Abstract
Delayed union and nonunion are a significant concern in long bone fractures and spinal fusions. Treatment of nonunion often entails multiple revision surgeries that further increase the financial, physical, and emotional burden on patients. The optimal treatment strategy for nonunions remains unclear in many cases, and the risk of complications after revision procedures remains high. This is in part due to our limited understanding of the biological mechanisms that inhibit proper bone healing and lead to nonunion. And yet, few preclinical models directly investigate how healing is impacted after establishment of nonunion, with most instead primarily focusing on treatment immediately after a fresh bone injury. Here, we utilized a critical size femoral defect model in rats where treatment was delayed 8 weeks post-injury, at which time nonunion was established. In this study, acute and delayed treatments with bone morphogenetic protein-2 (BMP-2) were assessed. We found that delayed treatment resulted in decreased bone formation and reduced mechanical strength compared to acute treatment, even when BMP-2 dose was increased by 2.5 times the acute treatment dose. Interestingly, serum cytokine analysis at 12 weeks post-treatment revealed signs of chronic immune dysregulation after delayed treatment. In particular, non-responders (rats that did not exhibit defect bridging) demonstrated higher overall expression of inflammatory cytokines, including TNFα and IL-1β, compared to responders. These findings suggest that re-establishing long-term immune homeostasis may be critical for successful bone healing, particularly after nonunion.
Keywords: nonunion, delayed treatment, BMP-2, chronic immune dysregulation
Although there has been significant progress in the treatment of bone injuries, the incidence of delayed union and nonunion remains high—up to 10% after long bone injuries and 40% after spinal fusions.1–3 The clinical diagnosis of nonunion is often made when the injured bone demonstrates no radiographical evidence of union after 6 months and no visible signs of healing progression for 3 consecutive months.4,5 Nonunions can be further categorized as hypertrophic or atrophic depending on the level of callus formation. There are several known causes of nonunion including mechanical instability, inadequate vascularization, periosteal disruption, infection, and poor soft tissue coverage.6,7 Most hypertrophic nonunion cases are believed to be due to insufficient stabilization,7,8 and are readily corrected with proper fixation. Atrophic cases are not as simple, and usually require more extensive fixation, resection of nonviable bone and fibrous tissue, and additional delivery of grafts/biologics.9 This process is often conducted on a trial-and-error basis over the course of multiple revision surgeries, incurring long recovery times and increasing financial burden. This current standard of care highlights the fact that the underlying biological mechanisms of nonunion remain poorly understood and merit further investigation in appropriate preclinical models.
The vast majority of studies using preclinical animal models to investigate bone healing involve creation of an injury followed immediately by treatment. This is inconsistent with current clinical practice where large bone defect injuries are often not given reparative treatment immediately (outside of wound stabilization) or even within the first weeks after injury. In fact, less than 7% of orthopedic surgeons surveyed support the idea of immediate bone grafting to treat segmental defects.10 Instead, the Masquelet technique has been increasingly used to treat large bone defects,11,12 particularly in cases where soft tissue coverage and wound closure can be managed.13 The success of Masquelet has been attributed to its ability to bias the local microenvironment towards a pro-healing state prior to delivery of any regenerative treatment. The induced vascularized membrane that forms has been demonstrated to secrete many potent growth factors such as VEGF, TGFβ1, and BMP-2 and can also prevent resorption of bone grafts.14,15 In addition, the membrane properties can be tuned by changing the spacer material used.16 However, complications such as nonunion still occur in 18–29% of cases treated with Masquelet,17,18 highlighting the need to better understand the biological factors that lead to poor bone healing. Nonetheless, the increasing adoption of delayed treatment techniques like Masquelet is not currently reflected in most preclinical models, which motivates work to establish models that are more representative of these clinical scenarios. For critically-sized large bone defect models, in particular, there remains a great opportunity to characterize some of the biological changes that occur with delayed treatment and to provide further insight into some of the mechanisms that can lead to delayed union and nonunion.
In this study, we sought to establish a model of chronic nonunion using a critically-sized femoral bone defect in rats, wherein treatment was delayed until 8 weeks following the initial injury. This time point was chosen because we have historically seen mineralized capping of the bone ends by 8 weeks in this model for defects that do not heal, which is analogous to one of the clinical hallmarks of long bone nonunion. We hypothesized that delayed treatment would result in impaired bone healing compared to acute treatment. Moreover, we also hypothesized that poor healing in this chronic nonunion model would be associated with a dysregulated long-term immune response. Bone regeneration was evaluated through longitudinal radiographs and quantitative micro-computed tomography (μCT). The biomechanical strengths of the regenerated bones were assessed by torsional testing to failure. Histological characterization was used to further elucidate differences in the newly formed bone between acute and delayed treatment. Finally, end point characterization of immune cells and inflammatory cytokines were performed on isolated spleens and sera, respectively, for the delayed treatment samples.
MATERIALS AND METHODS
Animals
For these studies, 13-week-old female SASCO Sprague Dawley rats (Charles River Laboratories, Inc.) were used. Rats were pair housed in individually ventilated caging (Tecniplast) with a tunnel and gnawing blocks (Bio-Serv) for enrichment. Bedding was a mixture of corn cob and processed paper. Purina Mills International #5001 was fed ad libitum. Filtered tap water treated with ultraviolet light was provided ad libitum in bottles. Sentinel results from Charles River Laboratories International Rat Prevalent PRIA testing were negative for all pathogens in the housing room. All animals were allowed to acclimate for at least 2 weeks before any procedures were performed. Following each procedure, a divider was temporarily placed in the cage for better monitoring of post-operative recovery. Animals were randomly allocated to treatment groups. All procedures in this study were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee in compliance with Federal regulations governing the protection of animals in research.
Alginate BMP-2 Preparation
RGD-functionalized alginate (FMC BioPolymer) was reconstituted in MEM alpha (Thermo Fisher Scientific) to create a 2% w/v solution, as described previously.19 Recombinant human bone morphogenetic protein 2 (BMP-2, Pfizer Inc.) was reconstituted in a solution of 0.1% rat serum albumin (Sigma–Aldrich) in 4 mM hydrochloric acid and mixed with the alginate solution to yield 2 or 5 μg BMP-2 per 150 μl of final solution. This alginate/BMP-2 solution was gelled with the addition of calcium sulfate (Sigma–Aldrich) at a 1:25 volume ratio. Hydrogels were prepared under sterile conditions inside a laminar flow hood and stored overnight at 4°C before use in surgery the next day.
