The pharmacological strategy to manage chronic obstructive pulmonary disease (COPD), as recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), is to initiate treatment with long-acting bronchodilators, namely long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), alone or in combination (1). For patients with frequent COPD exacerbations and significant dyspnea despite these bronchodilators, treatment is intensified to triple therapy by adding inhaled corticosteroids (ICSs) (1). These recommendations have remained quite stable over time, although the 2019 recommendations introduce the use of blood eosinophil levels in the decision to add ICSs (2).
A global phenomenon, however, is the large gap between these recommendations and clinical practice, particularly in respect to the overuse of ICSs. In the United States, the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) found that 50% of patients were treated with nonindicated ICS-containing regimens (3). The POPE (Phenotypes of COPD in Central and Eastern Europe) study found that over 50% of nonexacerbators were using ICSs, including 37% on triple therapy (4). Apart from the absence of effectiveness, a major concern around such nonindicated ICS overuse is the increased risk of pneumonia and of other adverse events associated with ICS (5).
In response to these worldwide trends, the 2019 GOLD recommendations introduced the notion of withdrawing ICSs for these patients and stepping down to long-acting bronchodilators (2). This follows randomized trials and commentaries on the safety of ICS withdrawal in COPD (6–9). Recently, the European Respiratory Society presented evidence-based guidelines for ICS withdrawal in COPD (10).
In this issue of the Journal, Han and colleagues (pp. 1237–1243) report on a reanalysis of the IMPACT (Informing the Pathway of COPD Treatment) trial according to baseline ICS use to provide data on the effects of ICS withdrawal in COPD (11). The trial recruited over 10,000 patients with moderate to very severe COPD and a recent history of exacerbation, including patients with a history of asthma. At screening, 71% of the study patients were on an ICS-containing treatment (40% on triple therapy) for at least 3 months. Thus, IMPACT includes a large number (n = 7,360) of patients who had ICS abruptly withdrawn and were randomly assigned to either an ICS-based treatment (i.e., continue ICS) or a LAMA–LABA bronchodilator (i.e., withdraw ICS), providing an opportunity for Han and colleagues to assess the effects of ICS withdrawal on the risk of COPD exacerbation.
Han and colleagues present their results in terms of comparing triple with LAMA–LABA therapy, but we favor the alternative presentation based on reversing the estimates to produce the effects of ICS withdrawal, namely comparing LAMA–LABA with triple therapy, which more accurately reflects the paper’s title. Thus, among ICS users at baseline, the rate of moderate or severe exacerbations was significantly increased by 41%, and the rate of severe exacerbations was increased by 54% with LAMA–LABA (ICS withdrawal) compared with triple therapy (ICS continuation). This analysis of the IMPACT trial suggests that ICS withdrawal has a significant detrimental effect by increasing the frequency of moderate and severe exacerbations.
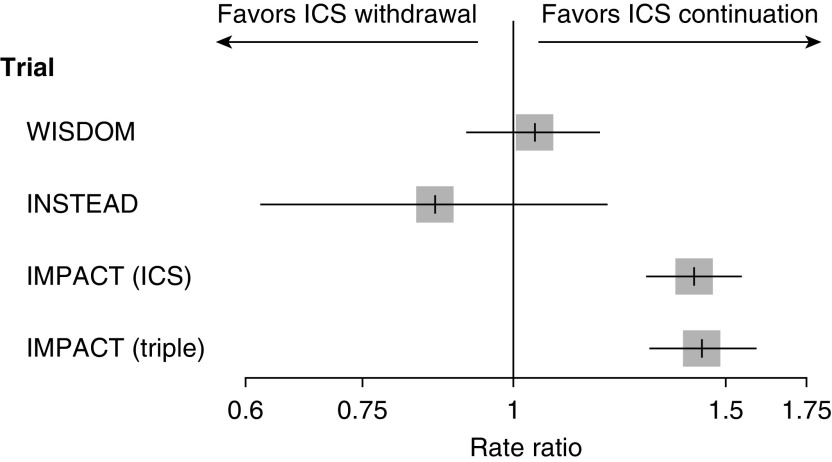
Two trials aimed specifically at evaluating the effects of ICS withdrawal in COPD provide useful comparisons. The WISDOM (Withdrawal of Inhaled Steroids during Optimized Bronchodilator Management) trial enrolled close to 2,500 patients with severe or very severe COPD and a history of at least one COPD exacerbation (6). All patients received triple therapy during a 6-week run-in period, after which they were randomly assigned to continue triple therapy or to withdraw the ICS component gradually over a 12-week period. Over the 12-month follow-up, the rate of moderate or severe COPD exacerbations was no different between ICS withdrawal and continuation groups (rate ratio, 1.04; 95% confidence interval [CI], 0.92–1.18; my calculation). The INSTEAD (Indacaterol: Switching Non-exacerbating Patients with Moderate COPD