Abstract
Despite the continuous deployment of new treatment strategies and agents over many decades, most disseminated cancers remain fatal. Cancer cells, through their access to the vast information of the human genome, have a remarkable capacity to deploy adaptive strategies for even the most effective treatments. We note there are two critical steps in the clinical manifestation of treatment resistance. The first, which is widely investigated, requires molecular machinery necessary to eliminate the cytotoxic effect of the treatment. However, the emergence of a resistant phenotype is not in itself clinically significant. That is, resistant cells affect patient outcomes only when they succeed in the second step of resistance by proliferating into a sufficiently large population to allow tumor progression and treatment failure. Importantly, proliferation of the resistant phenotype is by no means certain and, in fact, depends on complex Darwinian dynamics governed by the costs and benefits of the resistance mechanisms in the context of the local environment and competing populations. Attempts to target the molecular machinery of resistance have had little clinical success largely because of the diversity within the human genome—therapeutic interruption of one mechanism simply results in its replacement by an alternative. Here we explore evolutionarily informed strategies (adaptive, double-bind, and extinction therapies) for overcoming treatment resistance that seek to understand and exploit the critical evolutionary dynamics that govern proliferation of the resistant phenotypes. In general, this approach has demonstrated that, while emergence of resistance mechanisms in cancer cells to every current therapy is inevitable, proliferation of the resistant phenotypes is not and can be delayed and even prevented with sufficient understanding of the underlying eco-evolutionary dynamics.
Metastatic cancer can be viewed as a speciation event where one or several cells of a multicellular organism (e.g., humans) proliferate and become the unit of natural selection, essentially becoming a new protozoan. Throughout this review, we use “ecological” to refer to changes in the abundance, distribution, and tumor ecosystem of the cancer cell populations (Maley et al. 2017). We use “evolutionary” to refer to changes in the heritable traits of cancer cell populations that can occur via genetic mutations, epigenetic changes, chromosomal rearrangements, gene duplication, aneuploidy, etc. (Ganem et al. 2007; Sun et al. 2017; Pienta et al. 2020).
For the cancer cells, the tumor represents their ecosystem. Within a patient, the cancer cells exhibit ecological dynamics defined as spatial and temporal changes in the distribution and abundance of the different cancer cell subpopulations. Furthermore, the cancer cell lineages exhibit evolutionary dynamics defined as changes in the heritable phenotypes of the subpopulations. Evolutionary dynamics often occur in response to natural selection, particularly regarding the evolution of therapy resistance.
The combined ecological and evolutionary dynamics are remarkably robust to therapeutic perturbations. In part, this is due to cellular diversity as spatial heterogeneity in the genotypic and phenotypic properties of tumor cells inevitably alter their response to applied treatments. In part, this is due to variations in the tumor environment, largely governed by variations in blood flow, which can alter both the delivery of a systemic agent and local concentrations of factors (e.g., oxygen) that may alter the efficacy of the treating agent (Stylianopoulos et al. 2018). Finally, in part, the response and resistance of cancer cells is governed by their complex interactions with adjacent host cells, which can provide local sanctuaries that permit tumor cells to survive treatment that would ordinarily be lethal.
In the absence of the evolution of resistance, most drugs and therapies would cure patients. In the case of first-line therapies, this is because the drugs have been designed specifically to eliminate the predominant cancer cells within the patient's tumor(s). In that sense, most drugs are ecologically successful. Given the current traits of the cancer cells, the drugs either directly kill cancer cells or prevent them from proliferating. In response, the cancer cell population experiences a negative growth rate and the patient's tumor burden declines as either a partial or complete response to the therapy. But, essentially, all drugs that have been developed prove to be evolutionarily unsuccessful, particularly for patients suffering from the metastatic disease. The general mechanism for failure is simply that the traits of cancer cells are not fixed. Rather, because of heritable variation, cancer cells can respond and adapt via evolution by natural selection to novel hazards and opportunities. Thus, through evolution, the patient's cancer, even with a rapidly declining population, can experience “evolutionary rescue” (Bell 2017), which allows a small number of cells to survive and then proliferate.
