Abstract
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, with ∼71 million chronically infected individuals worldwide. Treatment of patients with HCV-related liver disease has advanced considerably thanks to the development of new direct-acting antiviral drugs that are now administered as highly potent, safe, and well-tolerated combinations with a high barrier to resistance. International organizations, such as the European Association for the Study of the Liver, the American Association for the Study of Liver Diseases jointly with the Infectious Diseases Society of America, or the World Health Organization have published detailed treatment guidelines. With these therapies becoming more and more widely available, elimination of hepatitis C as a public health threat by 2030 can now be envisaged in several countries. In other regions, better screening, diagnosis, and linkage to care will be necessary to achieve this ambitious goal.
Chronic hepatitis C virus (HCV) infection affects ∼71 million people worldwide (European Union HCV Collaborators 2017; Polaris Observatory HCV Collaborators 2017). In patients with chronic hepatitis C, the hepatic injury ranges from minimal lesions to extensive fibrosis, cirrhosis, and/or hepatocellular carcinoma (HCC). Chronic HCV infection is also associated with a number of extrahepatic manifestations. Worldwide mortality related to the complications of chronic hepatitis C has been estimated to be 580,000 in 2017 (Roth et al. 2017).
HCV infection is curable by antiviral therapy. Cure of HCV infection is characterized by the sustained virological response (SVR), that is, undetectable HCV RNA 12 weeks (SVR12) or 24 weeks (SVR24) after the end of antiviral therapy. The chance of late post-SVR relapse is very low. HCV infection cure results in improvement or disappearance of liver necroinflammation and fibrosis in patients who do not have cirrhosis. Hepatic fibrosis may also regress in patients with cirrhosis. Recent data have shown that the cumulative incidence of HCC and liver-related mortality are significantly reduced in patients who clear HCV, as compared with untreated patients, especially in those with cirrhosis (Nahon et al. 2017; Carrat et al. 2019). Viral elimination also induces reversal of most HCV-induced extrahepatic manifestations (Negro 2018).
Anti-HCV therapy has advanced dramatically during the last two decades. The interferon (IFN)-α era was associated with injectable drugs, long duration of therapy, the requirement for ribavirin, complex treatment algorithms, frequent on-treatment monitoring, high rates of sometimes severe adverse events, and suboptimal SVR rates in most patient populations. Treatment of HCV infection is now based on the use of highly efficacious, well-tolerated fixed-dose pangenotypic direct-acting antiviral (DAA) combinations. Improvements include shorter duration (8–12 weeks in most instances), very high SVR rates (>95%), and rare, modest side effects.
This review summarizes current knowledge and treatment recommendations for patients with chronic hepatitis C.
GOAL OF HCV THERAPY
As stated by the European Association for the Study of the Liver (EASL) in its 2018 recommendations, the goal of therapy is to cure HCV infection to (1) prevent the complications of HCV-related liver and extrahepatic diseases, including hepatic necroinflammation, fibrosis, cirrhosis, decompensation of cirrhosis, HCC, severe extrahepatic manifestations, and death; (2) improve quality of life and remove stigma; and (3) prevent onward transmission of HCV (European Association for the Study of the Liver 2018).
The end point of therapy is the SVR. It is recommended to use a sensitive molecular method with a lower limit of detection ≤15 international units (IUs)/mL to assess the SVR12 or the SVR24. However, a qualitative assay with a lower limit of detection ≤1000 IU/mL (3.0 Log IU/mL) or the HCV core antigen assay can also be used to assess the virological response 24 weeks after the end of therapy (European Association for the Study of the Liver 2018). In patients with advanced fibrosis and cirrhosis, surveillance for HCC must be continued (European Association for the Study of the Liver 2018).
