Abstract
Rationale: Urinary TIMP-2 (tissue inhibitor of metalloproteinases-2) and IGFBP7 (insulin-like growth factor–binding protein 7) can predict acute kidney injury (AKI) in patients with sepsis.
Objectives: To address critical questions about whether biomarkers can inform the response to treatment and whether they might be used to guide therapy, as most sepsis patients present with AKI.
Methods: We measured [TIMP-2] · [IGFBP7] before and after a 6-hour resuscitation in 688 patients with septic shock enrolled in the ProCESS (Protocol-based Care for Early Septic Shock) trial. Our primary endpoint was stage 3 AKI, renal replacement therapy, or death within 7 days.
Measurements and Main Results: The endpoint was reached in 113 patients (16.4%). In patients with negative [TIMP-2] · [IGFBP7] at baseline, those who became positive (>0.3 U) after resuscitation had three-times higher risk compared with those who remained negative (21.8% vs. 8.5%; P = 0.01; odds ratio [OR], 3.0; 95% confidence interval [CI], 1.31–6.87). Conversely, compared with patients with a positive biomarker at baseline that were still positive at Hour 6, risk was reduced for patients who became negative (23.8% vs. 9.8%; P = 0.01; OR, 2.15; 95% CI, 1.17–3.95). A positive [TIMP-2] · [IGFBP7] after resuscitation was associated with worse outcomes in both patients with and without AKI at that time point. The clinical response to resuscitation, as judged by the Acute Physiology and Chronic Health Evaluation II score, was weakly predictive of the endpoint (area under the curve, 0.68; 95% CI, 0.62–0.73) and improved with addition of [TIMP-2] · [IGFBP7] (0.72; 95% CI, 0.66–0.77; P = 0.03). Different resuscitation protocols did not alter biomarker trajectories, nor did they alter outcomes in biomarker-positive or biomarker-negative patients. However, biomarker trajectories were associated with outcomes.
Conclusions: Changes in urinary [TIMP-2] · [IGFBP7] after initial fluid resuscitation identify patients with sepsis who have differing risk for progression of AKI.
Clinical trial registered with www.clinicaltrials.gov (NCT 00510835).
Keywords: acute kidney injury, cell-cycle arrest biomarkers, sepsis, dialysis, survival
At a Glance Commentary
Scientific Knowledge on the Subject
As urinary concentrations of TIMP-2 (tissue inhibitor of metalloproteinases-2) and IGFBP7 (insulin-like growth factor–binding protein 7) increase, the risk for acute kidney injury (AKI), dialysis, and death rise. We sought to validate prior observations on the relationship between measures of these biomarkers together with clinical criteria for AKI with the risk for death, dialysis, or progression to severe AKI within 7 days and examine the effects of resuscitation on this relationship in patients with septic shock.
What This Study Adds to the Field
When [TIMP-2] · [IGFBP7] (the product of TIMP-2 and IGFBP7) falls after fluid resuscitation, risk for progression to these outcomes decreases. Conversely, the persistence of high concentrations of [TIMP-2] · [IGFBP7] was strongly associated with death, dialysis, or progression to severe AKI.
Acute kidney injury (AKI) is a common complication in critically ill patients (1–3), and sepsis is the most common cause for critical illness in hospitalized patients (4). We previously reported in the ProCESS (Protocol-based Care for Early Septic Shock) trial that the incidence of AKI is nearly 70% in patients with septic shock; among these study patients, about 75% of AKI episodes were clinically apparent at presentation to the emergency department (5, 6). Importantly, progression to severe AKI, dialysis, and death in these patients occurs in nearly 30% (7), and it is important to assess the risk for developing these outcomes.
We have previously shown that the urinary biomarkers TIMP-2 (tissue inhibitor of metalloproteinases-2) and IGFBP7 (insulin-like growth factor–binding protein 7) predict the onset of moderate-to-severe AKI within 12 hours, which precedes the rise of serum creatinine (sCr) (8). [TIMP-2] · [IGFBP7] (the product of TIMP-2 and IGFBP7) performs well in identifying higher risk of AKI in both medical and surgical patients (9, 10); it is not affected by other acute or chronic comorbidities, including chronic kidney disease (11); and it adds to clinical models that predict AKI in critically ill patients (12). Recently, we found that measurements of these markers provide additional prognostic information when added to clinical assessment (13). Here, we hypothesize that changes in clinical management might alter these biomarker patterns and affect corresponding clinical outcomes.
To design interventional trials, we sought to validate our prior results in an independent cohort and to assess the response of [TIMP-2] · [IGFBP7] to treatment in patients with septic shock. Specifically, we tested three hypotheses. First, we tested the hypothesis that urinary [TIMP-2] · [IGFBP7] would change over the course of a 6-hour resuscitation, and second, we tested the hypothesis that these changes would be associated with differing rates of short-term outcomes (severe AKI, dialysis, or death within 7 d) in a cohort of patients with septic shock. We examined biomarkers alone and in conjunction with clinical findings. Third, we tested the hypothesis that alternate resuscitation strategies would have differing effects on biomarker trajectories. Some of the results of these analyses have been previously reported in abstract form (14).