Evolutionary rescue and treatment failure happens because (1) there is a small preexisting population of resistant cancer cells that now grow and fill the space left by the now dying sensitive cells; (2) as the populations of sensitive cancer cells are collapsing, there is both selection for and time to produce mechanisms of resistance (Murtaza et al. 2013); and (3) residual populations of cancer cells may have been exposed to sublethal concentrations of drug, allowing them to gradually acclimate to the drug resulting in evolution of a fully resistant phenotype (similar to the laboratory procedure for developing resistant cancer cell lines by slowly increasing drug doses over a matter of months) (Rosa et al. 2014). As has been noted by others, despite the realization that cancer drugs fail because of evolution, concepts from evolution by natural selection are virtually absent (<1%) from publications on cancer-treatment outcomes (Nooter and Herweijer 1991; Aktipis et al. 2011).
In general, there are two approaches to investigating therapy resistance. The most common avenue of research focuses on identifying the specific molecular mechanisms of resistance. This is perhaps best illustrated in the extensive literature on membrane extrusion pumps. P-glycoproteins (PgPs) (Bosch and Croop 1996), for example, use ATP to actively remove cytotoxic agents from the tumor cell cytoplasm and send them back into the environment. This approach has the advantage of identifying molecular mechanisms that can be targeted and thus defeated. However, despite decades of research in well-recognized adaptive mechanisms such as the multidrug resistance (MDR) genes (Nooter and Herweijer 1991), this approach has not led to clinically significant therapy. In large part, this is the result of the remarkable diversity of adaptive strategies available through the human genome. Thus, successful therapeutic intervention targeting one resistance mechanism simply selects for a second available strategy (Yang et al. 2013). Such MDR is commonly seen in breast cancer, among others, and can include resistance to both chemotherapeutic agents and immune therapies (Chen and Sikic 2012; Tang et al. 2016; Kadkol et al. 2019). Using drugs in combination or sequentially can prolong progression-free survival but does not seem to prevent the evolution of fully resistant cancer cells. In contrast, treatment of pediatric acute lymphocytic leukemia by sequencing of several drugs consistently cures the cancer prior to complete MDR. We will return to this treatment strategy as an example of evolution-based extinction therapy (Gatenby et al. 2019, 2020; Reed et al. 2020) below.
Importantly, however, we note that the expression of a resistance mechanism does not ensure that the resistant population will rapidly proliferate leading to tumor progression. Thus, while the mechanisms of adaptation to the toxic effects of therapy are molecular, the clinical relevance of resistance is entirely dependent on proliferation of resistant populations. The evolution of resistance becomes the first step in therapy failure. For clinically relevant failure, the whole cancer population must recover ecologically as resistant cells replace sensitive ones, or resistant cells expand from micrometastases. The speed of relapse will be influenced by the magnitude of the resistance cost to the cancer cell, the effectiveness of the resistance mechanism, and the favorability of their local environment and their competition with other cellular populations (Enriquez-Navas et al. 2015, 2016).
Here we review treatment strategies that explicitly incorporate evolutionary principles to limit proliferation of resistant and prolong time to progression. Even when considered incurable, conventional cancer therapies still aim for a therapy regimen that maximizes the rate and magnitude of response. The goal is to kill as many cancer cells as possible within the shortest amount of time. Thus, cancer treatments are typically administered at the maximum tolerated dose (MTD). Indeed, this principle is so universally accepted that the goal of phase 1 trials—necessary for clinical translation of any cancer drug—is explicitly determining the MTD, which is then used in all subsequent investigations. While killing the maximum number of tumor cells with the greatest possible drug dose is intuitively appealing, we propose that it is usually evolutionarily unwise. This reflects the principle noted above. MTD applies the greatest evolutionary force for resistance to emerge, and, ecologically, it provides competitive release (Newton and Ma 2019) for the resistant cancer cells that no longer may be suppressed by the sensitive cells that had previously prevailed in the tumor. If the therapy regimen does not effect a cure, then it simply speeds Darwinian dynamics toward a resistant population that makes disease progression inevitable. Without a paradigm shift in the way we view cancer therapy, evolution of therapy resistance shall remain a leading cause of patient death.