PRETREATMENT ASSESSMENT
Classical Pretreatment Assessment
Other causes of chronic liver disease and factors likely to affect the natural history or progression of liver disease should be investigated. They include other blood-borne viruses (hepatitis B virus and human immunodeficiency virus), alcohol consumption, renal impairment, and signs of a metabolic syndrome. Liver disease severity must be assessed before therapy by means of noninvasive methods such as liver stiffness measurement or blood biomarkers (European Association for Study of the Liver, Asociacion Latinoamericana para el Estudio del Higado. 2015), to identify patients with advanced fibrosis (METAVIR score F3) or cirrhosis (METAVIR score F4) who need posttreatment surveillance for HCC every 6 months.
Treatment is indicated in the presence of HCV RNA or HCV core antigen. Measuring their amounts has no use, because the level of replication does not influence treatment choices. In contrast, determining the HCV genotype and subtype remains useful to tailor the treatment regimen and its duration. Indeed, with pangenotypic DAA regimens, patients with cirrhosis and genotype 3 infection need to adapt their treatment. Also, rare subtypes of frequent genotypes have been isolated in patients from African or Asian origin and poorly respond to current HCV treatment regimens because of the presence in their genomes of natural polymorphisms conferring reduced susceptibility to HCV DAAs (Krishnan et al. 2018; Fourati et al. 2019; Smith et al. 2019). However, these subtypes are generally not recognized by widely used HCV genotyping assays. Because access to reliable HCV resistance testing is limited and highly efficacious treatments are available for patients with detectable preexisting resistance-associated substitutions (RASs) at baseline, systematic testing for HCV resistance before treatment is not recommended (Pawlotsky 2016).
Careful assessment of possible interactions of HCV DAAs with drugs taken by the patients must be performed before therapy and the necessary adaptations must be made. The website developed by the University of Liverpool (see hep-druginteractions.org) provides an invaluable comprehensive resource for the pretherapeutic assessment of potential drug–drug interactions and decision-making.
Simplified Pretreatment Assessment
Pretreatment assessment of patients with chronic hepatitis C can be simplified to ensure better linkage to care. Simplified assessment includes the presence of HCV replication (HCV RNA or HCV core antigen testing) and drug–drug interactions. Checking for the presence of advanced fibrosis (F3) or cirrhosis (F4) is useful only if treatment for HCC is available. A simple noninvasive marker score, such as FIB-4 or APRI, can be used.
TREATMENT INDICATIONS
All treatment-naive and -experienced patients with HCV infection, who are willing to be treated and who have no contraindications for treatment, should be treated (European Association for the Study of the Liver 2018) (also, see who.int/hepatitis/publications/global-hepatitis-report2017/en and hcvguidelines.org). This includes adolescents aged 12 years and above. In children younger than 12 years, treatment should be deferred until DAAs, including pangenotypic regimens, are approved for this age group (European Association for the Study of the Liver 2018).
Treatment must be considered without delay in patients with significant fibrosis (METAVIR score F2 or F3) or cirrhosis (METAVIR score F4), including decompensated cirrhosis, patients with clinically significant extrahepatic manifestations, patients with HCV recurrence after liver transplantation, patients at risk of a rapid evolution of liver disease because of concurrent comorbidities, and individuals at high risk of transmitting HCV (European Association for the Study of the Liver 2018).
AVAILABLE HCV DAA DRUGS AND COMBINATIONS
The HCV DAAs available in 2020 and recommended for use by international guidelines are listed in Table 1.
Table 1.