Surgical Procedures
Anesthesia was induced and maintained using isoflurane (Henry Schein Animal Health) inhalation. Prior to each procedure, all animals were given a subcutaneous injection of sustained-release buprenorphine (ZooPharm) for analgesia. Briefly, an anterolateral skin incision was made in the thigh followed by blunt dissection to separate the overlaying muscles to reach the femur bone. Limited extension of this muscle window allowed for placement of a radiolucent polysulfone fixation plate for internal stabilization. Critically-sized 8 mm defects were created in the mid-diaphysis of the femur using an oscillating saw. For the acutely treated animals, a poly-caprolactone (PCL, Sigma Aldrich) nanofiber mesh was carefully placed around the newly exposed bone ends and alginate loaded with BMP-2 was delivered via syringe injection through the mesh perforations. Subsequently the muscle and skin were closed using 4–0 vicryl suture and wound clips, respectively. In contrast, for the animals receiving delayed treatment, the bone defects were initially left empty (no treatment) and the muscle and skin were closed. At 8 weeks, a second procedure was performed on these animals where the original incision was re-opened to expose the fixation plate and femur. An oscillating saw was used to remove any mineralized end capping of the defects and any soft tissue ingrowth within the defect space was cleared to allow for placement of the PCL nanofiber mesh. Finally, alginate/BMP-2 was delivered and the muscle and skin were closed as before.
Radiography and Micro-Computed Tomography
To qualitatively assess longitudinal bone regeneration, 2D in vivo digital radiographs were acquired with an MX-20 digital machine (Faxitron X-ray Corp) at 2, 4, 8, and 12 weeks post-treatment. Bridging scores were assigned to each radiograph by two blinded investigators where bridging was defined as continuous bone spanning the entire defect space (from bone end to bone end). In instances of disagreement, a third blinded investigator served as tiebreaker. This binary scoring system for bridging was used as it best corresponds to the transition point in biomechanical function associated with bridging. Longitudinal bone formation was quantitatively evaluated using 3D micro-computed tomography (μCT) at 4, 8, and 12 weeks post-treatment. In vivo scans of femurs were performed using the vivaCT40 (Scanco Medical) at a 38 μm voxel size, 55 kVp voltage, and a 145 μA current. A threshold corresponding to 50% of native cortical bone density was applied to segment bone mineral and identify newly regenerated bone, as established previously.20 The volume of interest (VOI) consisted of the central 5.89 mm (155 slices) of the 8 mm defect. The sample sizes for each group were: Acute, 2 μg (n = 10), Delayed, 2 μg (n = 12), Delayed, 5 μg (n = 12).
Biomechanical Testing
Torsional testing to failure was performed as previously described.20,21 Femurs were excised at 12 weeks post-surgery, wrapped in PBS-soaked gauze, and stored at −20°C until testing could be performed. On the day of testing, samples were thawed, the surrounding soft tissues were excised, and the femurs were first μCT-scanned, as described above. Subsequently, the fixation plate was removed so that the native bone ends could be potted in Wood’s metal (Alfa Aesar). The potted femurs were tested to failure in torsion at a rotation rate of 3° per second using the EnduraTEC ELF3200 axial/torsion testing system (Bose). Failure strength was determined by locating the peak torque within the first 60° of rotation. Torsional stiffness was calculated by finding the slope of the linear region before failure in the torque-rotation plot. The sample sizes for each group were: Acute, 2 μg (n = 10), Delayed, 2 μg (n = 12), Delayed, 5 μg (n = 12), Intact Control (n = 30).
Histological Analyses
Representative samples from each treatment group (including responder and non-responder for delayed treatment groups) were harvested post-mechanical testing and fixed in 10% neutral buffered formalin at room temperature for 48 h. Samples were then switched to PBS and sent to HistoTox Labs (Boulder, CO) for decalcification, processing, and Hematoxylin and Eosin (H&E) and Safranin-O staining. Images of stained sections were taken on a Nikon Eclipse E600 upright microscope at 10× magnification using Q Imaging software. All images were taken in the middle of the bone defect.
Serum and Spleen Collection
Blood was collected by cardiac stick (~3ml per sample) at 12 weeks post-treatment, and transferred into serum collection tubes (Thermo Fisher Scientific) and allowed to clot at room temperature for 30 min before storage at 4°C overnight. The following day, all tubes were centrifuged at 1500g for 10 min and the yellow (straw) serum was collected and stored at −20°C. Spleens harvested at 12 weeks post-treatment were minced and frozen in cryopreservation media (80% FBS, 20% DMSO) at −80°C overnight before being transferred to a liquid nitrogen dewar for storage.
Univariate and Multivariate Analyses of Serum Cytokines
Sera collected at 12 weeks post-treatment were diluted to 80% and analyzed with the Milliplex MAP Rat Cytokine/Chemokine Magentic kit (Millipore Sigma). The assays were read using a MAGPIX Luminex instrument (Luminex). Conventional univariate analysis by t-test was performed on the median fluorescent intensity values read by the machine (with background subtracted). Discriminant partial least squares regressions (D-PLSR) were conducted in MATLAB (Mathworks) using the partial least squares algorithm by Cleiton Nunes (available on the Mathworks File Exchange). The data were z-scored (mean subtracted and normalized to standard deviation for each cytokine) before being passed into the algorithm. This multivariate method requires scale-free data so that the analysis would not be biased towards cytokines with extremely high values. An orthogonal rotation in the LV1-LV2 plane was used to define the axis of best separation between responders and non-responders (rats that did/did not demonstrate radiographic defect bridging, respectively). A Monte Carlo sub-sampling using 1000 iterations was used to characterize standard deviation on the individual signals involved in LV1 of the D-PLSR model (total responder/non-responder dataset). For each iteration, 83% (20/24) of the samples used to construct the total D-PLSR model were randomly sampled, and a new D-PLSR model was constructed. To correct for sign reversals, each sub-sampled LV1 was multiplied by the sign of the scalar product of the new LV1 and the corresponding LV1 from the total model. The same orthogonal rotation used for the total model was applied to the LV1s from each iteration, and mean and standard deviation was computed for each signal across all iterations. The sample sizes for each group were: Non-responders (n = 9) and Responders (n = 15).