The rationale and intuitive appeal of MTD is that it seems to offer the potential for cure if the cancer population can be eradicated before resistance evolves. If the patient is cured before resistance can evolve, then it is an obvious success. Furthermore, if rarely occurring or combinations of mutations are required for the existing cancer cells to become resistant, then reducing their population to the smallest level possible may slow the rate at which the necessary mutations arise. Random mutations associated with resistance are more likely in a large than a small population. Such logic can fail on two counts. First, evolving partial resistance may precede the evolution of complete resistance. This can happen when the resistance trait is already encoded in the genome allowing up-regulation of receptors or membrane pumps (Sartorius and Krammer 2002; Shayan et al. 2017). Or it can happen when the cancer cells use phenotypic plasticity to transition into a more resistant state such as cancer stem cells, quiescent cells, persister cells (Ramirez et al. 2016), or poly-aneuploid cancer cells that permit some to survive. These form the nucleus from which more robust resistance mechanisms can then evolve as also seen in bacteria and yeast (Benveniste and Davies 1973). Second, there is growing evidence that resistant phenotypes already exist at low frequency within the pretreatment tumor. Intrinsic resistance describes the innate resistance of the cancer cells to therapy or the existence of a preexisting resistant subpopulation. Acquired resistance refers to the emergence of more resistant phenotypes as natural selection exerts directional selection for increasingly resistant phenotypes that were not preexisting within the tumor (Sarmento-Ribeiro et al. 2019). The preexistence of resistant phenotypes have been identified for checkpoint inhibitors (Sarmento-Ribeiro et al. 2019), osimertinib (Taniguchi et al. 2019), and BET inhibitors in colorectal cancer (Ma et al. 2017).
As noted by prior authors (Enriquez-Navas et al. 2015), cancer cells can achieve rapid evolutionary changes by loss-of-function mutations, gene duplications, and gain-of-function by simply epigenetically accessing genes of the entire human genome that otherwise would not be expressed in cells of the particular tissue of origin. This likely contributes to resistance even after initial therapy has reduced the cancer population to a mere fraction of its pretreatment size. For example, simply increasing RNA expression (Heim and Lage 2005) for proteins conferring resistance can allow cancer cells to acclimate to the therapy and begin the process of evolving more robust resistance mechanisms. PgP (Schneider et al. 1989; Fletcher et al. 2010; Wind and Holen 2011) provides such an example. PgP expression increases under stressful conditions such as hypoxia and low Ph. And it may provide cancer cells with a degree of phenotypic plasticity for responding to novel chemical stressors including some chemotherapies.
Poly-aneuploid cancer cells (Amend et al. 2019) provide another example for why even small cancer cell populations can evolve and mount an ecological recovery. Their frequency increases in response to stress including therapy. Such cells up-regulate varied metabolic pathways relative to their more fragile diploid state, and then they accelerate evolution toward resistant forms. Remarkably, after surviving the stressor, poly-aneuploid cells can resume a diploid state that is now therapy-resistant (Amend et al. 2019). Environmentally mediated drug resistance (Lin et al. 2019) can act in concert to the aforementioned pathways to resistance. The cancer cells may find safety in pockets of normal cells, or from the detoxifying activities of cancer-associated fibroblasts (Li et al. 2015b).
CANCER THERAPY AND ECO-EVOLUTIONARY DYNAMICS
Cancers are a disease of Darwinian dynamics where natural selection favors cancer cell lineages that have phenotypes that maximize their fitness (survival and proliferation rates) given the circumstances. The circumstances represent the ecology of the cancer cells within their tumor environments. Hence the cancer cells and their habitats represent an open, complex adaptive system (Schwab and Pienta 1996). It is open because the cancer cells rely entirely on nutrients supplied by the host, and the cancer cells do and must signal and communicate with the surrounding normal cells including vasculature, fibroblasts, and various cells of the immune system. The system is complex in that there are multiple interacting components, in addition to the cancer cells, that also exhibit nonlinear, interacting dynamics (the normal cell types, for instance). It is adaptive in that the cancers evolve phenotypes that improve their fitness, and the host as a variety of homeostatic feedbacks and mechanisms that attempt to maintain and restore normal states and functions. In response to perturbations of either the components or their interactions, one can expect to see nonintuitive, unexpected, and unintended consequences (Levin 2003). Such will be the case for cancer therapies that act to perturb the cancer cells and their tumor ecosystem.
Traditional cancer therapies rely on an intuitively appealing premise. It is assumed that best results emerge from applying therapies that aim to kill the maximum number of cancer cells without intolerably negative effects on the patient (Norton and Simon 1986; Rodrigues and de Arruda Mancera 2013). Both drug development and therapy seek to determine and then administer the MTD. This leads to several therapy regimens. One protocol achieves maximum cell death by administering regular doses of the therapeutic agent at levels limited only by concern for fatal toxicity. Such dosing may be punctuated by “drug holidays” where therapy is reduced or stopped when adverse patient effects reach unacceptable levels. In some cases, drug holidays are preplanned leading to a “metronomic” strategy (Gnoni et al. 2015). Such a strategy can either administer lower doses of therapy but more frequently or higher doses of therapy but with temporary breaks. Metronomic regimens try to reduce toxicity to the patient while permitting a higher cumulative amount of administered drug.