Hepatitis C virus (HCV) direct-acting antivirals (DAAs) approved in Europe and the United States
| Product | Presentation | Posology |
|---|---|---|
| Pangenotypic drugs or drug combinations | ||
| Sofosbuvir | Tablets containing 400 mg of sofosbuvir | One tablet once daily |
| Sofosbuvir/velpatasvir | Tablets containing 400 mg of sofosbuvir and 100 mg of velpatasvir | One tablet once daily |
| Sofosbuvir/velpatasvir/voxilaprevir | Tablets containing 400 mg of sofosbuvir, 100 mg of velpatasvir and 100 mg of voxilaprevir | One tablet once daily |
| Glecaprevir/pibrentasvir | Tablets containing 100 mg of glecaprevir and 40 mg of pibrentasvir | Three tablets once daily |
| Genotype-specific drugs or drug combinations | ||
| Sofosbuvir/ledipasvir | Tablets containing 400 mg of sofosbuvir and 90 mg of ledipasvir | One tablet once daily |
| Paritaprevir/ombitasvir/ritonavir | Tablets containing 75 mg of paritaprevir, 12.5 mg of ombitasvir and 50 mg of ritonavir | Two tablets once daily |
| Dasabuvir | Tablets containing 250 mg of dasabuvir | One tablet twice daily (morning and evening) |
| Grazoprevir/elbasvir | Tablets containing 100 mg of grazoprevir and 50 mg of elbasvir | One tablet once daily |
TREATMENT OF PATIENTS WITHOUT CIRRHOSIS AND PATIENTS WITH COMPENSATED (CHILD–PUGH A) CIRRHOSIS
Only IFN-free, DAA-based regimens must be used to treat HCV-infected patients. Whenever possible, combination regimens comprising two drugs are preferred to triple combination regimens, to minimize the risk of side-effects and drug–drug interactions (European Association for the Study of the Liver 2018). Treatment indications depend on the HCV genotype/subtype, the severity of liver disease, and/or prior therapy.
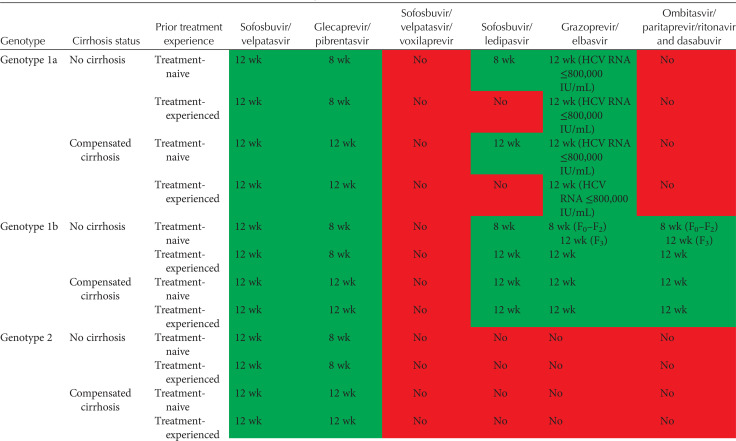
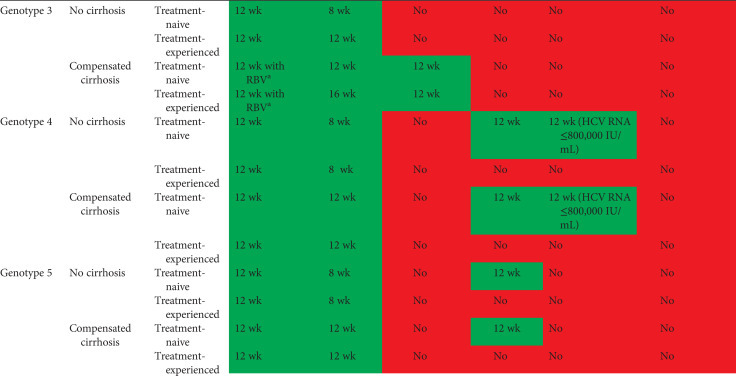
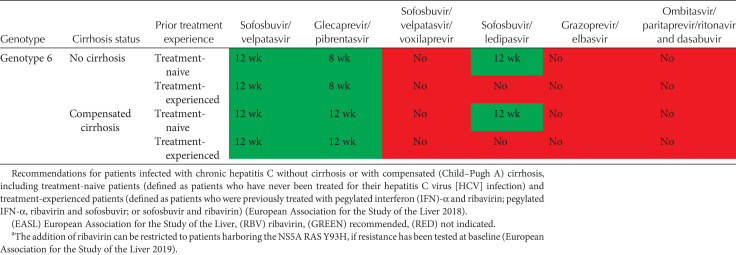
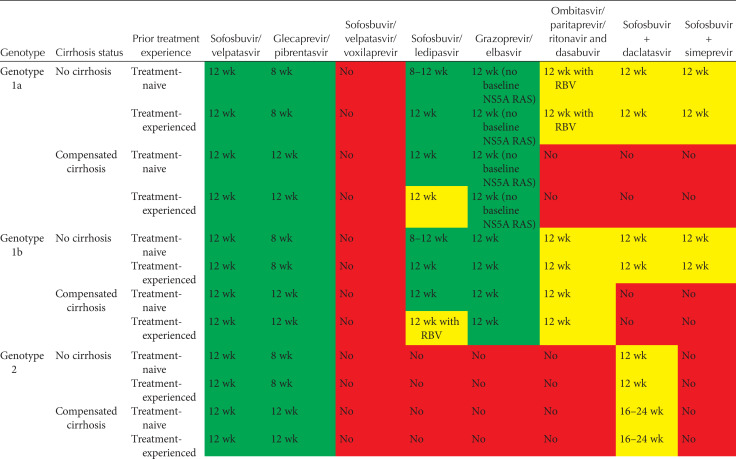
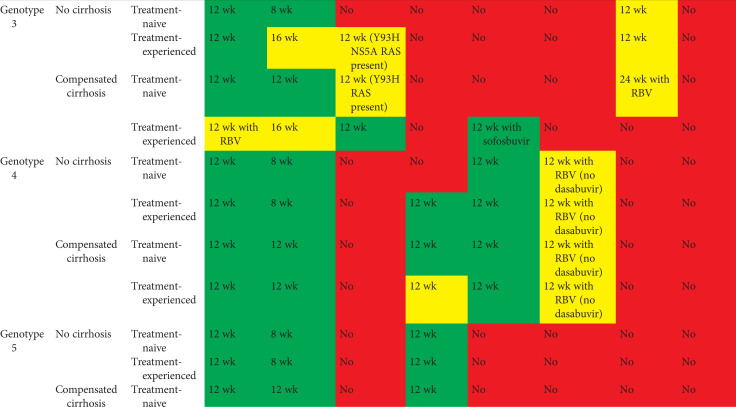