Characterization of Immune Cells by Flow Cytometry
Cryopreserved spleens were thawed and digested in 2 ml Opti-MEM I reduced serum medium (Thermo Fisher Scientific) with 2 mg/ml collagenase B (Sigma–Aldrich) for an hour at room temperature. The digested tissues were then passed through 40 μm cell strainers (Thermo Fisher Scientific) and centrifuged at 500g for 5 min. Red blood cells were removed by brief incubation in RBC lysis buffer (eBioscience) and the remaining cells were resuspended in flow cytometry staining buffer (PBS containing 2% FBS) for antibody staining. Cells were stained with anti-Rat antibodies (Thermo Fisher Scientific) for CD3, CD4, CD8, FoxP3, His48, CD11b, His36, and B220 using manufacturer’s recommended protocols, and all samples were then run through an Accuri C6 flow cytometer (BD Biosciences). All data were exported for analysis in FlowJo software. The sample sizes for each group were: Non-responders (n = 9) and Responders (n = 14).
Statistical Analyses
All data are reported as mean ± standard error of the mean. Significance was determined by two-tailed t-test or analysis of variance (ANOVA) as appropriate, with multiple comparisons made by Tukey’s post-hoc test. In cases of unequal variances (according to F test/Bartlett’s test), the t-test with Welch’s correction or Kruskal–Wallis with Dunn’s post-hoc test were used instead. Significance was determined by a p < 0.05. All statistical calculations were performed using GraphPad Prism 7 software. Sample sizes were determined by performing a power analysis in G*Power software based on bone volume and maximum torque results obtained from previous studies.
RESULTS
Delayed Treatment Results in Impaired Bone Regeneration
Radiographs at 12 weeks post-treatment demonstrated clear differences between acute and delayed treatment (Fig. 1). All acute 2 μg treated defects achieved radiographic bridging while only 50% of delayed 2 μg and 75% of delayed 5 μg treated defects did so. These qualitative observations were supported by μCT quantification of new bone formation. The acutely-treated defects had significantly more bone formation at weeks 4, 8, and 12 compared to both delayed treatment groups (Fig. 2A). There were no observed differences in bone mineral density between any treatment groups, but density increased over time for all groups as expected (Fig. 2B).
Figure 1.
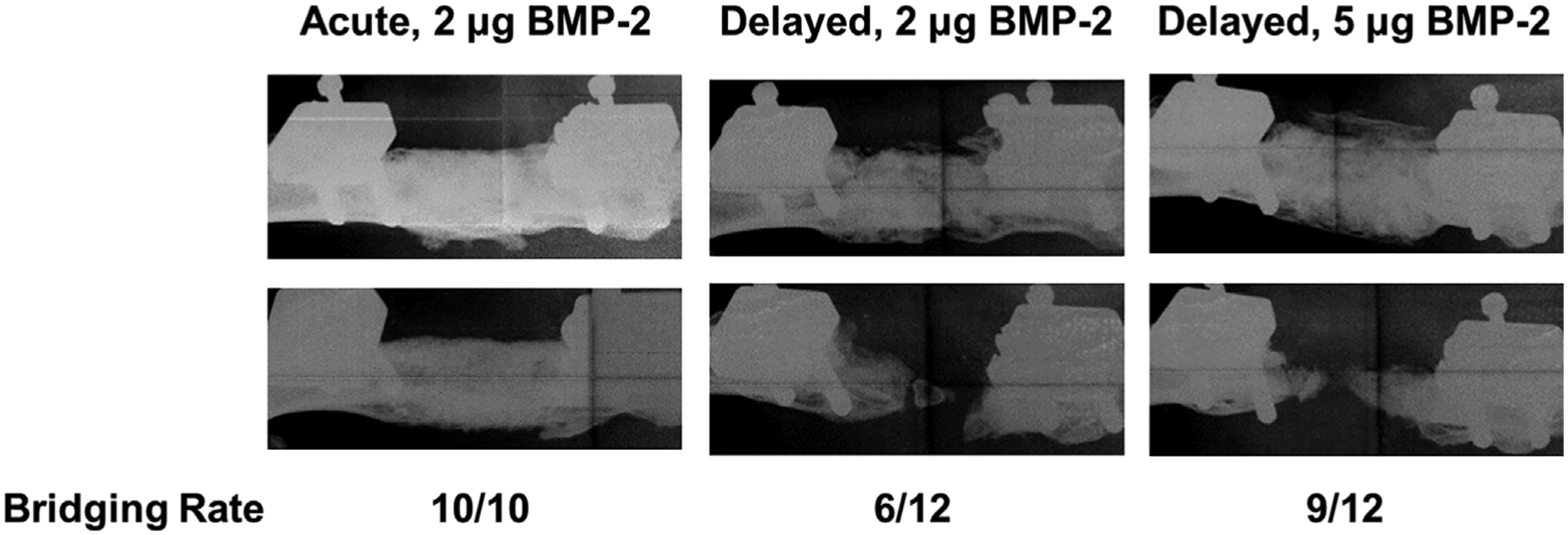
Acute treatment results in qualitatively better bone formation compared to either delayed treatment group. Radiographs at 12 weeks post-treatment (two representative images from each group shown here) revealed much more variability in bone formation with delayed treatment. This was reflected in the bridging scores for each group as 100%, 50%, and 75% of defects were bridged by 12 weeks for acute 2 μg, delayed 2 μg, and delayed 5 μg treatment groups, respectively.
Figure 2.