Methods
Study Design
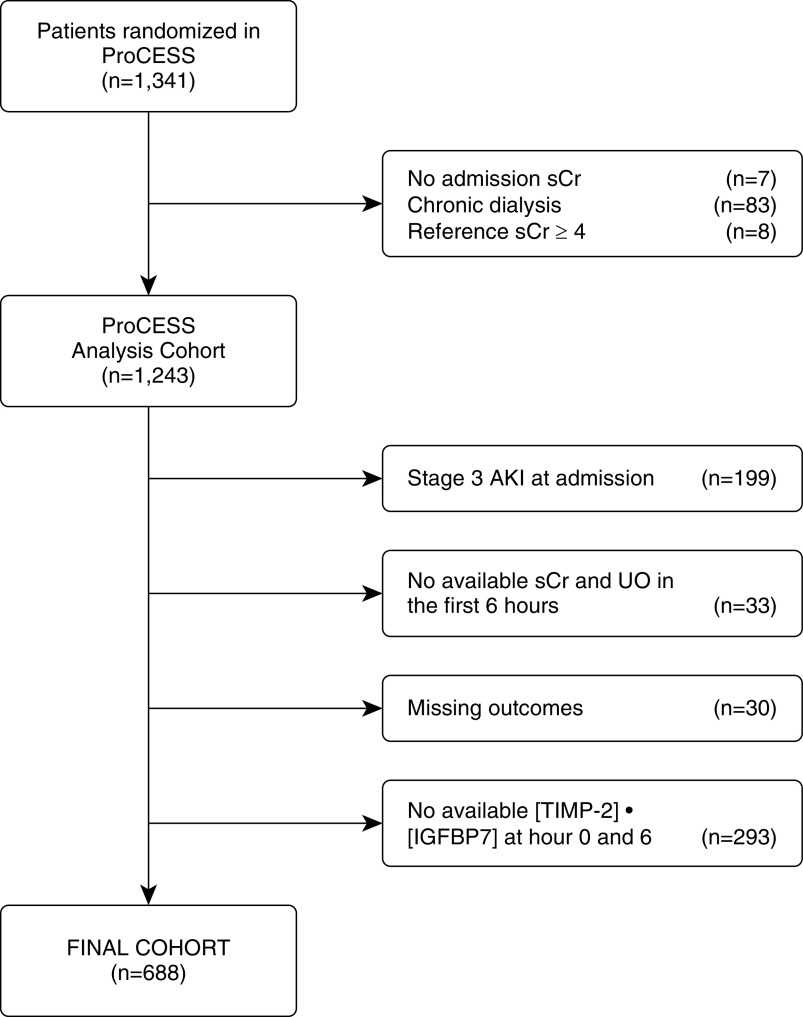
The ProCESS trial was a multicenter randomized clinical trial that investigated three resuscitation strategies in patients with septic shock, as previously described (5). In this secondary analysis, we included all patients with complete clinical data and who had provided urine samples for analysis of [TIMP-2] · [IGFBP7] at the time of enrollment and after 6 hours of resuscitation. We excluded patients with stage 3 AKI at admission, those without complete data on sCr and urine output (UO) in the first 6 hours after enrollment, those without information on outcomes (AKI stage, dialysis, or death), and those without biomarker information at one or both time points (Figure 1). For the summarized list of reasons for missing biomarkers, see Table E1 in the online supplement. We collected patient demographics, prior health history, sCr, and hourly UO in electronic case-report forms. We obtained urine samples at the time of enrollment and after 6 hours of resuscitation. The [TIMP-2] · [IGFBP7] measurement was performed using a clinical immunoassay (NephroCheckTM Test; Astute Medical) by technicians blinded to the clinical and outcome data. We measured sCr using an isotope dilution mass spectrometry–traceable assay at the clinical laboratories at each site as part of routine clinical chemistry testing. The study was approved by the institutional review board at the University of Pittsburgh, and each participating site approved the study protocol for the parent study.
Figure 1.
Study cohort. AKI = acute kidney injury; ProCESS = Protocol-based Care for Early Septic Shock; sCr = serum creatinine; [TIMP-2] · [IGFBP7] = the product of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor–binding protein 7; UO = urine output.
Outcome Measures
Our primary outcome was the development of Kidney Disease: Improving Global Outcomes (KDIGO) stage 3 AKI, initiation of renal replacement therapy (RRT), or death within the first 7 days of enrollment. We examined the composite outcome of death or RRT through 1-year in survival analysis. We determined in-hospital need of RRT and death using hospital records and postdischarge outcomes by linkage to the National Death Index and United States Renal Data System (15). We classified AKI using both sCr and UO criteria (16). We assessed AKI in the first 6 hours and within the first week after enrollment and patients were classified as having AKI when sCr and/or UO met KDIGO criteria for at least AKI stage 1. For additional details on baseline creatinine assessment, see the online supplement. [TIMP-2] · [IGFBP7] measurements were reported in a categorical way (positive or negative) using the “high-sensitivity” cutoff [≤0.3 (ng/ml)2/1,000] in the primary analysis and the “high-specificity” cutoff [>2.0 (ng/ml)2/1,000] in sensitivity analyses (17, 18).
Statistical Analysis
We used the Pearson chi-square test or Fisher exact test to compare categorical variables between patients who developed the primary endpoint and those who did not; for continuous variables, we compared values using Kruskal-Wallis rank tests or t tests as nonparametric and parametric methods respectively. Kaplan-Meier analysis was performed to assess differences in the risk of death and dialysis up to 1 year stratified by the four combinations of the biomarker patterns, and weighted log-rank tests (the generalized Wilcoxon, Tarone-Ware, and Peto-Peto-Prentice tests) were calculated. We calculated the area under the curve (AUC) of [TIMP-2] · [IGFBP7] in addition to standard criteria for severity of illness (Acute Physiology and Chronic Health Evaluation II [APACHE II] score) at both Hour 0 and Hour 6. We analyzed the number of patients assigned to the three treatment arms and the proportion of patients who developed the primary endpoint among the four biomarker patterns. We also compared the proportion of patients who received each specific intervention among the biomarker trajectories. The survival analysis was conducted by using Stata 14 (StataCorp), and the rest of the analyses were conducted by using R version 3.5.3 (R Foundation for Statistical Computing). For additional details, see the online supplement.
Results
Patient Characteristics and Frequency of the Primary Endpoint
The final analysis cohort included 688 patients (Figure 1). A total of 113 patients (16.4%) achieved the composite primary endpoint; 80 patients developed AKI stage 3, 2 of whom received RRT (one patient on Day 1 and the second one on Day 2) and 24 of whom died. An additional 33 deaths occurred in the first 7 days of enrollment in patients who did not reach stage 3 AKI (18 reached the maximum stage 2, 8 reached stage 1, and 7 never developed AKI). In addition, patients dying without AKI died quite early in their stay, the majority in the first 72 hours and all within the first week. Baseline characteristics of the patient population stratified by biomarker trajectories are summarized in Table 1. No differences existed in age, race, or ethnicity among the four groups. Male sex predominated in patients who were [TIMP-2] · [IGFBP7] positive at Hour 0 and [TIMP-2] · [IGFBP7] negative at Hour 6 (subgroup +/−) and patients who were [TIMP-2] · [IGFBP7] positive at both Hour 0 and Hour 6 (subgroup +/+) (59.5% and 56.1%, respectively). Patients with positive biomarkers at time 6 also tended to have higher severity of disease at the time of presentation (higher APACHE II score and Sequential Organ Failure Assessment score) and higher rate of chronic kidney disease (10.9% in patients who were [TIMP-2] · [IGFBP7] negative at Hour 0 and [TIMP-2] · [IGFBP7] positive at Hour 6 [subgroup −/+], 10.2% in subgroup +/+) compared with patients who were [TIMP-2] · [IGFBP7] negative at both Hour 0 and Hour 6 (subgroup −/−) and subgroup +/−. Furthermore, they presented with higher median serum lactate (1.91 [interquartile range, 1.25–3.17] in subgroup −/+; 2.71 [interquartile range, 1.6–4.5] in subgroup +/+). Over the 6-hour intervention, 274 patients (39.8%) developed AKI by KDIGO criteria (sCr, UO). At Hour 0, 231 patients (33.5%) had [TIMP-2] · [IGFBP7] ≤ 0.3, whereas [TIMP-2] · [IGFBP7] > 0.3 was reported in the remaining 457 patients (66.5%). At Hour 6, 339 patients (49.3%) had [TIMP-2] · [IGFBP7] ≤ 0.3, whereas the test was >0.3 in the other 349 patients (50.7%). At Hour 0, 270 patients already had AKI (148 patients with AKI stage 1 and 122 patients with AKI stage 2), whereas this number increased to 274 at Hour 6 (196 patients with AKI stage 1, 71 patients with AKI stage 2, and 7 patients with AKI stage 3). At Day 7, the percentage of patients who developed AKI increased further (132 with AKI stage 1, 201 with AKI stage 2, and 80 patients with AKI stage 3).