Tables 2 and 3 show current EASL recommendations and American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidance, respectively, for treatment of patients without cirrhosis or with compensated cirrhosis, including “treatment-naive” patients (i.e., patients who have never been treated for their HCV infection) and “treatment-experienced” patients (i.e., patients previously treated with pegylated IFN-α and ribavirin; pegylated IFN-α, ribavirin and sofosbuvir; or sofosbuvir and ribavirin). These recommendations are based on a large number of phase II and III trial data, as well as on real-world experience.
Table 2.
EASL recommendations 2018 for treatment of HCV-infected patients
Table 3.
AASLD/IDSA guidance for HCV-infected patients
Importantly, licensed generic drugs and drugs in agreement with the Medicines Patent Pool have been shown to generate similar results to the original compounds (Freeman and Hill 2016). The presence of the drug at the appropriate dosage must be verified by the provider and guaranteed to the prescriber and patient. Effective and safe generics are a crucial resource in resource-limited countries.
TREATMENT OF PATIENTS WITH SEVERE LIVER DISEASE AND PATIENTS IN THE POSTLIVER TRANSPLANT SETTING
Patients with Decompensated Cirrhosis
The use of protease inhibitors is contraindicated in patients with Child–Pugh B and C decompensated cirrhosis and in patients with compensated cirrhosis and a history of prior decompensation, because of higher drug exposure and risk of toxicities. Thus, treatment of patients with decompensated cirrhosis should be based on the combination of sofosbuvir and an NS5A inhibitor (sofosbuvir/velpatasvir, sofosbuvir/ledipasvir, or sofosbuvir plus daclatasvir if these regimens are not available).
Patients with decompensated cirrhosis without HCC awaiting liver transplantation with a MELD score ≥18–20 should be transplanted first without antiviral treatment, provided that the waiting time on the transplant list is below 6 months, and their HCV infection should be treated after liver transplantation (European Association for the Study of the Liver 2018).