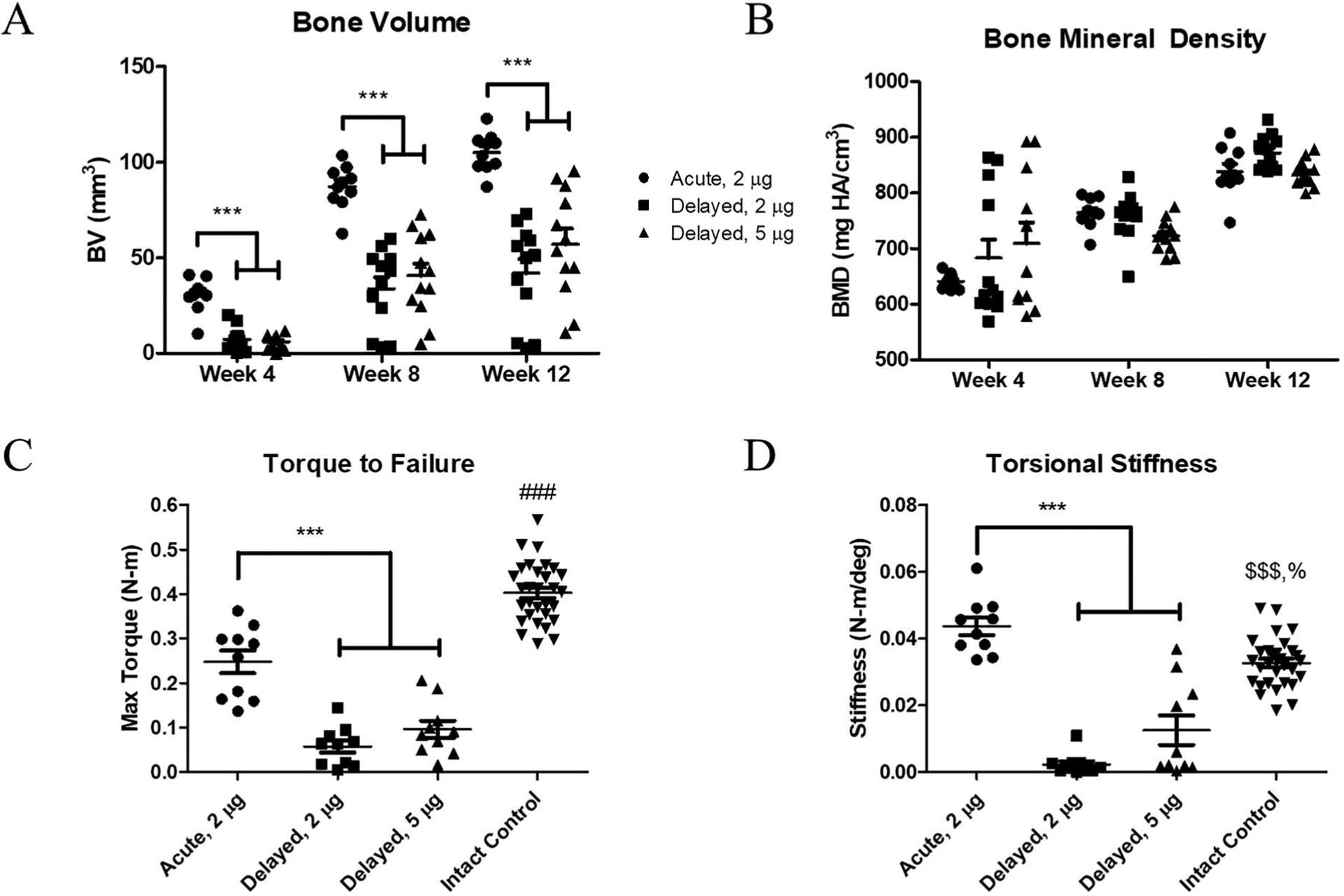
Acutely-treated defects demonstrated better bone regeneration as quantified by μCT and biomechanical testing. Acute treatment with 2 μg BMP-2 yielded more bone formation at all time points compared to delayed treatment, at both 2 and 5 μg (A). There were no observed differences in bone mineral densities (B). Acutely-treated defects also demonstrated higher max torque (C) and torsional stiffness (D) compared to both delayed treatment groups at week 12 post-treatment. Compared to intact control femurs, regenerated defects given acute treatment exhibited decreased max torque and increased torsional stiffness. ***p < 0.001 as indicated, $$$p < 0.001 versus Delayed, 2 μg, %p < 0.05 versus Delayed, 5 μg, ###p < 0.001 versus all other treatment groups by ANOVA with Tukey’s post-hoc test or the nonparametric equivalent Kruskal–Wallis with Dunn’s post-hoc test in cases of unequal variances (Torsional Stiffness, D), n = 10–12/treatment groups, n = 30 for intact control group.
Bone Defects Receiving Delayed Treatment Exhibit Reduced Mechanical Strength
Functional assessment of the newly regenerated bones by mechanical testing was consistent with the μCT analysis results. The femurs that received acute treatment demonstrated greater torque to failure (Fig. 2C) and higher torsional stiffness (Fig. 2D) compared to both delayed treatment groups. All treated groups had significantly lower torque to failure compared to intact control femurs at 12 weeks post-treatment. However, the acutely-treated defects were significantly stiffer than intact controls at this time point.
Diminished Areas of New Bone Following Delayed Treatment
H&E staining (Fig. 3) revealed several interesting morphological differences. Acute treatment resulted in many larger areas of new bone formation throughout the defect space, while delayed treatment (at both 2 and 5 μg BMP-2 doses) resulted in smaller pockets of new bone. Furthermore, H&E also suggested minimal presence of hypertrophic chondrocytes in acutely-treated defects at 12 weeks post-treatment, whereas chondrocytes were more apparent in the delayed treated samples, particularly the delayed 2 μg group. These areas of cartilage formation were confirmed by Saf-O staining (Fig. 3), which showed abundant cartilage present in the non-responder sample of the delayed 2 μg group. Interestingly, Saf-O staining revealed that alginate remaining in the defect with acute treatment was primarily located within newly mineralized bone, whereas residual alginate fragments in delayed treatment samples were mostly isolated or surrounded by soft tissue.
Figure 3.

Bone defect histology at 12 weeks post-treatment showed clear differences between the treatment groups. Representative hematoxylin and eosin staining (the images on the left for each pair) revealed more widespread and larger areas of new bone formation with acute treatment compared to either delayed treatment group. Safranin-O staining (the images on the right for each pair) indicated greater presence of chondrocytes still remaining within the defect space for delayed treatment, particularly at the 2 μg BMP-2 dose level.