Table 1.
Baseline Characteristics of Study Population Stratified by Biomarker Trajectories
| All Patients (N = 688) [Median (IQR) or n (%)] | Subgroup −/− (n = 176) [Median (IQR) or n (%)] | Subgroup −/+ (n = 55) [Median (IQR) or n (%)] | Subgroup +/− (n = 163) [Median (IQR) or n (%)] | Subgroup +/+ (n = 294) [Median (IQR) or n (%)] | P Value* | |
|---|---|---|---|---|---|---|
| Age, yr | 62 (50–74) | 61 (49.5–72) | 64 (55–72.5) | 61 (50–71.5) | 64.5 (50–78) | 0.15 |
| Sex, M | 367 (53.3) | 80 (45.5) | 25 (45.5) | 97 (59.5) | 165 (56.1) | 0.03 |
| Race | 0.22 | |||||
| White | 491 (71.4) | 127 (72.2) | 41 (74.5) | 114 (69.9) | 209 (71.1) | |
| Black or African American | 153 (22.2) | 39 (22.2) | 13 (23.6) | 43 (26.4) | 58 (19.7) | |
| Other | 44 (6.4) | 10 (5.7) | 1 (1.8) | 6 (3.7) | 27 (9.2) | |
| Ethnicity | 0.63 | |||||
| Hispanic | 78 (11.3) | 20 (11.4) | 4 (7.3) | 16 (9.8) | 38 (12.9) | |
| Non-Hispanic | 610 (88.7) | 156 (88.6) | 51 (92.7) | 147 (90.2) | 256 (87.1) | |
| APACHE II score | 19 (15–23) | 17 (13–21) | 19 (14.5–25) | 18 (14–22.5) | 20 (16–24) | <0.001 |
| SOFA score | 6 (4–9) | 5 (3–7) | 7 (4.5–10) | 5 (4–7) | 7 (5–10) | <0.001 |
| Comorbidities | ||||||
| Charlson comorbidity index | 2 (1–4) | 2 (1–3.25) | 1 (1–3) | 2 (1–4) | 2 (1–4) | 0.89 |
| Hypertension | 406 (59.0) | 104 (59.1) | 32 (58.2) | 94 (57.7) | 176 (59.9) | 0.97 |
| Chronic respiratory disease | 155 (22.5) | 41 (23.3) | 12 (21.2) | 39 (23.9) | 63 (21.4) | 0.93 |
| Cancer | 134 (19.5) | 38 (21.6) | 10 (18.2) | 28 (17.2) | 58 (19.7) | 0.77 |
| Renal impairment | 50 (7.3) | 6 (3.4) | 6 (10.9) | 8 (4.9) | 30 (10.2) | 0.01 |
| Acute congestive heart failure | 70 (10.2) | 21 (11.9) | 3 (5.5) | 17 (10.4) | 29 (9.9) | 0.61 |
| Prior myocardial infarction | 75 (10.9) | 21 (11.9) | 6 (10.9) | 14 (8.6) | 34 (11.6) | 0.74 |
| Cerebral vascular disease | 75 (10.9) | 16 (9.1) | 9 (16.4) | 17 (10.4) | 33 (11.2) | 0.50 |
| Peripheral vascular disease | 58 (8.4) | 17 (9.7) | 5 (9.1) | 10 (6.1) | 26 (8.8) | 0.66 |
| Chronic dementia | 56 (8.1) | 7 (4.0) | 3 (5.5) | 20 (12.3) | 26 (8.8) | 0.03 |
| Hepatic cirrhosis | 40 (5.8) | 7 (4.0) | 5 (9.1) | 8 (4.9) | 20 (6.8) | 0.38 |
| Peptic ulcer disease | 34 (4.9) | 5 (2.8) | 4 (7.3) | 9 (5.5) | 16 (5.4) | 0.41 |
| AIDS and related syndromes | 15 (2.2) | 5 (2.8) | 1 (1.8) | 4 (2.5) | 5 (1.7) | 0.87 |
| Enrollment criteria | ||||||
| Hyperlactatemia | 399 (58.0) | 79 (44.9) | 33 (60) | 99 (60.7) | 188 (63.9) | <0.001 |
| Refractory hypotension | 373 (54.2) | 108 (61.4) | 29 (52.7) | 79 (48.5) | 157 (53.4) | 0.12 |
| Physiologic variables | ||||||
| Serum lactate, mmol/L | 2.00 (1.14–3.48) | 1.70 (0.90–2.98) | 1.91 (1.25–3.17) | 1.62 (1.04–3.10) | 2.71 (1.60–4.50) | <0.001 |
| Systolic blood pressure, mm Hg | 99 (84–120) | 101 (85–122) | 100 (84–117) | 99 (83–123) | 97 (83–119) | 0.46 |
| MAP, mm Hg | 70 (60–87) | 73 (63–88) | 72 (60–86) | 69 (59–87) | 69 (60–86) | 0.19 |
| Anemia | 104 (15.12) | 26 (14.8) | 11 (20) | 23 (14.1) | 44 (15.0) | 0.70 |
| Fluid overload > 10% over first 72 h | 45 (6.5) | 7 (3.9) | 4 (7.2) | 7 (4.3) | 27 (9.2) | 0.08 |
| [TIMP-2] · [IGFBP7], (ng/ml)2/1,000 | — | 0.1 (0.05–0.17) | 0.16 (0.08–0.22) | 0.85 (0.48–1.92) | 1.71 (0.84–4.1) | <0.001 |
Definition of abbreviations: APACHE II = Acute Physiologic Assessment and Chronic Health Evaluation II; IGFBP7 = insulin-like growth factor–binding protein 7; IQR = interquartile range; MAP = mean arterial pressure; SOFA = Sequential Organ Failure Assessment; TIMP-2 = tissue inhibitor of metalloproteinases-2.
