Abstract
Advances in single-cell RNA-seq (scRNA-seq) and computational analysis have enabled the systematic interrogation of the cellular composition of tissues. Combined with tools from developmental biology, cell biology, and genetics, these approaches are revealing fundamental aspects of tissue geometry and physiology, including the distribution, origins, and inferred functions of specialized cell types, and the dynamics of cellular turnover and differentiation. By comparing different tissues, such studies can delineate shared and specialized features of cell types and their lineage. Here, we compare two developmentally related murine epithelia, the airway and the small intestinal epithelia, which are both derived from the embryonic endodermal gut tube. We examine how airway and intestine generate and functionalize common archetypal cell types to fulfill similar shared physiologic functionalities. We point to cases in which similar cell types are repurposed to accommodate each tissue's unique physiologic role, and highlight tissue-specific cells whose specializations contribute to the distinct functional roles of each organ. We discuss how archetypal and unique cell types are incorporated within a cellular lineage, and how the regulation of the proportions of these cell types enables tissue-level organization to meet functional demands and maintain homeostasis.
Epithelia are sheets of polarized cells that serve to partition the body's internal milieu from the outside environment. During vertebrate development, all three primordial germ layers give rise to epithelia that serve a panoply of essential functions, including barrier maintenance, absorption and secretion, sensation, and immunity.
The diverse cells comprising a tissue must function together in a well-orchestrated manner to meet the tissue's functional demands and maintain homeostasis (Kotas and Medzhitov 2015). During evolution, distinct epithelia have redeployed several archetypal cell types. We broadly define an archetypal cell as a cell type that, despite its occurrence in distinct tissues, is easily recognized because of the presence of a shared set of recurring molecular and/or morphologic motifs that allow the given archetypal cell to subtend a shared epithelial function that is required in diverse physiologic contexts. Not all epithelia take advantage of every archetype cell. Moreover, epithelia also rely upon “unique” cell types (Chan et al. 2009).
Despite the fact that cells are the fundamental units of tissue structure, epithelial classification thus far has not been based on cell-type composition per se. Instead, the classical categorization of epithelia is based on tissue architecture and location in the body (Table 1; Wheater and Burkitt 1987). In the near future, we expect that epithelia will be increasingly understood through the lens of a newfound molecular understanding of each component cell. Just as cytology moved from morphologic to molecular definitions of cell types, we can now anticipate moving from morphologic to molecular definitions of tissues as composite structures.
Table 1.
Structural classification of epithelia
| Structural type | Organ |
|---|---|
| Simple cuboidal | Small ducts of kidney and pancreas |
| Simple columnar (ciliated) | Female reproductive tract |
| Simple columnar (nonciliated) | Small intestine, Stomach |
| Pseudostratified columnar (ciliated) | Large airways of the respiratory system |
| Simple squamous | Alveolar lung, endothelium, mesothelium |
| Stratified squamous | Oral cavity, pharynx, esophagus |
| Stratified squamous (keratinizing) | Skin |
| Stratified cuboidal | Excretory ducts of salivary glands and sweat glands |
| Transitional | Urothelium |
Data based on Wheater and Burkitt (1987).
The molecular definition of tissues is now beginning with the systematic identification of all the component cell types of a tissue through comprehensive profiling of each cell type's characteristic expression patterns (Regev et al. 2017). In particular, massively parallel single-cell genomics, especially single-cell RNA-seq (scRNA-seq) and single-cell assay for transposase accessible chromatin-seq (scATAC-seq), currently provide the most readily available methods for comparing distinct cell types, even for rare cells, and aligning cells by their features across tissues. Profiling methods allow us to recover comprehensive sets of expressed marker genes with no prior knowledge, in contrast to the one to four preselected markers classically deployed in developmental biology and histopathology. Furthermore, computational analysis of scRNA-seq and scATAC-seq data allows for the modeling of complex composite properties of tissues, such as networked intercellular signaling within a tissue and lineage (Chen et al. 2019; Luecken and Theis 2019). In this way, we can assemble an aggregate picture of how component cells contribute to specific physiologic functions, how those cells establish an ancestor–descendent order, whether those cell states are plastic, how these properties change with perturbation or disease, and, finally, how intercellular interactions within the tissue and between tissues orchestrate global physiology (Kotas and Medzhitov 2015). With the advances in spatial genomics methods (Ståhl et al. 2016; Moor and Itzkovitz 2017; Rodriques et al. 2019), we should further expect enhanced integrated understanding of the cellular and histological levels.