from Salmeterol/Fluticasone to Indacaterol) trial included 581 patients with moderate COPD and no COPD exacerbations in the previous year who received a LABA ICS for at least 3 months and then were randomly assigned to a LAMA or LABA ICS (7). Over the 6-month follow-up, the rate of moderate or severe COPD exacerbations was no different between ICS withdrawal and continuation (rate ratio, 0.86; 95% CI, 0.62–1.20). In contrast, the current reanalysis of the IMPACT trial suggests that the corresponding “ICS withdrawal” rate ratio is 1.41 (95% CI, 1.30–1.54) among prior users of any ICS and 1.43 (95% CI, 1.30–1.59) among prior users of triple therapy (Figure 1) (11).
Figure 1.
Rate ratio and 95% confidence interval of moderate or severe chronic obstructive pulmonary disease exacerbations comparing inhaled corticosteroid (ICS) withdrawal with ICS continuation from the WISDOM (Withdrawal of Inhaled Steroids during Optimized Bronchodilator Management), INSTEAD (Indacaterol: Switching Non-exacerbating Patients with Moderate COPD from Salmeterol/Fluticasone to Indacaterol), and IMPACT (Informing the Pathway of Chronic Obstructive Pulmonary Disease Treatment) trials, with the latter computed among all 7,360 users of ICSs and among the 2,406 users of triple therapy at baseline (6, 7, 11).
What could explain these large differences? First, the patient populations were different. WISDOM enrolled patients with an FEV1 of less than 50% predicted and a history of at least one COPD exacerbation in the previous year, which is identical to the first type of patients selected in IMPACT. However, IMPACT also included a second type of patients, with an FEV1 of 50% to 80% predicted and two or more moderate exacerbations or one severe exacerbation in the previous year. Thus, 100% of this GOLD 2 subgroup in IMPACT were frequent exacerbators compared with an expected 22%, as observed in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) cohort (12). Moreover, a history of asthma was permitted in IMPACT, but no such information was provided in WISDOM.
The INSTEAD trial included patients with an FEV1 of 50% to 80% predicted, no exacerbation in the previous year, and no history of asthma, whose ICS-based treatment for more than 3 months was not recommended. In contrast, for patients with this degree of airway obstruction, the IMPACT trial required frequent exacerbations and allowed a “history of asthma,” a significant risk factor for moderate and severe COPD exacerbations (12–14). A second explanation for the differences is the abruptness of ICS withdrawal in IMPACT compared with the gradual ICS dose reduction over the first 12 weeks of follow-up in WISDOM. The INSTEAD trial also had an abrupt withdrawal, though the patients did not have prior exacerbations and had been inappropriately treated with ICS.
A promising aspect of the IMPACT trial is its varied patient population. Indeed, the presence of moderate to very severe airway obstruction, frequent and less frequent exacerbations, history of asthma, and blood eosinophil counts are all significant risk factors for COPD exacerbations, thus allowing the identification of profiles of patients who would benefit from ICS withdrawal or continuation (12–15).
Another potentially strategic marker is the occurrence of an early exacerbation. Indeed, we previously showed that the difference in the occurrence of the first exacerbation between LAMA–LABA and triple therapy is only observed in the first month of follow-up, with equal occurrence in the subsequent 11 months (16, 17). This suggests that there is a subset of patients who are harmed early by ICS withdrawal, whereas the remaining patients are equally safe when stepped down to LAMA–LABA therapy. Figure E1 of Han and colleagues shows that early exacerbators (in the first month) represent approximately 20% of the 1,481 patients randomly allocated to LAMA–LABA, whereas for the remaining 80%, the effectiveness is similar between LAMA–LABA and triple therapy. The analysis of Han and colleagues that excludes early exacerbations, rather than early exacerbators (likely the frequent exacerbators), fall short of addressing this issue (11).