Patients with decompensated cirrhosis without HCC awaiting liver transplantation with a MELD score <18–20 should be treated before liver transplantation. The treatment regimen should be sofosbuvir and velpatasvir with ribavirin for 12 weeks (all genotypes) or sofosbuvir and ledipasvir for 12 weeks with ribavirin (genotypes 1, 4, 5, and 6). In these patients who may not tolerate ribavirin very well, ribavirin can be started at 600 mg daily and subsequently adjusted. Treatment can be administered for 24 weeks if ribavirin is contraindicated (European Association for the Study of the Liver 2018).
Patients with decompensated cirrhosis not on the waiting list for liver transplantation should be treated urgently with the same regimens as patients with an indication for liver transplantation (sofosbuvir/velpatasvir or sofosbuvir/ledipasvir for 12 weeks with ribavirin or for 24 weeks without ribavirin) to improve their liver function and survival (European Association for the Study of the Liver 2018).
Patients with Hepatocellular Carcinoma
In patients with HCC and an indication for curative liver transplantation, HCV infection can be treated before transplantation only if it does not interfere with the management of HCC. Otherwise, HCV treatment should be delayed until after transplantation. In both cases, treatment should follow the above-mentioned rules (European Association for the Study of the Liver 2018).
In patients with treated HCC without an indication for liver transplantation, recent data indicate that HCV infection treatment has no impact on subsequent HCC recurrence (ANRS Collaborative Study Group on Hepatocellular Carcinoma 2016). These patients, who generally have underlying cirrhosis, should be treated for their HCV infection. They subsequently require lifelong surveillance by means of ultrasound to detect HCC recurrence of de novo occurrence (European Association for the Study of the Liver 2018).
HCV Infection after Liver Transplantation
Very high SVR rates have been reported in HCV-infected patients with infection recurrence postliver transplantation, especially if they have been treated before the development of cirrhosis (Charlton et al. 2015; Manns et al. 2016; Reau et al. 2017). Thus, these patients should be treated as early as possible after transplantation, ideally when they are stabilized after ∼3 months (European Association for the Study of the Liver 2018).
Immunosuppressant drug levels must be monitored during and after anti-HCV therapy and adapted as needed. The combination of sofosbuvir with an NS5A inhibitor (velpatasvir, ledipasvir, or daclatasvir, according to the HCV genotype) is preferred because of the lack of major effect on immunosuppressant drug levels. Ribavirin should be added with caution in patients with decompensated cirrhosis. The fixed-dose combination of glecaprevir and pibrentasvir will be preferred in patients with advanced chronic kidney disease (stage 4 or 5 and/or on hemodialysis) in the posttransplant setting; immunosuppressant drug levels may need to be adjusted during and after this therapy (European Association for the Study of the Liver 2018).
Organs from HCV-infected donors can now be transplanted to HCV RNA-positive recipients in many settings, provided that rapid posttransplant antiviral therapy is guaranteed following the rules described for liver transplant recipients with HCV recurrence (European Association for the Study of the Liver 2018).
TREATMENT OF HCV-RELATED EXTRAHEPATIC MANIFESTATIONS
The above-recommended HCV DAA treatments have been shown to improve the clinical manifestations of mixed cryoglobulinemia (Saadoun et al. 2016, 2017; Sise et al. 2016; Bonacci et al. 2017; Comarmond et al. 2017; Emery et al. 2017; Gragnani et al. 2017). They should also be used in patients with HCV-associated non-Hodgkin lymphoma (European Association for the Study of the Liver 2018).
In patients with mild-to-moderate renal impairment (eGFR ≥ 30 mL/min/1.73 m2), any of the above-recommended regimens can be used. In contrast, in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) and in patients with end-stage renal disease on hemodialysis, the fixed-dose combination of gleparevir and pibrentasvir should be preferred as a first-line therapy.