THE AIRWAY AND INTESTINAL EPITHELIAL ENSEMBLES
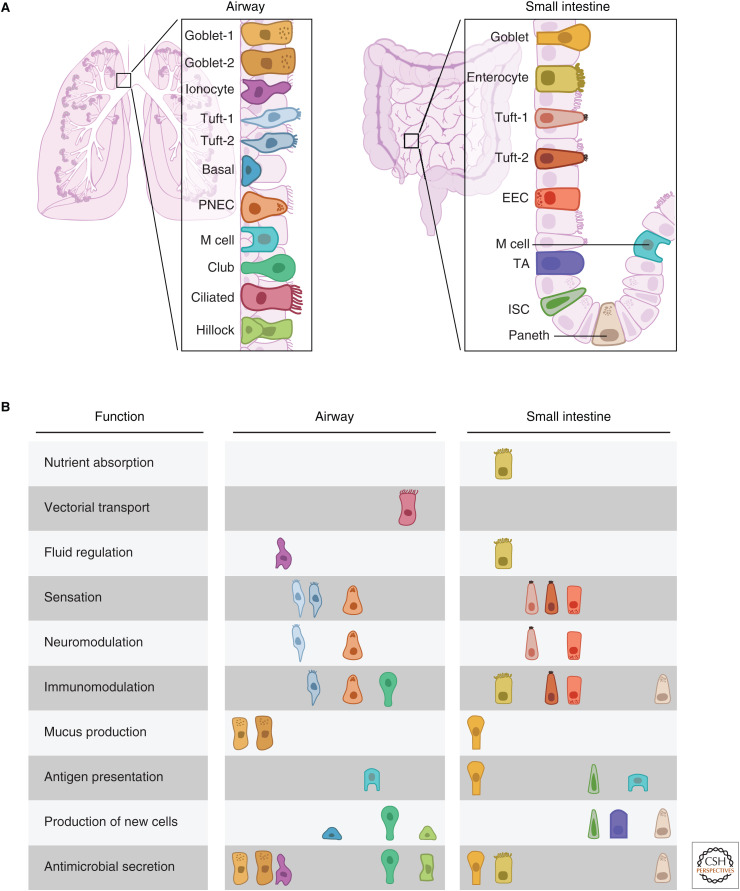
The airway and intestinal epithelia share many essential functions, including barrier defense and the management of microbes and immune cells, but they also fulfill entirely unique physiological roles. Cells of the small intestinal epithelium are arranged in a simple columnar fashion, accommodating digestion and nutrient absorption. In contrast, the cells of the airway epithelium are arranged in a simple pseudostratified epithelium, consisting of a single layer of cells attached to the basement membrane (Fig. 1A). The airway epithelium appears stratified because of the varying height of nuclei and because not all airway epithelial cells reach the luminal surface. This epithelium is generally classified as a simple epithelium, but the basally located stem cells are shielded from direct exposure to the lumen by differentiated luminal cells. This arrangement of cells allows for mucus production and clearance. At a higher order, the pseudostratified epithelium of the airway occurs as a simple cylindrical sheet that lines the mucosal surface of the respiratory tubes (Fig. 1A, left panel). In contrast, the columnar epithelium of the gut is folded into crypts and villi, presumably to protect the buried stem cell population (Kaiko et al. 2016) while also maximizing the surface area of enterocyte cells that absorb nutrients from the gut lumen (Fig. 1A, right panel).
Figure 1.
Functional specialization of epithelial subtypes. (A) Schematic comparison of the tissue architecture and cell types of murine airway (left) and small intestinal (right) epithelia. (B) Compartmentalization of epithelial tissue functions mapped onto component cell types in the two epithelia. (PNEC) pulmonary neuroendocrine cells, (EEC) enteroendocrine cell, (TA) transit-amplifying, (ISC) intestinal stem cell.
Reflecting their physiological distinctions, the airway and the gut epithelia share multiple archetype cell types that are adapted to their organ-specific location, as well as some unique cell types. In some cases, an aggregate tissue function, such as immune regulation, is distributed over multiple archetype cells in complex ways that likely relate to organ-specific functionality. In some cases, evolution has repurposed archetype cells with minor modifications, whereas in others, entirely new cell types arose that accommodate unique physiologic demands. We now describe and categorize by function the cells of these two epithelia.
UNIQUE EPITHELIAL CELLS CHARACTERISTIC OF EITHER AIRWAY OR INTESTINE
Intestinal Enterocytes
Enterocytes are essential for performing nutrient absorption, the cardinal physiologic function of the intestinal epithelium, and to transport ions and water across the small intestinal epithelium to maintain fluid homeostasis. Consistent with this, the enterocyte is also the most abundant cell type in the intestinal epithelium, covering a large fraction of the villus surface. To support their unique function of high-volume nutrient absorption, enterocytes possess microvilli, morphologic specializations that increase the cells’ surface area. As an abundant cell type, enterocytes must also contribute to barrier defense and produce antimicrobials, a feature shared by many cell types in both the airway and intestinal epithelia.
Airway Multiciliated Cells
The multiciliated cell, defined by motile cilia, is an archetype lung cell not found in the intestine. Motile cilia have a highly stereotyped structure, which is very similar to the flagella of the last common eukaryotic ancestor (Mitchell 2007). They are anchored along the luminal surface of ciliated cells and beat rhythmically to clear mucus and debris up and out of the airways in a process termed mucociliary clearance. Failure to clear mucus results in persistent lung infection (Tilley et al. 2015; Reiter and Leroux 2017). Motile ciliated cells fulfill similar purposes in other organs. For example, they are responsible for the flow of cerebrospinal fluid and transport along the fallopian tubes toward the uterus (Mitchison and Valente 2017). Despite their high degree of structural conservation across tissues and organisms, we speculate that ciliated cells from these distinct tissues may occur as flavors of a ciliated archetype cell adapted to different contexts. We hypothesize that scRNA-seq of ciliated cells across organs will reveal distinct expression programs that reflect the tuning of an archetype cell to a particular physiologic role.