If cancer is evolutionarily dynamic and responsive to natural selection then conventional assumptions about therapy have serious flaws (Axtell and Arends 1990; Gatenby 2009; Gatenby et al. 2009b; Silva et al. 2012; Renton et al. 2014; Jansen et al. 2015; Kam et al. 2015). The most desirable outcome of therapy is a complete cure. When traditional therapies have a high likelihood of killing all cancer cells then they should be applied. Dead cancer cells do not evolve. However, most metastatic cancers remain incurable and lethal under current practice. A therapy that aims to eradicate the cancer is futile. In this case, a therapy that aims to contain and control becomes the evolutionarily sound course of action. Maximum dose therapy may destroy the entire population of sensitive cells, but in the process imposes intense selection for resistant phenotypes. The resistant phenotypes become highly adaptive and proliferative both because of their resistance to the therapy and their release from competition from the hitherto more successful sensitive cancer cells (Axtell and Arends 1990; Renton et al. 2014).
Pest and weed management from agriculture provides an unlikely but welcome parallel problem (Oliveira et al. 2007; Neve et al. 2009). Like cancer therapy, the norm for decades was to apply pesticides at extremely high doses with the goal of either eradicating or at the very least achieving maximal immediate reductions of the pest (witness DDT). Like therapy, applications of biocides were limited by cost and by known side effects for human health, water quality, and the environment. Observations and field experiments on resistance in agricultural pests date to the early 1900s (Melander 1914). The diamondback moth (Plutella xylostella) and Colorado potato beetle (Leptinotarsa decemlineata) are resistant to all chemicals approved for their control (Georghiou and Taylor 1986; Zolfaghari et al. 2019). The bollworm (Helicoverpa zea) has recently demonstrated resistance to corn and cotton that had been genetically modified to kill the bollworm (Tabashnik et al. 2019).
In response, starting in 1968, it has been informal and now formal policy within the U.S. Department of Agriculture (USDA) to have resistant management plans that anticipate, measure, and mitigate the consequences of pests evolving resistance. Practice now places a greater emphasis on limiting the application of pesticides to maintain sensitivity in the pest and the long-term efficacy of the pesticide. The goal is to prevent the emergence of pesticide resistance while maintaining a low and acceptable level of crop damage (Oliveira et al. 2007; Neve et al. 2009).
Principles of Darwinian dynamics are also incorporated into the management and control of invasive species. For invasive species, the USDA mandates the collection of time series data to monitor the species’ ecological and evolutionary dynamics. Invasive species are known to evolve in response to their novel environment (akin to cancer cells adapting and shaping their microenvironments within the tumor) as well as to evolve resistance to chemical and biological control agents. Like the management of agricultural pests and invasive species, cancer therapy can use concepts and models of eco-evolutionary dynamics to develop resistance-management plans that use drugs more sparingly and judiciously (Cunningham 2019).
EVOLUTIONARILY INFORMED CANCER THERAPY
A resistance-management plan must consider the cancer cells’ strategies that confer resistance, competition between resistant and sensitive cancer cell populations, and the cost of resistance. How easily and at what penalty do the cancer cells circumvent the therapy? Avoiding death for any species is a strong selective force. Hence, cancer therapies that kill cancer cells will favor adaptive strategies of resistance among the survivors. Furthermore, the size of the human genome and its myriad of developmental programs whether epigenetically active or not likely offers evolving cancer multiple adaptive pathways for avoiding death. HIV has only nine protein-encoding genes (Li et al. 2015a). Fortunately, the disease can be controlled by targeting three or so of these genetically encoded pathways. Not so for cancer that may have access to the approximately 20,000 protein coding genes of the human genome. Thus, the set of evolutionarily feasible strategies available to a human cancer cell is likely orders of magnitude greater than those available to HIV.