Subgroups: −/− = [TIMP-2] · [IGFBP7] negative at both Hour 0 and Hour 6; −/+ = [TIMP-2] · [IGFBP7] negative at Hour 0 and [TIMP-2] · [IGFBP7] positive at Hour 6; +/− = [TIMP-2] · [IGFBP7] positive at Hour 0 and [TIMP-2] · [IGFBP7] negative at Hour 6; +/+ = [TIMP-2] · [IGFBP7] positive at both Hour 0 and Hour 6. Anemia was defined by Hb: <10 g/dl for males and <8 g/dl for females.
P values are shown for multiple comparison using ANOVA F test for continuous variables and the chi-square test or Fisher exact test for categorical variables.
Changes in [TIMP-2] · [IGFBP7] over Time and Relationship with Clinical Outcomes
We analyzed biomarker data at Hours 0 and 6 and defined the risk for the primary endpoint in four different subgroups of patients according to [TIMP-2] · [IGFBP7] status (Figure 2). We also provided the percentage of patients achieving each endpoint included in the composite one stratified by biomarker trajectories in Table E2. Patients in subgroup −/− had limited risk for developing the primary endpoint (8.5%); by contrast, in patients who first became positive at Hour 6 (subgroup −/+), the proportion of events was significantly higher (21.8% vs. 8.5%; P = 0.01). However, after adjusting for age, race, reference sCr, and nonrenal Sequential Organ Failure Assessment score, the odds ratio (OR) was not significant (2.15; 95% confidence interval [CI], 0.88–5.25). Patients in subgroup +/+ had the highest proportion of events (23.8%); interestingly, in patients who became negative at Hour 6 (subgroup +/−), the proportion of patients who developed the primary endpoint was significantly lower (9.8% vs. 23.8%; P < 0.001) compared with subgroup +/+ (adjusted OR, 2.15; 95% CI, 1.17–3.95; P = 0.016).
Figure 2.
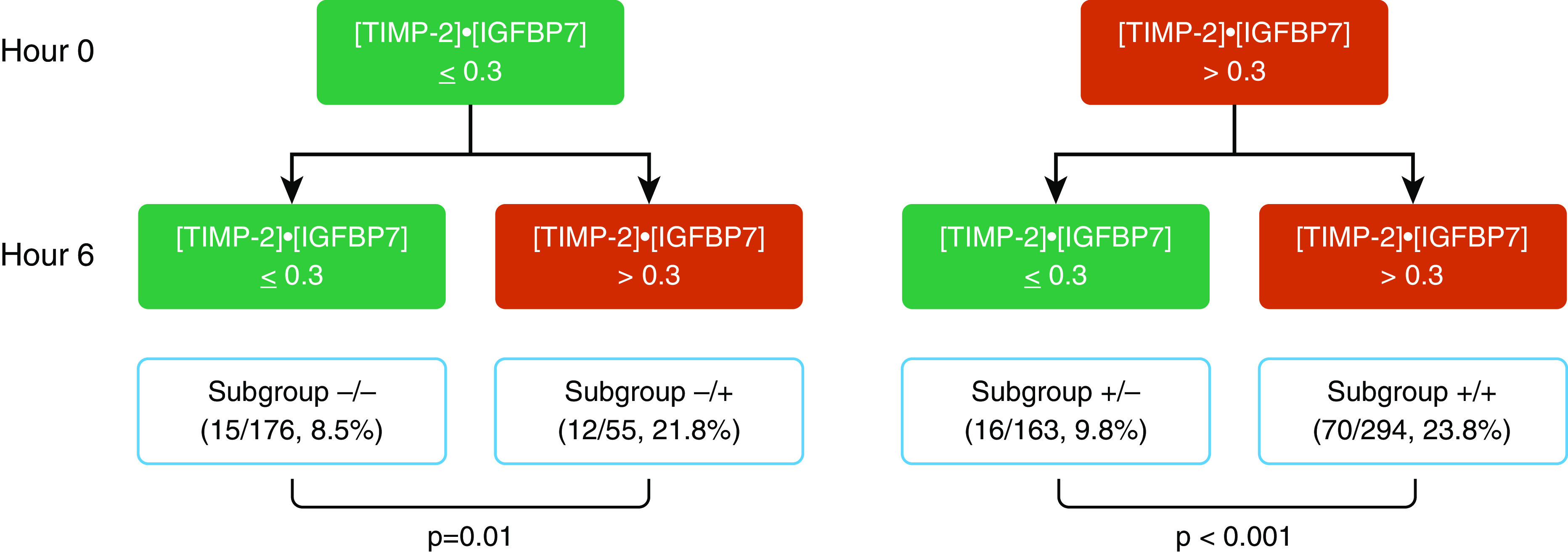
Risk assessment flow diagram combining [TIMP-2] · [IGFBP7] (the product of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor–binding protein 7) at Hour 0 and Hour 6. The red boxes indicate the positivity of [TIMP-2] · [IGFBP7] (biomarker values greater than the cutoff), whereas the green ones represent the condition with biomarker-negative results. For each combination, we reported the proportion of patients who achieved the primary endpoint. Subgroup −/− = [TIMP-2] · [IGFBP7] negative at both Hour 0 and Hour 6; Subgroup −/+ = [TIMP-2] · [IGFBP7] negative at Hour 0 and [TIMP-2] · [IGFBP7] positive at Hour 6; Subgroup +/− = [TIMP-2] · [IGFBP7] positive at Hour 0 and [TIMP-2] · [IGFBP7] negative at Hour 6; Subgroup +/+ = [TIMP-2] · [IGFBP7] positive at both Hour 0 and Hour 6.