There is no intestinal cell directly comparable with the ciliated cell. In the intestine, the propulsion of luminal contents is mediated by an entirely different mechanism: surrounding smooth muscle contraction (Feher 2012). Indeed, ciliated cells are unlikely to be able to produce sufficient force to transport the bulky contents of the intestine.
Airway Epithelial Ionocytes
Pulmonary ionocytes are rare epithelial cells in the airways whose identity was only recently described by scRNA-seq of mouse and human airways, and whose exact function requires further scrutiny (Hawkins and Kotton 2018; Montoro et al. 2018; Plasschaert et al. 2018; Travaglini and Krasnow 2018). Ionocytes are characterized by the specific expression of the transcription factor (TF) FOXI1, specialized subunits of proton-transporting V-ATPases, and the chloride/bicarbonate channel cystic fibrosis (CF) transmembrane conductance regulator (CFTR). The high expression of CFTR by ionocytes is consistent with prior observations of rare airway epithelial cells with particularly high CFTR expression (Engelhardt et al. 1992; Jiang and Engelhardt 1998).
Interestingly, FOXI1 and V-ATPase subunits are also coexpressed in intercalated cells of the kidney, FORE cells of the endolymphatic epithelium of the inner ear, and narrow and clear cells of the epididymis (Vidarsson et al. 2009). All of these cells express V-ATPase subunits that localize to the plasma membrane and secrete protons into the extracellular fluid. We speculate that pulmonary ionocytes similarly regulate the composition and properties of the fluid and mucus layer at the surface of the mammalian airway epithelium (Montoro et al. 2018). The conservation of functional markers of ionocytes in distinct mammalian tissues suggests that they are an archetype cell that regulates hydration, pH, and ion transport in several tissue contexts and across phyla.
The specific and high expression of CFTR by ionocytes contrasts with the diffuse expression of CFTR in many of the cell types within the intestinal epithelium (Haber et al. 2017; Montoro et al. 2018). Despite the different expression patterns of CFTR in the intestine and airway, both tissues are loci of disease in CF patients (Jiang and Engelhardt 1998; De Lisle and Borowitz 2013). How these different patterns of CFTR tissue expression reflect the distinct physiology of these tissues remains to be clarified.
CELLS FOUND IN BOTH THE INTESTINAL AND AIRWAY EPITHELIA
Intestinal and Airway Tuft Cells
Tuft cells, also called brush cells or solitary chemosensory cells, were first recognized in the airway as cells with apical microvillar appendages. These microvilli, which are strikingly different from those of enterocytes (Banerjee et al. 2018), were believed to represent a sensory appendage, but the precise role of tuft cells continued to be mysterious for decades (Reid et al. 2005). Airway tuft cells express genes associated with taste reception, neuromodulation, and immunomodulation, suggesting that they may survey chemicals in the airway lumen and evoke airway defenses (Krasteva et al. 2011). Tuft cells in the small intestine similarly express various components of chemosensory pathways (Bezençon et al. 2008). Immunologically, they initiate type 2 immune responses to helminth infections (Gerbe et al. 2016; Howitt et al. 2016; von Moltke et al. 2016), by regulating the activity of group 2 innate lymphoid cells (ILC2s) via secretion of the cytokine interleukin-25 (IL-25). This effect is amplified by a dramatic increase in tuft cell numbers following infection.
scRNA-seq of tuft cells in the airways and small intestine (Haber et al. 2017; Montoro et al. 2018) allows cross-organ comparison, and reveals remarkable similarity in expression profiles of tuft cells in the two tissues. This includes expression of fate-specifying TFs, chemosensory G-protein-coupled receptors (GPCRs), and immunomodulatory cytokines (Schneider et al. 2019). Both airway and intestinal tuft cells are the predominant epithelial source of Il25. IL-25 is a central mediator of the inflammatory response in allergic asthma, so its production by tuft cells implicates them in asthma pathophysiology. T helper 2 (Th2)-mediated responses associated with allergic asthma are believed to reflect an aberrant deployment of Th2 immunity in the control of lung parasitic infection, implicating conserved functionality within a shared archetype cell population. Thus, despite the distinct luminal environments of the intestine and airway, the tuft cell archetype is likely involved in both tissues in bridging chemosensation, immunity, and the need to defend against specific types of pathogens.
Tuft cells in both epithelia occur as one of two specialized subsets: a tuft-1 subset more strongly expressing chemosensation and neuromodulation genes, and a tuft-2 subset expressing inflammatory genes. Both express shared tuft-cell TFs, but each subset is also associated with distinct TFs (Montoro et al. 2018). Whether cells of each subset are capable of interconversion remains to be seen. Because they share many features and are conserved in both intestinal and airway epithelia, we propose that tuft-1 and tuft-2 subsets represent different flavors of the same archetype cell. Furthermore, just as specialized subsets of many immune cell types are capable of fulfilling distinct physiological functions (Fang et al. 2018), we speculate that tuft cell specialization is an important feature of their functionality.