Pro-Inflammatory Serum Cytokines Are Upregulated in Non-Responders
At 12 weeks post-treatment, serum was collected for all samples that received delayed treatment and subsequently analyzed across a panel of inflammatory cytokines (Fig. 4). Discriminant partial least squares regression (D-PLSR) was used to identify a profile of cytokines that together distinguished between responders and non-responders, that is, whether or not the defect was successfully bridged (Fig. 5A). The analysis revealed an axis, LV1, that separated responders to the left and non-responders to the right. The LV1 axis (Fig. 5B) described cytokines that were upregulated with non-responders (positive bars) as well as responders (negative bars). Overall, non-responders demonstrated increased expression of inflammatory cytokines such as IL-1β, GM-CSF, IL-2, and IFNɣ, as well as the anti-inflammatory cytokine IL-10 (Fig. 5B). Univariate analysis of individual top LV1 correlates revealed that non-responders had significantly higher levels of TNFα and IL-1β in serum at 12 weeks post-treatment (Fig. 6). No significant differences were observed between BMP-2 doses (not shown).
Figure 4.
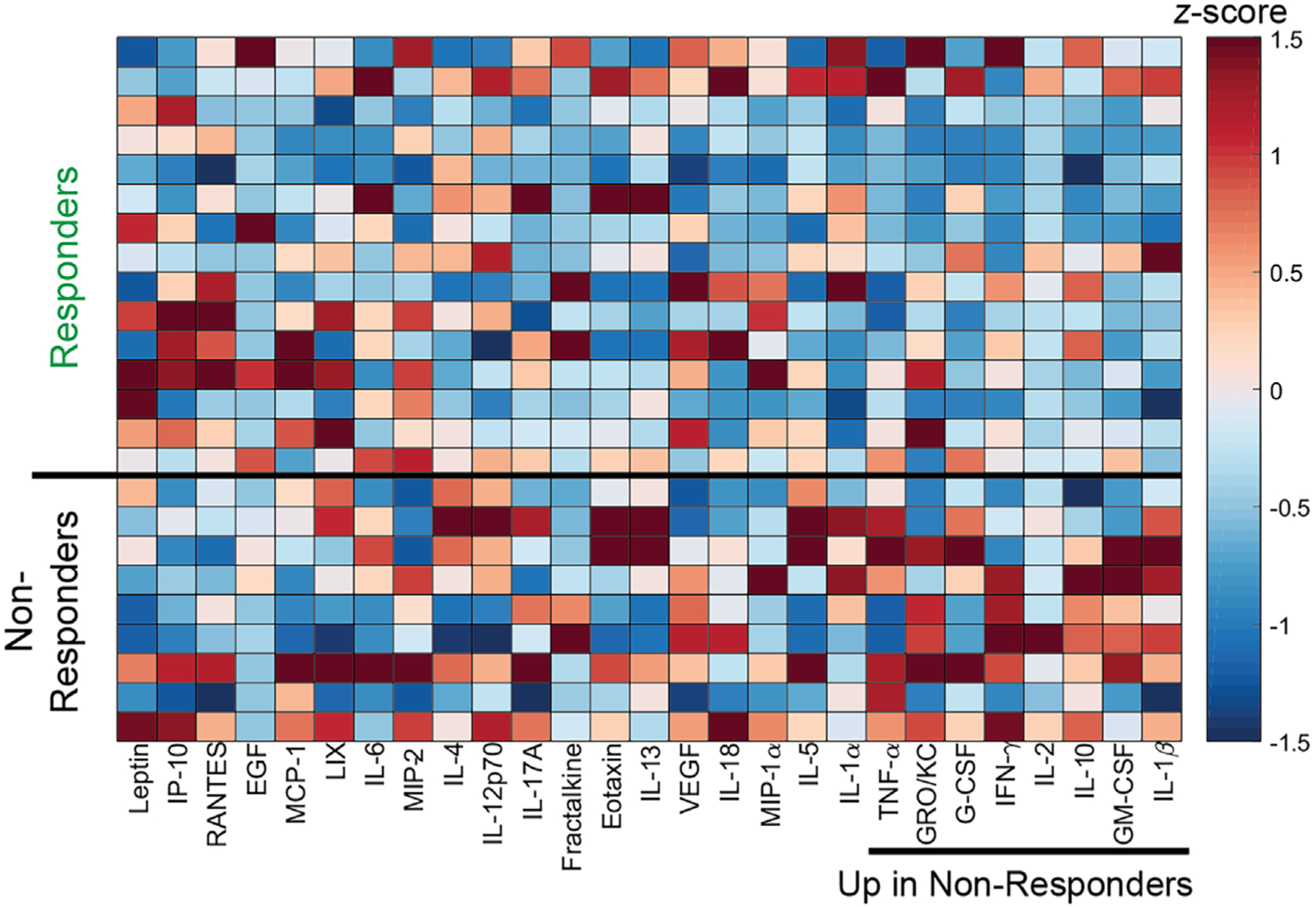
Heatmap of z-scored cytokine expression profiles shows differences between responders (R) and non-responders (N). Serum cytokine expression at 12 weeks post-treatment for delayed treatment samples was quantified via multiplex Luminex assay. For these analyses, samples were pooled and then categorized into responders or non-responders according to whether the defects exhibited radiographic bridging by 12 weeks.
Figure 5.