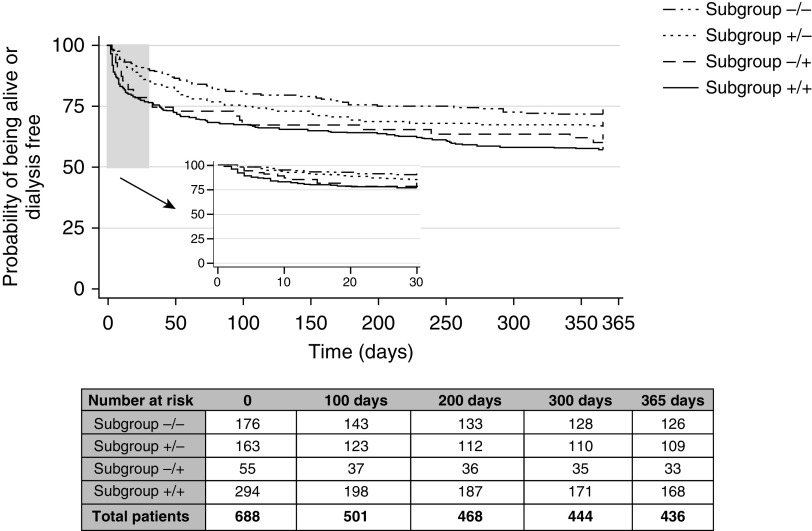
We found similar results in sensitivity analysis when using the high-specificity cutoff for [TIMP-2] · [IGFBP7] [>2.0 (ng/ml)2/1,000] and the OR for developing the endpoint remained greater in patients in subgroup −/+ compared with −/− (adjusted OR, 1.69, 95% CI, 0.83–3.44) and in patients in subgroup +/+ compared subgroup +/− (adjusted OR, 2.65, 95% CI, 1.20–5.88) (see Figure E1A in the online supplement). In sensitivity analysis excluding death from the outcome, [TIMP-2] · [IGFBP7] at Hour 6 remained strongly associated with stage 3 AKI or RRT in this cohort (Figure E1B). One-year survival and dialysis risk stratified by the results of [TIMP-2] · [IGFBP7] at the time of enrollment and 6 hours later is shown in Figure 3. Patients in the subgroup −/− had a lower risk for death/dialysis at 1 year, whereas subgroups −/+ and +/+, both characterized by positive Hour 6 [TIMP-2] · [IGFBP7], had a significantly higher risk to develop the composite outcome (log-rank test < 0.05) (Table E3).
Figure 3.
Kaplan-Meier analysis at 1 year by [TIMP-2] · [IGFBP7] (the product of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor–binding protein 7) concentrations between Hour 0 and Hour 6. Subgroup −/− = [TIMP-2] · [IGFBP7] negative at both Hour 0 and Hour 6; Subgroup −/+ = [TIMP-2] · [IGFBP7] negative at Hour 0 and [TIMP-2] · [IGFBP7] positive at Hour 6; Subgroup +/− = [TIMP-2] · [IGFBP7] positive at Hour 0 and [TIMP-2] · [IGFBP7] negative at Hour 6; Subgroup +/+ = [TIMP-2] · [IGFBP7] positive at both Hour 0 and Hour 6.
Combining Biomarkers and Clinical Assessment
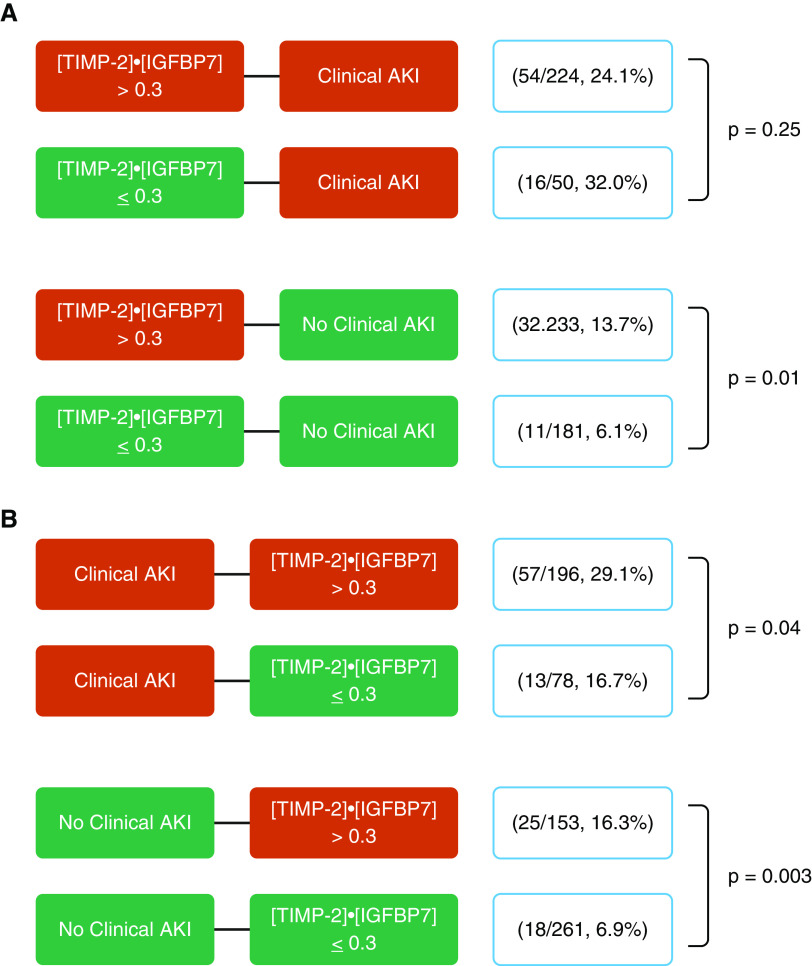
We also examined the association of [TIMP-2] · [IGFBP7] in addition to clinical criteria for AKI at the two time points with the development of the primary endpoint (Figure 4). At Hour 6, clinical AKI was diagnosed by only creatinine criteria in 168 patients, whereas 59 patients had AKI by only UO, and 47 patients had AKI using both criteria. At Hour 0, the risk for the primary endpoint was low (6.1%) in biomarker-negative patients with no clinical AKI. However, even among patients without clinical evidence of AKI within the first 6 hours, patients with a positive [TIMP-2] · [IGFBP7] result at Time 0 were more than twice as likely to develop the endpoint compared with those who tested negative (13.7% vs. 6.1%; P = 0.01; adjusted OR, 2.34; 95% CI, 1.12–4.90). In contrast, for patients with clinical AKI on presentation, a positive [TIMP-2] · [IGFBP7] result did not identify worse outcomes compared with patients with a negative biomarker result. There was a significant interaction between [TIMP-2] · [IGFBP7] at Time 0 and AKI within the first 6 hours on the primary endpoint, such that the relationship between the biomarker test result and the endpoint was attenuated in the presence of AKI (AKI–[TIMP-2] · [IGFBP7] interaction; OR, 0.27; 95% CI, 0.10–0.73; P = 0.01).