Intestinal Enteroendocrine Cells (EECs) and Airway Pulmonary Neuroendocrine Cells (PNECs)
Both EECs and PNECs were once believed to arise from the neural crest, but both cell types have since been shown to be of endodermal origin and are locally maintained (Kuo and Krasnow 2015; Noguchi et al. 2015; Montoro et al. 2018). Rare enteroendocrine cells of the intestinal epithelium are specialized for sensing the luminal environment and transmitting information locally and systemically. The expression of many different GPCRs on their cell surfaces enables EECs to detect luminal nutrients and various endogenous and pathogen-associated metabolites (Gribble and Reimann 2016; Yu et al. 2019). EECs transduce these stimuli and relay signals to enteric neurons, endothelial cells, and neighboring epithelial cells via secreted cytokines and peptide hormones (McCauley 2020). EECs also send long-range signals through the bloodstream. Historically, EECs were classified by their expression of a particular dominant hormone. Recent scRNA-seq analysis of the small intestinal epithelium revealed a greater diversity of EEC subsets than previously believed. Instead of an association with one hormone, each EEC subset is distinguished by expression of a particular combination of hormones (Haber et al. 2017).
Intestinal EECs and airway PNECs share many functional attributes, including chemosensory capabilities (Gu et al. 2014). PNECs also produce peptide hormones, but in contrast to the numerous distinct hormone production programs that demarcate distinct EEC subsets, PNECs of the airway preliminarily appear to be less diverse (Montoro et al. 2018). Although a PNEC-derived peptide hormone may shape the type 2 immune response to asthma (Sui et al. 2018; Wallrapp et al. 2019), it is unclear why airway PNECs would display a more limited hormone repertoire than their counterparts in the intestine.
Aside from hormone expression, PNEC diversity is also reflected in their physical distribution as either solitary cells or as clusters of neuroepithelial bodies (NEBs). These clustered structures are notably absent in the intestinal epithelium, but are reminiscent of the aggregated NECs that compromise pancreatic islets. NEBs are often located at airway branch points, in which they may be ideally poised to sample the concentrations of oxygen and volatile compounds in inspired and expired gases. Of note, some PNECs found in NEBs act as facultative multipotent stem cells (Ouadah et al. 2019). Molecular signatures that distinguish structurally distinct PNEC populations are yet to be defined and spatial transcriptomics approaches should help resolve these.
Additional examples of conserved cells found in both airway and intestinal epithelia, but that we do not discuss in detail, include lymphoid-associated antigen-presenting M cells and mucus-producing goblet cells (Table 2; Fig. 1B).
Table 2.
The distribution of epithelial tissue functions onto component cell types corresponding to Figure 1
| Function | Airway cell type | Specific role | Small intestinal cell type | Specific role |
|---|---|---|---|---|
| Nutrient absorption | N/A | Enterocyte | Absorption of carbohydrates (e.g., Lct) and lipids, bile resorption | |
| Vectorial transport | Ciliated cells | Directional propulsion of mucus | N/A | |
| Fluid regulation | Ionocyte | Chloride (Cftr), sodium (ENaC), potassium (Kcnma1) transport | Enterocyte | Sodium (Slc5a1, Sglt1) |
| Sensation | Tuft cells | Chemosensation, possible innervation | Tuft cells | Chemosensation of parasites (Trpm5, Gnat3) |
| PNEC | Oxygen sensation, stretch, innervation | Enterochromaffin cells | Detection of irritants, metabolites, and catecholamines | |
| Neuromodulation | PNEC | Hormones, innervated | Enterochromaffin | Serotinergic activation of sensory nerves (Tph1, Tac1) |
| Tuft-1 | Neurotransmitter production (ChAT) | Enteroendocrine | Regulation of satiety (Ghrelin, Pyy) and mood (Gastrin, Cck) | |
| Tuft-1 | Neurotransmitter production (ChAT) | |||
| Immunomodulation | PNEC | Allergic response (Cgrp, Gaba) | Enteroendocrine cells | Cck, Glp-1 substance P, neurokinin A, and neurokinin B |
| Tuft-2 | Allergic response (leukotrienes, IL-25, Tslp) | Tuft-2 | Leukotrienes, Il25, Tslp | |
| Club | Tnf | Paneth | Tnfα | |
| Enterocytes | Il18 | |||
| Mucus production | Goblet | Mucins (Muc5b, Muc5ac) | Goblet cells | Mucins (Muc2) |
| Antigen presentation | Microfold cells | Microfold cells Intestinal stem cells | ||
| Goblet | ||||
| Production of new cells | Basal cells | Production of club and rare cell types | Intestinal stem cells | Production of transit-amplifying cells, Paneth cells |
| Production of differentiation factors to club cells | Transit-amplifying | Production of differentiated cells | ||
| Club cells | Production of ciliated cells and goblet cells | Paneth cells | Stem cell niche factors WNT (Wnt3,11), Notch (Dll4) | |
| Hillock basal cells | Production of hillock club cells | |||
| PNEC | Reserve stem cell | |||
| Antimicrobial secretion | Ionocytes | Secretion of cochlin | Paneth cells | Antimcrobial peptides—α defensins, lysozyme, angiogenin4, Mmp7, interlectin-1 |
| Club | Surfactants (Sftpd, Scgb3a2), antimicrobial (Ltf, Lyz2) | Enterocytes | Antimcrobial peptides—C-type lectins (Reg3b, Reg3g) | |
| Hillock club | Surfactants (Sftpd), antimicrobial (Ltf, Lyz2) | |||
| Goblet | Goblet | Antiparasitic immunity—Retnlb |
EPITHELIAL FUNCTIONS DISTRIBUTED AMONG DIVERGENT CELL TYPES IN A TISSUE-SPECIFIC PATTERN
Some epithelial functions are distributed among divergent cell types in a pattern unique to each tissue (Table 2; Fig. 1B). For instance, both the airways and the intestines must produce antimicrobial agents to defend against pathogens. Many cell types participate in generating each tissue's respective battery of antimicrobial agents, likely attributable to the myriad types of pathogens. These cells include, but are not limited to, Paneth cells, enterocytes, and goblet cells in the intestinal epithelium, and club cells, hillock club cells, goblet cells, and ionocytes in the airway epithelium (Table 2; Fig. 1). These unique arrangements might reflect the need to contend with distinct pathogens and toxins associated with either food consumption or airbreathing.