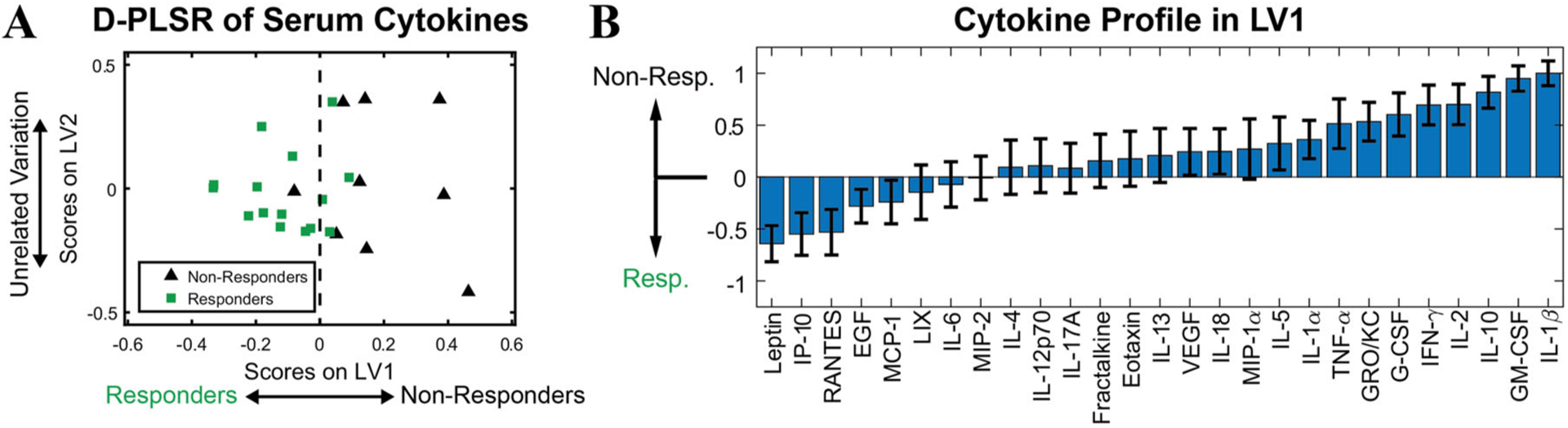
Discriminant partial least squares regression (D-PLSR) of z-scored serum cytokine expression was performed for delayed treatment samples at 12 weeks post-treatment. The regression established latent variable 1 (LV1) that separates samples into responder and non-responder groups (A). LV1 defines the profile of cytokines correlated with each group and shows that non-responders were positively correlated with expression of many inflammatory and anti-inflammatory cytokines, including IL-1β, GM-CSF, and IL-10 (B).
Figure 6.
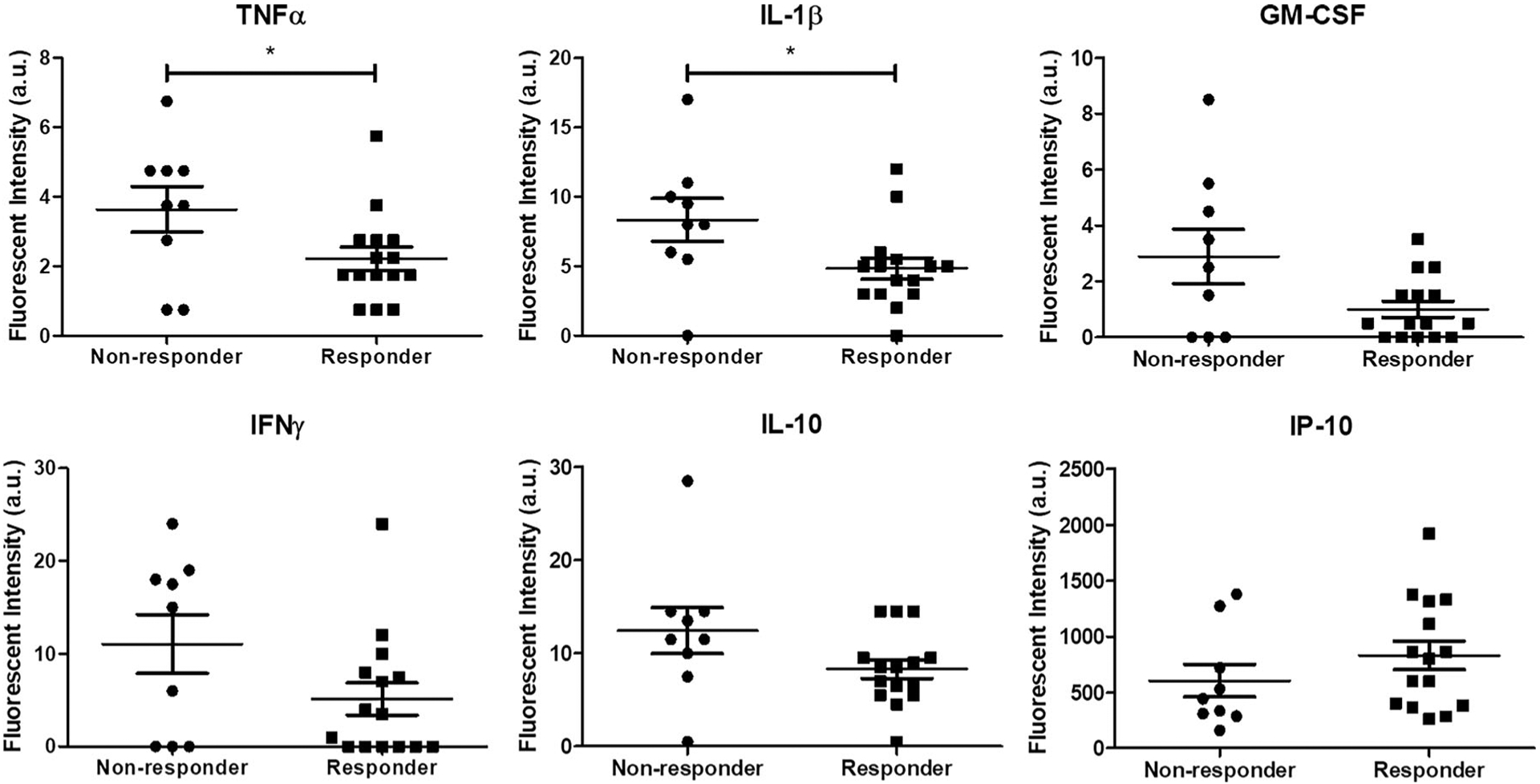
Sera from non-responders demonstrated elevated expression of inflammatory cytokines at 12 weeks post-treatment. Cytokine differences between non-responders and responders for six out of the twenty seven cytokines tested are shown here. TNFα and IL-1β levels in non-responders were significantly higher than those in responders. No other significant differences were observed. *p < 0.05 as indicated by two-tailed t-test or t-test with Welch’s correction in cases of unequal variances (GM-CSF, IL-10), n = 9–15/group.