Figure 4.
Risk assessment flow diagram for [TIMP-2] · [IGFBP7] (the product of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor–binding protein 7) at (A) Hour 0 and at (B) Hour 6 in combination with serum creatinine and urine output in predicting the risk of the primary endpoint. The red boxes indicate the positivity of the criteria (when serum creatinine and urine output meet Kidney Disease: Improving Global Outcomes criteria for acute kidney injury (AKI) stage 1, or when [TIMP-2] · [IGFBP7] values are greater than the cutoff). The green boxes represent the condition of normal serum creatinine, urine output (no clinical AKI), and biomarker negativity. For each combination, we reported the proportion of patients who achieved the primary endpoint.
At 6 hours after enrollment, corresponding to the end of the resuscitation intervention, high concentrations of [TIMP-2] · [IGFBP7] were associated with adverse outcomes in septic patients, regardless of clinical evidence of AKI; the risk for the primary endpoint was significantly higher in patients with positive [TIMP-2] · [IGFBP7] results in both patients without clinical AKI (16.3% vs. 6.9%; P = 0.003; adjusted OR, 2.09; 95% CI, 1.06–4.10) and those with clinical AKI (29.1% vs. 16.7%; P = 0.04; adjusted OR, 1.59; 95% CI, 0.78–3.22). Here, there was no interaction between clinical AKI and [TIMP-2] · [IGFBP7] (OR, 0.78; 95% CI, 0.36–0.89; P = 0.60), so the biomarker was associated with the endpoint independently of AKI status. Finally, the risk for the primary endpoint was quite low (8 of 148; 5.4%) for patients testing negative at both time points and not manifesting AKI, whereas it was quite high (48 of 174; 27.6%) for those testing positive at 0 and 6 hours and manifesting AKI clinically.
Finally, we evaluated different models including sCr with or without [TIMP-2] · [IGFBP7] at the two time points. At Time 0, the AUC for the composite endpoint using creatinine alone was 0.53 (95% CI, 0.47–0.59; P = 0.13), whereas performance improved (P = 0.02) with the addition of [TIMP-2] · [IGFBP7] (AUC, 0.59; 95% CI, 0.53–0.65; P = 0.01). Similarly, at 6 hours, the addition of urinary [TIMP-2] · [IGFBP7] to sCr slightly improved (P = 0.02) the AUC (0.71; 95% CI; 0.65–0.76; P = 0.001) compared with creatinine alone (AUC, 0.67; 95% CI; 0.61–0.72; P = 0.001).
Combining Biomarkers and APACHE II Score
We also evaluated [TIMP-2] · [IGFBP7] in addition to the standard criteria for severity of illness (APACHE II) at both Time 0 and Time 6. At Time 0, the AUC for the composite endpoint using the APACHE II score was 0.61 (95% CI, 0.55–0.67; P = 0.001). Performance did not significantly (P = 0.23) improve with the addition of [TIMP-2] · [IGFBP7] (AUC, 0.63; 95% CI, 0.58–0.68; P = 0.04). Conversely, at 6 hours, the addition of urinary [TIMP-2] · [IGFBP7] to APACHE II significantly (P = 0.03) improved the AUC (0.72; 95% CI, 0.66–0.77; P = 0.001) compared with APACHE II alone (AUC, 0.68; 95% CI, 0.62–0.73; P = 0.001).
Treatments Received among [TIMP-2] · [IGFBP7] Subgroups
Among patients with positive Hour 0 [TIMP-2] · [IGFBP7] results, the proportion of patients who were still positive at Hour 6 was not influenced by treatment arms (early goal-directed therapy vs. protocol-based standard care vs. usual care, 64.3% vs. 64.9% vs. 64.8%), and no significant differences were found in the proportion of events (21.1% vs. 17.7% vs. 17.9%). Similarly, among patients with negative biomarker results at Hour 0, no significant effects on biomarker trajectories (positive Hour 6 [TIMP-2] · [IGFBP7], 31.3% vs. 21.6% vs. 16.9%) or endpoints (14.4% vs. 10.8% vs. 9.2%) were found (Table 2).
Table 2.
Analysis of Biomarker Trajectories and Outcomes by Treatment Arm
| Hour 0 | Treatment Arm | Hour 6 | Stage 3 AKI, RRT, or Death within 7 d | |
|---|---|---|---|---|
| [TIMP-2] · [IGFBP7] > 0.3 (n = 457) | EGDT (n = 137) | [TIMP-2] · [IGFBP7] > 0.3 (n = 88, 64.3%) | 22/88 (25%) | 29 (21.1%) |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 49, 35.7%) | 7/49 (14.3%) | |||
| PSC (n = 158) | [TIMP-2] · [IGFBP7] > 0.3 (n = 101, 64.9%) | 23/101 (22.8%) | 28 (17.7%) | |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 57, 36%) | 5/57 (8.8%) | |||
| Usual care (n = 162) | [TIMP-2] · [IGFBP7] > 0.3 (n = 105, 64.8%) | 25/105 (23.8%) | 29 (17.9%) | |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 57, 35.2%) | 4/57 (7%) | |||
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 231) | EGDT (n = 83) | [TIMP-2] · [IGFBP7] > 0.3 (n = 26, 31.3%) | 8/26 (30.7%) | 12 (14.4%) |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 57, 68.7%) | 4/57 (7%) | |||
| PSC (n = 83) | [TIMP-2] · [IGFBP7] > 0.3 (n = 18, 21.6%) | 2/18 (11.1%) | 9 (10.8%) | |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 65, 78.4%) | 7/65 (10.7%) | |||
| Usual care (n = 65) | [TIMP-2] · [IGFBP7] > 0.3 (n = 11, 16.9%) | 2/11 (18.1%) | 6 (9.2%) | |
| [TIMP-2] · [IGFBP7] ≤ 0.3 (n = 54, 83.1%) | 4/54 (7.4%) | |||
Definition of abbreviations: AKI = acute kidney injury; EGDT = early goal-directed therapy; IGFBP7 = insulin-like growth factor–binding protein 7; PSC = protocol-based standard care; RRT = renal replacement therapy; TIMP-2 = tissue inhibitor of metalloproteinases-2.