Intestinal stem cells (ISCs) and airway basal cells both serve as stem cells, but they have distinct markers, occupy unique niches, and use distinct signaling pathways to self-renew and differentiate. Additionally, they display very different stem cell kinetics that reflect high (intestine) or low (airway) homeostatic cell turnover. Furthermore, in the intestine, stem cells give rise to transit-amplifying (TA) cells in the crypt (Gehart and Clevers 2019) TA cells in turn serve as workhorse progenitors, rapidly proliferating and differentiating to support the rapid replacement of differentiated villus cell types. In contrast, airway club cells are long-lived progenitors found throughout the entire length of the airway. Perhaps because they are not continuously cycling (Clevers and Watt 2018), they are able to support a differentiated cell machinery that is used to detoxify contaminants.
INFERRING CELLULAR DYNAMICS AND LINEAGE RELATIONSHIPS IN EPITHELIA
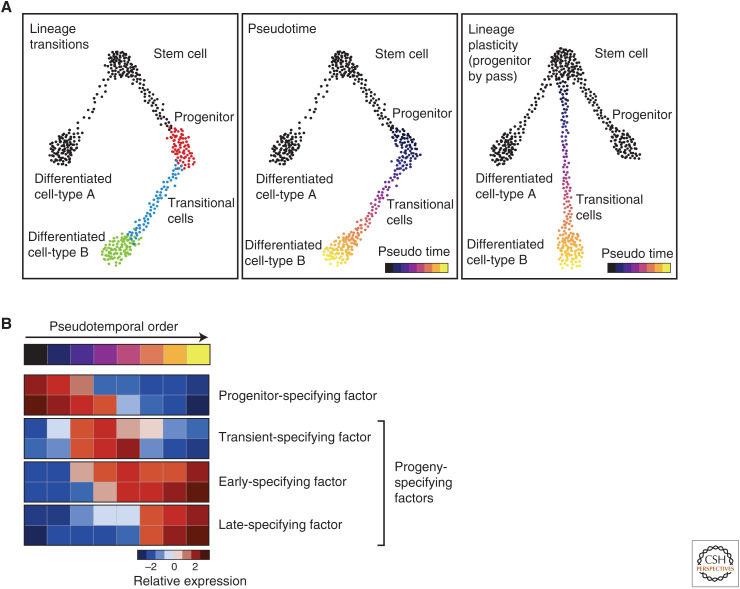
Epithelial homeostasis involves cell turnover and differentiation. In the airways and small intestine, this results in a spectrum of transitional cell states as a progenitor cell is converted into another cell type. These transitional cells are captured in scRNA-seq experiments and they can be used to model differentiation paths. Computational methods can order cells collected at a single time point into a pseudotemporal ordering that arranges transitional cells along trajectories in gene-expression space (Trapnell et al. 2014; Schiebinger et al. 2019). The ordering allows for inferences concerning parent–progeny relationships between cells (Fig. 2A). Pseudotemporal ordering cannot be used to directly infer directionality, but the inferred relationships can be directly verified using lineage tracing. In one such application, we previously performed genetic lineage tracing of airway epithelial stem cells followed by scRNA-seq of epithelial cells at various time points after the initial lineage labeling. This “pulse-seq” strategy allowed us to revise previous models of cellular differentiation (Montoro et al. 2018). For example, tuft cells were previously believed to represent long-lived cells, but were found to be continuously produced during homeostasis (Saunders et al. 2013). Furthermore, pulse-seq analysis established a new cellular hierarchy in which stem cells directly produce tuft cells, solitary PNECs, and ionocytes. The number of methods for tracking the clonal histories of cells is rapidly growing (Habib et al. 2016; Alemany et al. 2018; Raj et al. 2018; Spanjaard et al. 2018), and these will enable finer analyses of cell lineages and differentiation.
Figure 2.