DISCUSSION
The pathophysiology of nonunion remains poorly understood. While some causes, such as improper mechanical fixation, have been identified and are fairly easy to correct, most others including infection, poor vascularization, and other co-morbidities are much harder to address, and the optimal interventions for these cases remain unclear.2,22,23 Development of reproducible animal models to study impaired bone healing has been difficult, particularly when deliberate mechanical instability is not the main driving force of nonunion.24
Here, we utilized a critically-sized femoral defect in rats to establish a model of chronic nonunion by delaying treatment with BMP-2 until after nonunion has been established. We found that delayed treatment results in impaired bone regeneration compared to acute treatment and this deficit in healing was not overcome with a modest increase in BMP-2 dose. The acute 2 μg BMP-2 group still formed significantly more bone and had mechanically stronger regenerated femurs compared to the delayed 5 μg BMP-2 group. We have previously shown a clear dose dependency in BMP-2-mediated bone regeneration for this range of doses for acute treatment.25 These results suggest that the local healing environment of a regenerating bone may be substantially altered both during the acute response phase and after nonunion has been established. This observation is supported by other studies that investigated nonunion. In particular, recent clinical work analyzing human fracture biopsies demonstrated changes in the local expression of BMP and BMP inhibitors.26 Interestingly, the relative proportion of these factors was indicative of subsequent healing outcome. These findings were consistent with those made by another group, which also observed differences in BMP and BMP inhibitor expression in the chondrocytes present in fracture callus and non-union tissues.27 Furthermore, elevated levels of certain matrix remodeling proteins, such as MMP-7 and MMP-12, have also been implicated in fracture non-union in humans, and these proteins were shown to actively bind and degrade BMP-2 in vitro.28
While not directly assessed in this study, it is likely that the reparative capacity of local stem and osteoprogenitor cells are diminished after nonunion. Indeed, recent work by Bajada et al. demonstrated that stromal cells isolated from human fracture nonunions exhibited reduced osteogenic and mineral-forming capacity as well as increased senescence compared to control bone marrow mesenchymal stromal cells.29 Multiple other groups have also shown that proliferation of stem cells as well as osteoblasts is impaired during nonunion.30,31 Taken together, it appears that osteogenesis is impaired during nonunion due to a reduction in the progenitor cells available at the site of injury and diminished ability of those cells that are present to differentiate into osteoblasts and form bone.
Safranin-O staining showed greater presence of chondrocytes within the defect space for the delayed treatment group at 12 weeks post-treatment compared to the same time point for acutely-treated animals. This suggests an alteration in the normal endochondral ossification healing process, whereby treatment after nonunion induces a delayed or prolonged period of cartilage deposition that subsequently further delays the onset of mineralization. This may be verified in future studies by histological characterization at multiple time points during healing. It is unlikely that the increased presence of cartilage is due to poor vascularization since the nonunion sites were highly vascularized at the time of treatment and the bone end caps were removed to create a fresh wound site.
Multivariate protein analysis of week 20 serum samples for the delayed treatment groups revealed that non-responders correlated with increased expression of many inflammatory factors. These long-term results suggest a chronically dysregulated immune response in the animals that exhibited the worst healing responses. Our observation is consistent with prior work from other groups, which showed persistently elevated levels of pro-inflammatory cytokines, including TNFα and IL-1, were detrimental to bone healing by enhancing osteoclast and inhibiting osteoblast activity.32–34 Furthermore, others have described similar findings clinically in response to trauma.35–37 Vanzant et al. observed an increase in inflammatory gene expression with patients that experienced a complicated recovery (>14 days) compared to those that had a relatively uncomplicated recovery (<5 days) from multiple organ injury.38
A limitation of the immune characterization work performed in this study was that only a single terminal time point (12 weeks post-treatment) was analyzed. At this late time point, we found no significant differences in spleen immune cell populations (Supplemental Fig. S1). It is possible the bone injury alone in this model is not traumatic enough to cause severe systemic immune dysfunction. In fact, recent work in a burn injury model demonstrated that splenic inflammatory monocyte populations were only increased through 8 days postburn.39 Only in a severe model of sepsis has widespread splenic alterations been observed beyond 14 days.40 Apart from spleen characterization, immune cell profiling of a more proximal target to the femur, such as the inguinal lymph nodes, bone marrow, and even the surrounding quadriceps muscle may have been more informative.
It remains to be seen if delivering higher doses of BMP-2 would further enhance healing in this chronic nonunion model. While it is likely that higher doses would result in improved bridging rates, increasing BMP-2 dose also increases the risk for complications, such as heterotopic ossification and excessive inflammation. In fact, we have observed some of these adverse effects in previous studies that delivered 10 and 30 μg BMP-2 to the rat segmental defect.21,41 Based on historical experience,25,42 the 5 μg dose utilized in this study was chosen as the ideal minimum dose for healing in rats with low risk of complications.
More work is needed to validate long-term immune dysregulation in this model of chronic nonunion. Longitudinal immune characterization at multiple time points post-treatment would help elucidate the progression of immune dysfunction and may reveal time points for early intervention. Moreover, these results could potentially motivate new approaches for the clinical treatment of nonunion, such as immunomodulatory therapy prior or in conjunction with a bone-healing therapy to improve patient outcomes.
In conclusion, the study presented here established a chronic nonunion model involving delayed treatment to critical size femoral defects in rats. Our results qualitatively and quantitatively demonstrated impaired healing compared to acute treatment, and this impairment could not be overcome with a 2.5× increase in BMP-2 dose. Interestingly, our multivariate analysis of serum cytokines revealed that delayed treatment animals with the worst healing response were associated with increased circulating levels of pro-inflammatory cytokines, including TNFα and IL-1β. These results suggest that the long-term systemic immune response may be indicative of bone healing outcome, which motivates more in-depth investigation into the role of immune homeostasis in bone regeneration.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported under the AFIRM II (U.S. Armed Forces Institute of Regenerative Medicine) effort, Award No. W81XWH-14-2-0003. The U.S. Army Medical Research Acquisition Activity was the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. L.D.W. was supported in part by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32-GM008433). The authors would like to thank all members of the Guldberg lab for assistance with surgeries. The rhBMP-2 used in this study was provided by Pfizer Inc.