Finally, we performed an exploratory analysis evaluating whether there were specific treatment differences in the first 6 hours among patients with different biomarker profiles. Patients in subgroup +/+ were more likely to receive vasopressors (55.6% vs. 40.5%; P = 0.002) and blood transfusions (13% vs. 6.7%; P = 0.04) compared with patients in subgroup +/−, and they were more likely to receive mechanical ventilation (31.4% vs. 17.8%; P = 0.002) (Table E4). Similarly, among patients with negative [TIMP-2] · [IGFBP7] results at Hour 0, those who became positive at Hour 6 received more interventions compared with those who remained negative (central venous catheterization, 80% vs. 65.9%; P = 0.05; mean intravenous fluid, 3.3 vs. 2.6 L; P = 0.04; vasopressor use, 56.4% vs. 38.6%; P = 0.02; dobutamine use, 12.7% vs. 2.8%; P = 0.009; blood transfusions, 20% vs. 9.7%; P = 0.04; mechanical ventilation, 27.3% vs. 11.4%; P = 0.005). Conversely, the percentage of patients receiving potential nephrotoxic antibiotics (vancomycin, gentamicin, amikacin, tobramycin, streptomycin, and/or neomycin) did not differ among the four biomarker groups (68% of patients in subgroup −/−, 72.3% in subgroup −/+, 71.1% in subgroup +/−, and 72.4% in subgroup +/+; P = 0.85).
Discussion
Although sepsis is the most common cause of AKI in critically ill patients, and fluid resuscitation is the mainstay of therapy, no large studies have examined the role of AKI biomarkers in this setting. TIMP-2 and IGFBP7 are markers of kidney stress, which are released after various exposures (e.g., ischemic or inflammatory processes like sepsis). As shown by Emlet and colleagues (19), IGFBP7 is secreted mostly from proximal tubule cells, and TIMP-2 is expressed and secreted mostly by distal tubule cells. Their effects result in G1 cell-cycle arrest for a short period to avoid cell division when cells are damaged (8). We aimed to examine changing concentrations of [TIMP-2] · [IGFBP7] in response to resuscitation treatments in patients with septic shock. Although [TIMP-2] · [IGFBP7] was developed and tested for predicting moderate-to-severe AKI, many patients with sepsis already have AKI at ICU admission, so an important question to address is whether changing patterns of these biomarkers in response to resuscitation are associated with different odds of progression to severe AKI. The present study provides numerous new insights into the early progression of AKI in patients with septic shock. First, although patients developing the composite endpoint (death, dialysis, or stage 3 AKI within 1 wk) had a more chronic and acute burden of disease, neither severity of hypotension nor the proportion with hyperlactatemia differed among these patients. Similar findings were reported by Honore and colleagues (17), who found that [TIMP-2] · [IGFBP7] was not influenced by shock or other nonrenal organ failures. Second, among patients manifesting AKI, the results of biomarker testing at baseline did not influence the likelihood of developing the endpoint (Figure 4A), whereas the biomarker status after resuscitation did (Figure 4B). Recently, Ronco and colleagues (20) showed that, among patients with AKI, those having [TIMP-2] · [IGFBP7] positive results at the time of enrollment had a significantly increased risk for ICU mortality and the composite of continuous RRT and all-cause mortality. Our results extend this finding by showing that the timing of biomarker measurements may affect the interpretation of the results. Patients resolving their “kidney stress” by 6 hours had much lower odds of developing the endpoint, even if they had clinical AKI. However, the persistence of ongoing stress after 6 hours was associated with significantly increased odds of subsequent progression to stage 3, death, or dialysis. This is new information and was not examined in the initial studies for this biomarker test. Other prior studies have mainly looked at patients without evidence of AKI and have used these markers for risk prediction (8, 12). Third, among patients without clinical evidence of AKI and disease progression during the initial 6 hours, increased [TIMP-2] · [IGFBP7] (either before or after resuscitation) was associated with more than double the odds of developing the endpoint (Figures 4A and 4B). These results differ somewhat from those reported by Koyner and colleagues (21), in which [TIMP-2] · [IGFBP7] strongly correlated with death or dialysis over 9 months in critically ill patients only when they developed AKI (at least stage 1). However, Koyner and colleagues (21) looked at AKI over the first 72 hours of the ICU stay, whereas we only examined AKI manifesting on admission or over the first 6 hours. Only 7 of 113 (6%) patients in our study met the primary endpoint without developing even stage 1 AKI, and most of these patients died within the first 72 hours.
In this way, we have validated our recent results from an ancillary analysis of the Sapphire study, in which we found that UO, sCr, and [TIMP-2] · [IGFBP7] were all predictive of progression to the death, dialysis, or stage 3 within 7 days (13). Combinations of predictors increased the hazard ratios considerably (from 2.17 to 4.14 to 10.05, respectively). Here, we extend these findings to include clinical findings over the first 6 hours of active fluid resuscitation as well as serial measurements of the biomarkers. Patients negative for AKI (by both UO and sCr) and testing negative at both time points -were at low risk (5.4%), whereas double-positive patients (both time points) with AKI (by either UO or sCr) represented the highest risk (27.6%). Meersch and colleagues (10) reported relatively stable [TIMP-2] · [IGFBP7] values for 24 hours after cardiac surgery. In the present study, we include response to therapy and found that crossovers from positive to negative (163 patients; 23.7%) and negative to positive (55 patients; 8%) after resuscitation were frequent and associated with different clinical outcomes. However, sepsis is likely a very different insult (22), and treatments such as antibiotics (23) and fluids (24) may also worsen AKI. This is the first large study that focuses specifically on patients with septic shock and examines the biomarker signature before and after initial resuscitation—critical time points. A persistently positive biomarker result may require clinicians to change management and to determine why kidney stress has not resolved with initial fluid and antibiotics. Optimization of volume and hemodynamic status are required to reduce the risk of progression to severe AKI during the course of disease (25).