Modeling lineage and differentiation dynamics with single-cell RNA (scRNA)-seq data. (A) Identifying cellular transition states (left panel, blue dots) associated with differentiation from a progenitor cell type (left panel, red dots) to a differentiated cell type (left panel, green dots) using pseudotemporal ordering of cells (middle panel). Injury-induced lineage plasticity can be mapped by observing altered differentiation trajectories (right panel) as compared with those during homeostasis (left panel). (B) Pseudotemporal ordering allows inferences concerning the sequence of putative fate-specifying genes that govern differentiation from a progenitor cell to a mature differentiated cell.
Epithelial differentiation is dynamically modulated to increase the production of particular cell types in response to injury or infection. As mentioned above, tuft cell numbers are greatly increased in the small intestinal epithelium in response to parasitic infection (Gerbe et al. 2016; Howitt et al. 2016; von Moltke et al. 2016). Similarly, goblet cell numbers are increased in airway epithelial diseases like allergic asthma (Chen et al. 2009; Roy et al. 2014; Whitsett 2018). These processes likely represent the increased production of one particular cell type from its usual progenitor cell. Injury also evokes lineage plasticity, with complex reorchestrations of normal lineage paths. For example, airway club cell progenitors dedifferentiate into basal stem cells in response to basal stem cell depletion (Tata et al. 2013), and, in response to inhaled injury (Pardo-Saganta et al. 2015), stem cells in the airway directly produce differentiated ciliated cells, bypassing the normal secretory cell intermediate associated with homeostatic ciliated cell differentiation (Fig. 2A). Similar lineage plasticity is also seen in hematopoiesis, in which hematopoietic stem cells can directly produce differentiated myeloid cells by circumventing the intervening multipotent progenitor state (Yamamoto et al. 2018).
By analyzing changes in transcription along any pseudotemporal path of differentiation, one can infer which TFs are responsible for progressive differentiation (Fig. 2B; Biton et al. 2018). The sequential expression of differentiation factors has been of long-standing interest to developmental biologists and the ready identification of these putative fate-specifying genes should facilitate cellular reprogramming (Spence et al. 2011; Mou et al. 2012; Wong et al. 2012; Pagliuca et al. 2014).
ARCHETYPE CELLS AND EVOLUTION
Archetype cells are also identifiable across phyla. For example, rodlet cells are rare epithelial secretory cells that were identified in the intestines of teleost fish in 1892 (Leino 1974; Manera and Dezfuli 2004; Reite 2005). They were originally believed to be a protozoan parasite because of their unique extended cellular morphology, but they were later identified as bona fide intestinal epithelial cells. These mysterious cells greatly increase in number following helminth infections. Rodlet cells are also implicated in the recruitment of mast cells and eosinophilic granule cells through the agency of various secreted factors. Thus, rodlet cells are reported to be epithelial sensors of parasitic infection that then mediated an inflammatory response (Reite 2005). Many defining features of rodlet cells match those of tuft cells, including their extended cell morphology, their localization in the intestinal epithelium, their hyperplasia in response to helminth infection, and their observed role as sensors and mediators of host defense. Amazingly, the characterization of rodlet cells preceded the functional characterization of tuft cells in the gut by more than two decades. scRNA-seq now affords an opportunity to compare these and other cell types across evolutionary time.
In a remarkable parallel, ionocytes were only recently identified as Foxi1-expressing epithelial cells that regulate mammalian airway fluid homeostasis (Montoro et al. 2018; Plasschaert et al. 2018; Travaglini and Krasnow 2018). Although cells with similar gene expression were found in the mammalian kidney, epididymis, and inner ear a decade before (Vidarsson et al. 2009), a comparison with archetype cells in frogs and fish laid a foundation for our interpretation of ionocyte function in the airway. In fish gills, mitochondria-rich ionocytes are specified by Foxi1 and express apical plasma membrane V-ATPases. These fish ionocytes were so named because they contribute to ion and fluid homeostasis through the secretion and absorption of ions (Esaki et al. 2009). In frog multiciliated skin, ionocytes are again specified by a Foxi1 ortholog and express apical plasma membrane V-ATPases. Frog ionocytes contribute to pH and osmoregulation through the regulation of protons, bicarbonate, and chloride (Quigley et al. 2011). Indeed, these prior insights were crucial in putting forward the hypothesis that pulmonary ionocytes act as regulators of airway hydration (Montoro et al. 2018).
As an atlas of cells comprising the human body is assembled (Regev et al. 2017), we will be able to revisit the question of archetype cells across human organs and indeed across species. This body of data will certainly form the basis for a broader understanding of cell-type evolution. These same evolutionary insights will serve as a foundation for establishing methods to regulate archetype cells throughout the body in the case of systemic disease, and subtle differences in archetype cells may point the way toward deciphering tissue-specific biology and therapy.