At later-stage cancers, maximum dose therapy will not eliminate all cancer cells, and recurrence via resistant cancer cells becomes inevitable. Under these circumstances, optimal therapy requires slowing or containing the proliferation of resistant populations. When treating to contain, two general principles emerge. First, the ecological and evolutionary dynamics of the resistant population can be controlled by either changing the environment of the tumor or indirectly by managing competing populations of sensitive cancer cells. Second, the physician can use foresight to anticipate and steer the cancer, whereas the cancer cells cannot anticipate the future and can only respond to current (or past) actions of the physician. As the leader in a leader–follower game, the physician (leader) can constantly adjust the dosing and mix of drugs to exploit the ecological and evolutionary dynamics of the cancer cells (followers) (Stankova et al. 2018).
In nature, some antipredator adaptations come at a high and persistent cost (constitutive defenses). Porcupines or armadillos are burdened by the extra weight of their unwieldy quills and carapaces, respectively. In addition to foraging more slowly, these forms of deterrence require a continual maintenance cost. Other defenses can be facultative such as organisms avoiding risky times and places or spending a portion of time vigilant for predators (Brown et al. 1999). The individual can modulate these defenses over time, but they incur costs of lower feeding efficiencies and missed opportunities to occupy habitats or acquire resources. In these cases, resistance to predators carries a cost.
Other antipredator adaptations are virtually cost free. This is generally the case for camouflage coloration by insect prey attempting to avoid bird predation. Peppered moths will have some coloration and this coloration will evolve to match the color of the tree trunks on which they perch (e.g., industrial melanism [Cook and Saccheri 2013]). The long-term evolutionary effectiveness of a biological control agent such as birds or a pesticide depends heavily on the cost of the prey's antipredator strategies. The more costly, the more effective the control agent. This will be true for evolutionarily informed approaches to cancer therapy.
Understanding the cost of resistance to cancer cells of their resistance strategies becomes key to devising appropriate therapies. In avoiding the immune system, the cost of camouflage to a cancer cell by modifying surface proteins may be costly or not—costly if these proteins are vital to the cell in their current form—and not if such proteins are rather superfluous or maintain function even when tweaked. To avoid chemical therapies, cancer cells can become resistant by up-regulating molecular defense mechanisms. These may include membrane pumps, secretion of defensive compounds or modifications to intracellular metabolic pathways or detoxification pathways. The expression, maintenance, and utilization of these chemical defenses costs the cell resources that otherwise would contribute to proliferation or survivorship in other forms than just avoiding therapy. Furthermore, absent therapy, the maintenance costs and missed opportunity costs of these “antipredator” adaptations will indubitably render the resistant cells less competitive than sensitive cells.
Up-regulating the membrane transporter PgP may confer MDR, and it may be an example of a costly resistance strategy (Schneider et al. 1989; Fletcher et al. 2010; Wind and Holen 2011). As a membrane transporter, PgP eliminates a variety of intracellular toxins and wastes including chemotherapies. The cancer cell can reduce the effectiveness of many chemical drugs by increasing the number and activity of these transporters. But there are operating and maintenance costs to the cell. A membrane pump uses two ATPs for every molecule transported out of the cell (Szakács et al. 2014). In addition to the cost of using the transporter, the material used to maintain a high capacity is now unavailable for other cell functions or proliferation (Choi and Yu 2014). This cost of resistance likely increases under resource limitation. Many such cells vying for glucose, glutamine, or fatty acids to fuel these pumps will depress the resources available to each other. As evidence that up-regulating transporters is costly, cancer cells with MDR only become common following chronic exposure to the drugs, and the cell population may revert to primarily sensitive cells upon removal of the drugs (McDermott et al. 2014). Evidence suggests that resistance costs also occur when the mechanisms involve up-regulation of receptors in response to hormone therapies or rewiring of metabolic pathways in response to targeted therapies (e.g., tyrosine kinase inhibitors).
Both competition between sensitive and resistant cancer cell types and the cost of resistance can form the basis for evolutionarily informed therapies (Gatenby 2009; Gatenby et al. 2009b).
An emerging yet untested form of evolutionarily informed therapy is extinction therapy where the additional therapeutic agents are sequenced in relatively quick succession. The goal is to cure the patient while never using a drug long enough to permit the evolution of resistance. In theory, extinction therapy starts with a first-strike therapy that leaves the tumor fragmented and the remaining cancer cell populations small and isolated. Prior to relapse and near the nadir of the first-strike's efficacy, second-strike agents are applied in relatively quick succession. These exploit the unique vulnerabilities of small, isolated cancer populations and prevent their recovery and increase the likelihood that, one-by-one, these subpopulations of cancer cells will go extinct (Gatenby and Brown 2020; Gatenby et al. 2020).