Footnotes
Conflict of interest: None.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Boden SD. 2002. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (PhilaPa 1976) 27:S26–S31. [DOI] [PubMed] [Google Scholar]
- 2.Green E, Lubahn JD, Evans J. 2005. Risk factors, treatment, and outcomes associated with nonunion of the mid-shaft humerus fracture. J Surg Orthop Adv 14:64–72. [PubMed] [Google Scholar]
- 3.Tzioupis C, Giannoudis PV. 2007. Prevalence of long-bone non-unions. Injury 38:S3–S9. [DOI] [PubMed] [Google Scholar]
- 4.Goel A, Sangwan SS, Siwach RC, et al. 2005. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury 36:203–206. [DOI] [PubMed] [Google Scholar]
- 5.Hernigou P, Poignard A, Beaujean F, et al. 2005. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–1437. [DOI] [PubMed] [Google Scholar]
- 6.Kokubu T, Hak DJ, Hazelwood SJ, et al. 2003. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res 21:503–510. [DOI] [PubMed] [Google Scholar]
- 7.Megas P 2005. Classification of non-union. Injury 36: S30–S37. [DOI] [PubMed] [Google Scholar]
- 8.Iwakura T, Miwa M, Sakai Y, et al. 2009. Human hypertrophic nonunion tissue contains mesenchymal progenitor cells with multilineage capacity in vitro. J Orthop Res 27: 208–215. [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Kanakaris NK, Dimitriou R, et al. 2009. The synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop Relat Res 467:3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obremskey W, Molina C, Collinge C, et al. 2014. Current practice in the management of open fractures among orthopaedic trauma surgeons. Part B: management of segmental long bone defects. A survey of orthopaedic trauma association members. J Orthop Trauma 28:e203–e207. [DOI] [PubMed] [Google Scholar]
- 11.Giannoudis PV, Faour O, Goff T, et al. 2011. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury 42:591–598. [DOI] [PubMed] [Google Scholar]
- 12.Masquelet AC, Begue T. 2010. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 41:27–37. [DOI] [PubMed] [Google Scholar]
- 13.Muhlhausser J, Winkler J, Babst R, et al. 2017. Infected tibia defect fractures treated with the Masquelet technique. Medicine (Baltimore) 96:e6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelissier P, Masquelet AC, Bareille R, et al. 2004. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res 22:73–79. [DOI] [PubMed] [Google Scholar]
- 15.Christou C, Oliver RA, Yu Y, et al. 2014. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS ONE 9:e114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaio N, Martino A, Toth Z, et al. 2018. Masquelet technique: the effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J Biomech 72:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auregan JC, Begue T, Rigoulot G, et al. 2016. Success rate and risk factors of failure of the induced membrane technique in children: a systematic review. Injury 47:S62–S67. [DOI] [PubMed] [Google Scholar]
- 18.Morelli I, Drago L, George DA, et al. 2016. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury 47:S68–S76. [DOI] [PubMed] [Google Scholar]
- 19.Kolambkar YM, Dupont KM, Boerckel JD, et al. 2011. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 32:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oest ME, Dupont KM, Kong HJ, et al. 2007. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res 25:941–950. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A, Krishnan L, Tran L, et al. 2018. The effects of age and dose on gene expression and segmental bone defect repair after BMP-2 delivery. JBMR Plus 0 [Epub ahead of print]. 10.1002/jbm4.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills L, Tsang J, Hopper G, et al. 2016. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res 5:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santolini E, West R, Giannoudis PV. 2015. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury 46: S8–S19. [DOI] [PubMed] [Google Scholar]
- 24.Kratzel C, Bergmann C, Duda G, et al. 2008. Characterization of a rat osteotomy model with impaired healing. BMC Musculoskelet Disord 9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerckel JD, Kolambkar YM, Dupont KM, et al. 2011. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 32:5241–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong FN, Hoyland JA, Freemont AJ, et al. 2009. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards non-union. J Orthop Res 27:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kloen P, Lauzier D, Hamdy RC. 2012. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 51:59–68. [DOI] [PubMed] [Google Scholar]
- 28.Fajardo M, Liu CJ, Ilalov K, et al. 2010. Matrix metal-loproteinases that associate with and cleave bone morphogenetic protein-2 in vitro are elevated in hypertrophic fracture nonunion tissue. J Orthop Trauma 24:557–563. [DOI] [PubMed] [Google Scholar]
- 29.Bajada S, Marshall MJ, Wright KT, et al. 2009. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45:726–735. [DOI] [PubMed] [Google Scholar]
- 30.Tawonsawatruk T, Kelly M, Simpson H. 2014. Evaluation of native mesenchymal stem cells from bone marrow and local tissue in an atrophic nonunion model. Tissue Eng Part C Methods 20:524–532. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann A, Ritz U, Hessmann MH, et al. 2008. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone 42:894–906. [DOI] [PubMed] [Google Scholar]
- 32.Loi F, Cordova LA, Pajarinen J, et al. 2016. Inflammation, fracture and bone repair. Bone 86:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert L, He X, Farmer P, et al. 2000. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 141:3956–3964. [DOI] [PubMed] [Google Scholar]
- 34.Guo R, Yamashita M, Zhang Q, et al. 2008. Ubiquitin ligase Smurf1 mediates tumor necrosis factor-induced systemic bone loss by promoting proteasomal degradation of bone morphogenetic signaling proteins. J Biol Chem 283: 23084–23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord JM, Midwinter MJ, Chen YF, et al. 2014. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet 384:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentile LF, Cuenca AG, Efron PA, et al. 2012. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura F, Shimizu H, Yoshidome H, et al. 2010. Immunosuppression following surgical and traumatic injury. Surg Today 40:793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. 2014. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg 76:21–29; discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noel JG, Osterburg A, Wang Q, et al. 2007. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 28:684–693. [DOI] [PubMed] [Google Scholar]
- 40.Delano MJ, Scumpia PO, Weinstein JS, et al. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204:1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan L, Priddy LB, Esancy C, et al. 2017. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater 49: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolambkar YM, Boerckel JD, Dupont KM, et al. 2011. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone 49: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