Footnotes
Editors: Cristina Lo Celso, Kristy Red-Horse, and Fiona M. Watt
Additional Perspectives on Stem Cells: From Biological Principles to Regenerative Medicine available at www.cshperspectives.org
REFERENCES
- Alemany A, Florescu M, Baron CS, Peterson-Maduro J, van Oudenaarden A. 2018. Whole-organism clone tracing using single-cell sequencing. Nature 556: 108–112. 10.1038/nature25969 [DOI] [PubMed] [Google Scholar]
- Banerjee A, McKinley ET, von Moltke J, Coffey RJ, Lau KS. 2018. Interpreting heterogeneity in intestinal tuft cell structure and function. J Clin Invest 128: 1711–1719. 10.1172/JCI120330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak S. 2008. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509: 514–525. 10.1002/cne.21768 [DOI] [PubMed] [Google Scholar]
- Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, et al. 2018. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175: 1307–1320.e22. 10.1016/j.cell.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ET, Quon GT, Chua G, Babak T, Trochesset M, Zirngibl RA, Aubin J, Ratcliffe MJH, Wilde A, Brudno M, et al. 2009. Conservation of core gene expression in vertebrate tissues. J Biol 8: 33 10.1186/jbiol130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. 2009. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119: 2914–2924. 10.1172/JCI39731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lareau C, Andreani T, Vinyard ME, Garcia SP, Clement K, Andrade-Navarro MA, Buenrostro JD, Pinello L. 2019. Assessment of computational methods for the analysis of single-cell ATAC-seq data. Genome Biol 20: 241 10.1186/s13059-019-1854-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Watt FM. 2018. Defining adult stem cells by function, not by phenotype. Annu Rev Biochem 87: 1015–1027. 10.1146/annurev-biochem-062917-012341 [DOI] [PubMed] [Google Scholar]
- De Lisle RC, Borowitz D. 2013. The cystic fibrosis intestine. Cold Spring Harb Perspect Med 3: a009753 10.1101/cshperspect.a009753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. 1992. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248. 10.1038/ng1192-240 [DOI] [PubMed] [Google Scholar]
- Esaki M, Hoshijima K, Nakamura N, Munakata K, Tanaka M, Ookata K, Asakawa K, Kawakami K, Wang W, Weinberg ES, et al. 2009. Mechanism of development of ionocytes rich in vacuolar-type H+-ATPase in the skin of zebrafish larvae. Dev Biol 329: 116–129. 10.1016/j.ydbio.2009.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Li X, Dai J, Cole L, Camacho JA, Zhang Y, Ji Y, Wang J, Yang XF, Wang H. 2018. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol 11: 97 10.1186/s13045-018-0637-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. 2012. Intestinal and colonic motility. In Quantitative human physiology: an introduction, pp. 711–720. Academic, Cambridge, MA. [Google Scholar]
- Gehart H, Clevers H. 2019. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 16: 19–34. 10.1038/s41575-018-0081-y [DOI] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, et al. 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230. 10.1038/nature16527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F. 2016. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78: 277–299. 10.1146/annurev-physiol-021115-105439 [DOI] [PubMed] [Google Scholar]
- Gu X, Karp PH, Brody SL, Pierce RA, Welsh MJ, Holtzman MJ, Ben-Shahar Y. 2014. Chemosensory functions for pulmonary neuroendocrine cells. Am J Respir Cell Mol Biol 50: 637–646. 10.1165/rcmb.2013-0199OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, et al. 2017. A single-cell survey of the small intestinal epithelium. Nature 551: 333–339. 10.1038/nature24489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Li Y, Heidenreich M, Swiech L, Avraham-Davidi I, Trombetta JJ, Hession C, Zhang F, Regev A. 2016. Div-seq: single-nucleus RNA-Seq reveals dynamics of rare adult newborn neurons. Science 353: 925–928. 10.1126/science.aad7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins FJ, Kotton DN. 2018. Pulmonary ionocytes challenge the paradigm in cystic fibrosis. Trends Pharmacol Sci 39: 852–854. 10.1016/j.tips.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. 2016. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351: 1329–1333. 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Engelhardt JF. 1998. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur J Hum Genet 6: 12–31. 10.1038/sj.ejhg.5200158 [DOI] [PubMed] [Google Scholar]
- Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. 2016. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165: 1708–1720. 10.1016/j.cell.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, Medzhitov R. 2015. Homeostasis, inflammation, and disease susceptibility. Cell 160: 816–827. 10.1016/j.cell.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. 2011. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci 108: 9478–9483. 10.1073/pnas.1019418108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CS, Krasnow MA. 2015. Formation of a neurosensory organ by epithelial cell slithering. Cell 163: 394–405. 10.1016/j.cell.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino RL. 1974. Ultrastructure of immature, developing, and secretory rodlet cells in fish. Cell Tissue Res 155: 367–381. 10.1007/BF00222812 [DOI] [PubMed] [Google Scholar]
- Luecken MD, Theis FJ. 2019. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol 15: e8746 10.15252/msb.20188746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manera M, Dezfuli BS. 2004. Rodlet cells in teleosts: a new insight into their nature and functions. J Fish Biol 65: 597–619. 10.1111/j.0022-1112.2004.00511.x [DOI] [Google Scholar]
- McCauley HA. 2020. Enteroendocrine regulation of nutrient absorption. J Nutr 150: 10–21. 10.1093/jn/nxz191. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. 2007. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. In Eukaryotic membranes and cytoskeleton. Advances in experimental medicine and biology, Vol. 607, pp. 130–140. Springer, New York: 10.1007/978-0-387-74021-8_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison HM, Valente EM. 2017. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 241: 294–309. 10.1002/path.4843 [DOI] [PubMed] [Google Scholar]
- Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, et al. 2018. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560: 319–324. 10.1038/s41586-018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor AE, Itzkovitz S. 2017. Spatial transcriptomics: paving the way for tissue-level systems biology. Curr Opin Biotechnol 46: 126–133. 10.1016/j.copbio.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Lau FH, Musunuru K, et al. 2012. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10: 385–397. 10.1016/j.stem.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, Sumiyama K, Morimoto M. 2015. Directed migration of pulmonary neuroendocrine cells toward airway branches organizes the stereotypic location of neuroepithelial bodies. Cell Rep 13: 2679–2686. 10.1016/j.celrep.2015.11.058 [DOI] [PubMed] [Google Scholar]
- Ouadah Y, Rojas ER, Riordan DP, Capostagno S, Kuo CS, Krasnow MA. 2019. Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and Notch. Cell 179: 403–416.e23. 10.1016/j.cell.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. 2014. Generation of functional human pancreatic β cells in vitro. Cell 159: 428–439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, Zhao R, Rajagopal J. 2015. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16: 184–197. 10.1016/j.stem.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, Jaffe AB. 2018. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560: 377–381. 10.1038/s41586-018-0394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley IK, Stubbs JL, Kintner C. 2011. Specification of ion transport cells in the Xenopus larval skin. Development 138: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B, Gagnon JA, Schier AF. 2018. Large-scale reconstruction of cell lineages using single-cell readout of transcriptomes and CRISPR–Cas9 barcodes by scGESTALT. Nat Protoc 13: 2685–2713. 10.1038/s41596-018-0058-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, et al. 2017. Science forum: the human cell atlas. eLife 6: e27041 10.7554/eLife.27041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L, Meyrick B, Antony VB, Chang LY, Crapo JD, Reynolds HY. 2005. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med 172: 136–139. 10.1164/rccm.200502-203WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite OB. 2005. The rodlet cells of teleostean fish: their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol 19: 253–267. 10.1016/j.fsi.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Reiter JF, Leroux MR. 2017. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 18: 533–547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ. 2019. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363: 1463–1467. 10.1126/science.aaw1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. 2014. Muc5b is required for airway defence. Nature 505: 412–416. 10.1038/nature12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CJ, Reynolds SD, Finger TE. 2013. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol 49: 190–196. 10.1165/rcmb.2012-0485OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebinger G, Shu J, Tabaka M, Cleary B, Subramanian V, Solomon A, Gould J, Liu S, Lin S, Berube P, et al. 2019. Optimal-transport analysis of single-cell gene expression identifies developmental trajectories in reprogramming. Cell 176: 928–943.e22. 10.1016/j.cell.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, O'Leary CE, Locksley RM. 2019. Regulation of immune responses by tuft cells. Nat Rev Immunol 19: 584–593. 10.1038/s41577-019-0176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjaard B, Hu B, Mitic N, Olivares-Chauvet P, Janjuha S, Ninov N, Junker JP. 2018. Simultaneous lineage tracing and cell-type identification using CRISPR–Cas9-induced genetic scars. Nat Biotechnol 36: 469–473. 10.1038/nbt.4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, et al. 2016. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353: 78–82. 10.1126/science.aaf2403 [DOI] [PubMed] [Google Scholar]
- Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, et al. 2018. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360: eaan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223. 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley AE, Walters MS, Shaykhiev R, Crystal RG. 2015. Cilia dysfunction in lung disease. Annu Rev Physiol 77: 379–406. 10.1146/annurev-physiol-021014-071931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. 2014. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32: 381–386. 10.1038/nbt.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglini KJ, Krasnow MA. 2018. Profile of an unknown airway cell. Nature 560: 313–314. 10.1038/d41586-018-05813-7 [DOI] [PubMed] [Google Scholar]
- Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerbäck S. 2009. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H+-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS ONE 4: e4471 10.1371/journal.pone.0004471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, Locksley RM. 2016. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225. 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour REE, Thakore PI, Schnell A, Lambden C, Herbst RH, et al. 2019. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51: 709–723.e6. 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheater PR, Burkitt HG, Daniels VG. 1987. Functional histology: a text and colour atlas. Churchill Livingstone, London. [Google Scholar]
- Whitsett JA. 2018. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc 15: S143–S148. 10.1513/AnnalsATS.201802-128AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. 2012. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30: 876–882. 10.1038/nbt.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Wilkinson AC, Nakauchi H. 2018. Changing concepts in hematopoietic stem cells. Science 362: 895–896. 10.1126/science.aat7873 [DOI] [PubMed] [Google Scholar]
- Yu Y, Yang W, Li Y, Cong Y. 2020. Enteroendocrine cells: sensing gut microbiota and regulating inflammatory bowel diseases. Inflamm Bowel Dis 26: 11–20. 10.1093/ibd/izz217 [DOI] [PMC free article] [PubMed] [Google Scholar]