RETREATMENT OF PATIENTS WHO FAILED AFTER A PROTEASE INHIBITOR- AND/OR NS5A INHIBITOR-CONTAINING REGIMEN
HCV resistance testing before retreatment in patients who failed after any of the DAA-containing treatment regimens is useful to guide retreatment by probabilities of response according to the resistance profile (European Association for the Study of the Liver 2018).
Two phase III trials showed the safety and efficacy of the triple combination of sofosbuvir, velpatasvir, and voxilaprevir for 12 weeks in patients who failed to achieve SVR after treatment containing a protease and/or an NS5A inhibitor (Bourlière et al. 2017). The triple combination of sofosbuvir and the fixed-dose combination of glecaprevir and pibrentasvir is an interesting alternative for retreatment of difficult-to-cure patients, including those with complex NS5A RAS patterns and/or with advanced liver disease (excluding decompensated cirrhosis) who have experienced several unsuccessful courses of treatment (Wyles et al. 2019). In particularly difficult-to-cure patients, the addition of ribavirin and/or extension of treatment duration to 16–24 weeks may be beneficial, although data are lacking to support this recommendation.
COMPETING INTEREST STATEMENT
The author has received research support from Abbott and Vela Diagnostics. He has served as an advisor for Abbott, Abbvie, Gilead, Merck, and Siemens Healthcare.
Footnotes
Editors: Arash Grakoui, Jean-Michel Pawlotsky, and Glenn Randall
Additional Perspectives on Hepatitis C Viruses: The Story of a Scientific and Therapeutic Revolution available at www.perspectivesinmedicine.org
REFERENCES
- ANRS Collaborative Study Group on Hepatocellular Carcinoma. 2016. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol 65: 734–740. 10.1016/j.jhep.2016.05.045 [DOI] [PubMed] [Google Scholar]
- Bonacci M, Lens S, Londoño MC, Mariño Z, Cid MC, Ramos-Casals M, Sánchez-Tapias JM, Forns X, Hernández-Rodríguez J. 2017. Virologic, clinical, and immune response outcomes of patients with hepatitis C virus-associated cryoglobulinemia treated with direct-acting antivirals. Clin Gastroenterol Hepatol 15: 575–583.e1. 10.1016/j.cgh.2016.09.158 [DOI] [PubMed] [Google Scholar]
- Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, et al. 2017. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 376: 2134–2146. 10.1056/NEJMoa1613512 [DOI] [PubMed] [Google Scholar]
- Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, et al. 2019. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 393: 1453–1464. 10.1016/S0140-6736(18)32111-1 [DOI] [PubMed] [Google Scholar]
- Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O'Leary JG, Vargas HE, et al. 2015. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 149: 649–659. 10.1053/j.gastro.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Comarmond C, Garrido M, Pol S, Desbois AC, Costopoulos M, Le Garff-Tavernier M, Si Ahmed SN, Alric L, Fontaine H, Bellier B, et al. 2017. Direct-acting antiviral therapy restores immune tolerance to patients with hepatitis C virus-induced cryoglobulinemia vasculitis. Gastroenterology 152: 2052–2062.e2. 10.1053/j.gastro.2017.02.037 [DOI] [PubMed] [Google Scholar]
- Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, Wong D, Janssen HLA, Feld JJ. 2017. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol 112: 1298–1308. 10.1038/ajg.2017.49 [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. 2018. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 69: 461–511. 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. 2019. Reply to: “Sofosbuvir/velpatasvir for patients with chronic genotype 3 HCV infection with compensated cirrhosis: response to EASL recommendations on treatment of hepatitis C 2018”: EASL recommendations on treatment of hepatitis C 2018: precision on the treatment of patients with genotype 3a infection and compensated cirrhosis. J Hepatol 70: 562–564. 10.1016/j.jhep.2018.11.004 [DOI] [PubMed] [Google Scholar]
