519–R523. 10.1152/ajpregu.00207.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Gumz ML, Lynch IJ, Greenlee MM, Rudin A, Cain BD, & Wingo CS (2009). Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1). Journal of Biological Chemistry, 284(44), 30087–30096. 10.1074/jbc.M109.030718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Jacobs ME, Wingo CS, & Cain BD (2011). Endothelin-1 gene regulation. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 25(1), 16–28. 10.1096/fj.10-161612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, … Gumz ML (2012). The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension, 59(6), 1151–1156. 10.1161/HYPERTENSIONAHA.112.190892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar SS, Berman AM, Herzig MX, McHill AW, Bowles NP, Swanson CM, … Shea SA (2019). Circadian Rhythm of Vascular Function in Midlife Adults. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(6), 1203–1211. 10.1161/ATVBAHA.119.312682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren M, Trensz F, Pasquali A, Cattaneo C, Strasser DS, Hess P, … Clozel M (2017). Endothelin ET A Receptor Blockade, by Activating ET B Receptors, Increases Vascular Permeability and Induces Exaggerated Fluid Retention s. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther, 361, 322–333. 10.1124/jpet.116.234930 [DOI] [PubMed] [Google Scholar]
- Wang J, Hu H, Song J, Yan F, Qin J, Guo X, … Chen L (2019). Aldosterone induced up-expression of ICAM-1 and ET-1 in pancreatic islet endothelium may associate with progression of T2D. Biochemical and Biophysical Research Communications, 512(4), 750–757. 10.1016/j.bbrc.2019.03.149 [DOI] [PubMed] [Google Scholar]
- Welch AK, Jacobs ME, Wingo CS, & Cain BD (2013, January). Early progress in epigenetic regulation of endothelin pathway genes. British Journal of Pharmacology, Vol. 168, pp. 327–334. 10.1111/j.1476-5381.2012.01826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ge Y, Bukhari AAS, Zhu Q, Shen Y, Li M, … Liang X (2019). Estrogen negatively regulates the renal epithelial sodium channel (ENaC) by promoting Derlin-1 expression and AMPK activation. Experimental and Molecular Medicine, 51(5). 10.1038/s12276-019-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