Finally, our results raise the hypothesis that because these molecules have a rapid response time (min), as seen in both experimental and clinical studies (19, 26), interventions in the first 6 hours could target biomarker-positive patients to reduce the risk of death, dialysis, or progression to stage 3 AKI—an approach similar to that used in recent studies in which KDIGO-recommended (16) actions were applied to biomarker-positive patients (27, 28). In our study, treatments may have influenced [TIMP-2] · [IGFBP7] trajectories. Among patients with positive [TIMP-2] · [IGFBP7] results at admission, the proportion of patients who received vasopressors, blood transfusions, and mechanical ventilation was higher in those who were still positive after the study interventions. Similarly, among patients with negative [TIMP-2] · [IGFBP7] results at admission, those who became positive at Hour 6 received more therapy. It does not appear that patients with evolving AKI were “undertreated” compared with those who had a more favorable biomarker trajectory.
Importantly, increased use of interventions in patients in subgroups +/+ and −/+ may reflect greater severity of illness in these subgroups compared with patients in subgroups +/− and −/−. Analysis by treatment arm (in which the intervention was randomly assigned as opposed to being determined by clinical status) revealed that protocolized care (compared with usual care) did not alter biomarker trajectories, nor did it affect short-term outcomes. Patients with positive biomarker results at Time 0 were no more likely to resolve these markers by Hour 6 when randomly assigned to protocolized care. The same was true for patients who were negative at Hour 0; treatment allocation did not influence the likelihood of remaining negative at Hour 6. Similarly, protocolized care did not influence the likelihood of developing the composite endpoint, either overall or specifically for biomarker-positive or biomarker-negative (Hour 0) patients.
Our study has several strengths and limitations. First, the analysis comes from a large, multicenter randomized clinical trial, with early enrollment of patients, adequate availability of baseline information, timely collection of samples, and standardized definition of AKI and outcomes. Second, the study population is more homogenous than that of previous biomarker studies (high heterogeneity of AKI etiologies). Third, [TIMP-2] · [IGFBP7] was developed for predicting the onset of moderate-to-severe AKI within 12 hours after ICU admission. Fourth, we analyzed biomarkers before and after the initial 6-hour resuscitation. We were limited to [TIMP-2] · [IGFBP7] measures, and we did not take into account other potential exposures, such as drugs or contrast media, that could have influenced development of AKI. We did not evaluate potential subsequent episodes of AKI during follow-up that could have influenced the outcome at 1 year. Finally, the absence of a validation cohort is acknowledged.
In conclusion, the change in urinary [TIMP-2] · [IGFBP7] trajectories in response to fluid resuscitation are associated with significant changes in risk of progression to severe AKI, dialysis, or death in septic patients with AKI.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the U.S. Renal Data System for supplying the data reported here. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Footnotes
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK083961 and UH3 DK114861) and the National Institute of General Medical Sciences (P50 GM076659) of the NIH.
Author Contributions: D.T.H., D.M.Y., D.C.A., and J.A.K. performed the primary study. M.F. and J.A.K. developed the research idea and study design for the present analysis and wrote the paper. Z.X. performed the statistical analysis. A.S., K.S., P.M.P., L.S.C., D.T.H., D.M.Y., and D.C.A. provided critical review and revised the paper.
A complete list of ProCESS and ProGReSS-AKI Investigators is available at https://crisma.upmc.com/progressakistudy.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201906-1197OC on June 25, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the ProCESS and ProGReSS-AKI Investigators, Derek C. Angus, Lakhmir S. Chawla, David T. Huang, Christopher Keener, John A. Kellum, Nicole Lucko, Paul M. Palevsky, Francis Pike, Kai Singbartl, Ali Smith, Donald M. Yealy, Sachin Yende, Raghavan Murugan, Lan Kong, Sachin Yende, and Xiaoyan Wen
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA, Bellomo R, Ronco C. Kidney attack. JAMA. 2012;307:2265–2266. doi: 10.1001/jama.2012.4315. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Basile D, Liu KD, McKay D, Molitoris BA, Nath KA, et al. Kidney Research National Dialogue (KRND) AKI: a path forward. Clin J Am Soc Nephrol. 2013;8:1606–1608. doi: 10.2215/CJN.06040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, et al. ProCESS and ProGReSS-AKI Investigators. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 8.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunnerson KJ, Shaw AD, Chawla LS, Bihorac A, Al-Khafaji A, Kashani K, et al. Sapphire Topaz Investigators. TIMP2 · IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80:243–249. doi: 10.1097/TA.0000000000000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heung M, Ortega LM, Chawla LS, Wunderink RG, Self WH, Koyner JL, et al. Sapphire and Topaz Investigators. Common chronic conditions do not affect performance of cell cycle arrest biomarkers for risk stratification of acute kidney injury. Nephrol Dial Transplant. 2016;31:1633–1640. doi: 10.1093/ndt/gfw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 13.McCullough PA, Ostermann M, Forni LG, Bihorac A, Koyner JL, Chawla LS, et al. the Sapphire Investigators. Serial urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 and the prognosis for acute kidney injury over the course of critical illness. Cardiorenal Med. 2019;9:358–369. doi: 10.1159/000502837. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino M, Keener C, Smith A, Kellum JA. Cell-cycle arrest biomarkers TIMP2*IGFBP7 predict worse outcomes in septic patients without clinical evidence of AKI [abstract] J Am Soc Nephrol. 2017;28:27. [Google Scholar]
- 15.US Renal Data System. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2016. [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter. 2012;2(Suppl):1–138. [Google Scholar]
- 17.Honore PM, Nguyen HB, Gong M, Chawla LS, Bagshaw SM, Artigas A, et al. Sapphire and Topaz Investigators. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016;44:1851–1860. doi: 10.1097/CCM.0000000000001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Sapphire Investigators. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29:2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH, IV, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol. 2017;312:F284–F296. doi: 10.1152/ajprenal.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2) · IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019;95:1486–1493. doi: 10.1016/j.kint.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, et al. Sapphire Investigators. Tissue inhibitor metalloproteinase-2 (TIMP-2)·IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng ZY, Wang HZ, Srisawat N, Wen X, Rimmelé T, Bishop J, et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012;40:538–543. doi: 10.1097/CCM.0b013e31822f0d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis*. Crit Care Med. 2014;42:e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzzi LM, Bergler T, Binnall B, Engelman DT, Forni L, Germain MJ, et al. Clinical use of [TIMP-2] · [IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care. 2019;23:225. doi: 10.1186/s13054-019-2504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FT., IV Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157:1545–1553, e5. doi: 10.1016/j.jtcvs.2018.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267:1013–1020. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.