- European Association for Study of the Liver, Asociacion Latinoamericana para el Estudio del Higado. 2015. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63: 237–264. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- European Union HCV Collaborators. 2017. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol 2: 325–336. 10.1016/S2468-1253(17)30045-6 [DOI] [PubMed] [Google Scholar]
- Fourati S, Rodriguez C, Hézode C, Soulier A, Ruiz I, Poiteau L, Chevaliez S, Pawlotsky JM. 2019. Frequent antiviral treatment failures in patients infected with hepatitis C virus genotype 4, subtype 4r. Hepatology 69: 513–523. 10.1002/hep.30225 [DOI] [PubMed] [Google Scholar]
- Freeman JA, Hill A. 2016. The use of generic medications for hepatitis C. Liver Int 36: 929–932. 10.1111/liv.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragnani L, Piluso A, Urraro T, Fabbrizzi A, Fognani E, Petraccia L, Genovesi A, Giubilei L, Ranieri J, Stasi C, et al. 2017. Virological and clinical response to interferon-free regimens in patients with HCV-related mixed cryoglobulinemia: preliminary results of a prospective pilot study. Curr Drug Targets 18: 772–785. 10.2174/1389450117666160208145432 [DOI] [PubMed] [Google Scholar]
- Krishnan P, Pilot-Matias T, Schnell G, Tripathi R, Ng TI, Reisch T, Beyer J, Dekhtyar T, Irvin M, Xie W, et al. 2018. Pooled resistance analysis in patients with hepatitis C virus genotype 1 to 6 infection treated with glecaprevir-pibrentasvir in phase 2 and 3 clinical trials. Antimicrob Agents Chemother 62: e01249 10.1128/AAC.01249-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, et al. 2016. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 16: 685–697. 10.1016/S1473-3099(16)00052-9 [DOI] [PubMed] [Google Scholar]
- Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V, et al. 2017. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 152: 142–156.e2. 10.1053/j.gastro.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Negro F. 2018. Expanded benefits of curing the extrahepatic manifestations of HCV infection. Gut 67: 1917–1919. 10.1136/gutjnl-2018-316578 [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM. 2016. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 151: 70–86. 10.1053/j.gastro.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Polaris Observatory HCV Collaborators. 2017. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2: 161–176. 10.1016/S2468-1253(16)30181-9 [DOI] [PubMed] [Google Scholar]
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. 2017. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70: 1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D, Thibault V, Si Ahmed SN, Alric L, Mallet M, Guillaud C, Izzedine H, Plaisier A, Fontaine H, Costopoulos M, et al. 2016. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann Rheum Dis 75: 1777–1782. 10.1136/annrheumdis-2015-208339 [DOI] [PubMed] [Google Scholar]
- Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, de Saint Martin L, Comarmond C, Bouyer AS, Musset L, et al. 2017. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology 153: 49–52.e5. 10.1053/j.gastro.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N, et al. 2016. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 63: 408–417. 10.1002/hep.28297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Magri A, Bonsall D, Ip CLC, Trebes A, Brown A, Piazza P, Bowden R, Nguyen D, Ansari MA, et al. 2019. Resistance analysis of genotype 3 hepatitis C virus indicates subtypes inherently resistant to nonstructural protein 5A inhibitors. Hepatology 69: 1861–1872. 10.1002/hep.29837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reau N, Kwo PY, Rhee S, Brown RS, Agarwal K, Angus P, Gane E, Kao J-H, Mantry PS, Reddy KR, et al. 2017. MAGELLAN-2: safety and efficacy of glecaprevir/pibrentasvir in liver or renal transplant adults with chronic hepatitis C genotype 1-6 infection. J Hepatol 66: S90–S91. 10.1016/S0168-8278(17)30444-0 [DOI] [Google Scholar]
- Wyles D, Weiland O, Yao B, Weilert F, Dufour JF, Gordon SC, Stoehr A, Brown A, Mauss S, Zhang Z, et al. 2019. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection. J Hepatol 70: 1019–1023. 10.1016/j.jhep.2019.01.031 [DOI] [PubMed] [Google Scholar]