In all, this analysis of the IMPACT trial by Han and colleagues provides some useful information on the potential effects of ICS withdrawal on the risk of exacerbations in COPD. However, the skewed patient population resulted in greatly different results from trials specifically aimed at studying ICS withdrawal. Moreover, by pooling rather than splitting, this analysis fails to identify the key patient groups who could benefit from ICS withdrawal or from continuation. Indeed, stratified analyses by characteristics such as a history of asthma, GOLD grade severity of airway obstruction, exacerbation frequency, and the degree of eosinophilia, as well as a study of the early exacerbators could provide an informative model of precision medicine for COPD management (18, 19). Such a modern approach of targeted treatment could permit the identification of subsets of patients who will benefit from ICS withdrawal or its continuation, thus reducing unnecessary harms from the adverse effects of these drugs, particularly pneumonia (18).
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202006-2600ED on August 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease (2019 Report) Fontana, WI: Global Initiative for Chronic Obstructive Lung Disease; 2019 [accessed 2020 Sep 23]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf
- 3. Ghosh S, Anderson WH, Putcha N, Han MK, Curtis JL, Criner GJ, et al. Current and former investigators of the SPIROMICS sites and reading centers; Analysis of the Global Initiative for Chronic Obstructive Lung Disease Recommendations in SPIROMICS. Alignment of inhaled chronic obstructive pulmonary disease therapies with published strategies. Ann Am Thorac Soc. 2019;16:200–208. doi: 10.1513/AnnalsATS.201804-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koblizek V, Milenkovic B, Barczyk A, Tkacova R, Somfay A, Zykov K, et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE study. Eur Respir J. 2017;49:1601446. doi: 10.1183/13993003.01446-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J. 2015;45:525–537. doi: 10.1183/09031936.00128914. [DOI] [PubMed] [Google Scholar]
- 6. Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 7. Rossi A, van der Molen T, del Olmo R, Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44:1548–1556. doi: 10.1183/09031936.00126814. [DOI] [PubMed] [Google Scholar]
- 8. Suissa S, Rossi A. Weaning from inhaled corticosteroids in COPD: the evidence. Eur Respir J. 2015;46:1232–1235. doi: 10.1183/13993003.00282-2015. [DOI] [PubMed] [Google Scholar]
- 9. Calverley P. Knowing when to stop: inhaled corticosteroids and COPD. Eur Respir J. 2015;46:1236–1238. doi: 10.1183/13993003.01372-2015. [DOI] [PubMed] [Google Scholar]
- 10. Chalmers JD, Laska IF, Franssen FME, Janssens W, Pavord I, Rigau D, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55:2000351. doi: 10.1183/13993003.00351-2020. [DOI] [PubMed] [Google Scholar]
- 11. Han MK, Criner GJ, Dransfield MT, Halpin DMG, Jones CE, Kilbride S, et al. The effect of inhaled corticosteroid withdrawal and baseline inhaled treatment on exacerbations in the IMPACT study: a randomized, double-blind, multicenter clinical trial. Am J Respir Crit Care Med. 2020;202:1237–1243. doi: 10.1164/rccm.201912-2478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 13. Jamieson DB, Matsui EC, Belli A, McCormack MC, Peng E, Pierre-Louis S, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:187–192. doi: 10.1164/rccm.201211-2103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müllerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 15. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965–974. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 16. Suissa S, Ariel A. Triple therapy trials in COPD: a precision medicine opportunity. Eur Respir J. 2018;52:1801848. doi: 10.1183/13993003.01848-2018. [DOI] [PubMed] [Google Scholar]
- 17. Suissa S, Drazen JM. Making sense of triple inhaled therapy for COPD. N Engl J Med. 2018;378:1723–1724. doi: 10.1056/NEJMe1716802. [DOI] [PubMed] [Google Scholar]
- 18. Suissa S, Ernst P. Precision medicine urgency: the case of inhaled corticosteroids in COPD. Chest. 2017;152:227–231. doi: 10.1016/j.chest.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 19. Sidhaye VK, Nishida K, Martinez FJ. Precision medicine in COPD: where are we and where do we need to go? Eur Respir Rev. 2018;27:180022. doi: 10.1183/16000617.0022-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.