EXPLOITING CANCER'S ECOLOGICAL AND EVOLUTIONARY DYNAMICS
There are three crucial elements that make cancers and their treatment highly dynamic. First, the distribution and abundance of cancer clones. These can be viewed as coexisting cytotypes, with perhaps distinctive niches (Lloyd et al. 2016) that can change rapidly. Second, through feedbacks between cancer cell activities, the host and intratumoral properties, the tumor ecosystem can rapidly change in size, character, and spatial heterogeneity. Third, the heritable traits of some or all the cancer cell types may change directly in response to therapy or in response to changes in the tumor environment. The first two dynamics describe the ecological dynamics of cancers and the tumor environment, and the third describes the evolutionary dynamics of changing frequencies and heritable phenotypes of the cancer cytotypes themselves.
Therapy acts on all three of these dynamics in intended and unintended ways. The ecological collapse of the whole tumor population is generally the intended and most conspicuous effect of initial treatment. Less conspicuous but in some ways more importantly, the environment of most surviving cancer cells will have been highly disrupted. The surviving cells will not be a random sample of the original population, and the places where they survive will be a biased subset of the original tumor microenvironments. With progression, the tumor burden has recovered ecologically. But, at this point of second-line therapies, the composition of cancer cell types, their phenotypes, and their microenvironments will be quite different from what they had been. The persistent or recurrent cancer cells will possess new phenotypic and genotypic profiles.
Cancer therapy becomes somewhat analogous to predator–prey coevolution. The deer flees (cancer cells) and the wolf pursues (therapy [Bakker 1983]). In nature, the reciprocal responses of prey to predator and vice versa are dynamic, and so cancer therapy must be. Dynamic treatment strategies should anticipate and stay ahead of the intratumoral evolution. This will require frequent measures of proven biomarkers that ideally provide information on both the ecological and evolutionary state of the cancer (Fischer et al. 2015). The patient may not be cured of cancer but can now live with the cancer. In addition to keeping the patient alive, the objective must include quality and longevity of life, cumulative dosing of therapies (radiation, chemo-agents, and/or immunotherapy), and the patient's sense of well-being both during and in the absence of therapy.
Adaptive Therapy
One such approach is adaptive therapy (AT) (Gatenby 2009; Gatenby et al. 2009b). The philosophy behind AT has similarities to integrated pest management where the goal in applying biocides to agricultural pests is not to kill them all, but rather to maintain an acceptable and low level of crop damage while ensuring the long-term evolutionary efficacy of the pesticides. In a similar manner, by considering the next evolutionary moves of the cancer cell populations, we should be able to enhance and maintain the longevity and efficacy of existing anticancer drugs. Such an approach will differ from conventional chemotherapy strategies. The aim of AT is to maximize progression-free survival. While desirable, the principle aim of AT is not a reduction in cancer burden. Furthermore, AT should maintain a nonlethal and non-lifestyle-compromising tumor burden by administering a minimum of drug that is well below the MTD. Finally, drugs and dosing schedules should be constantly adjusted to the current state of the tumor and its cancer cells. In this way, the therapy regimen is constantly changing and updated to steer and exploit the cancer's evolutionary dynamics. Thus, a dynamically active and acceptable tumor burden can be maintained.
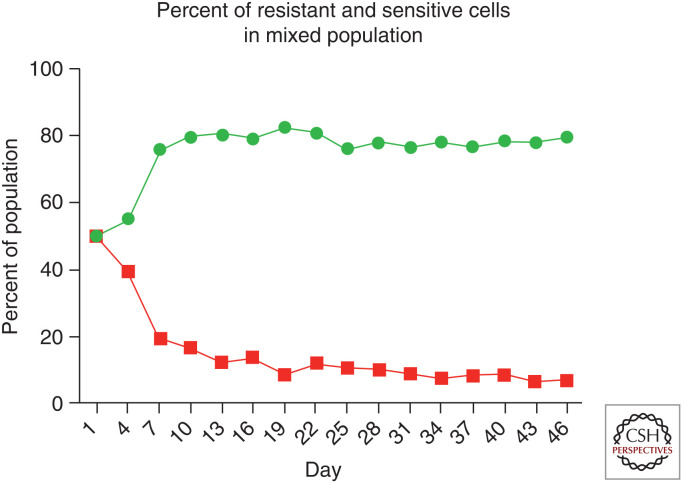
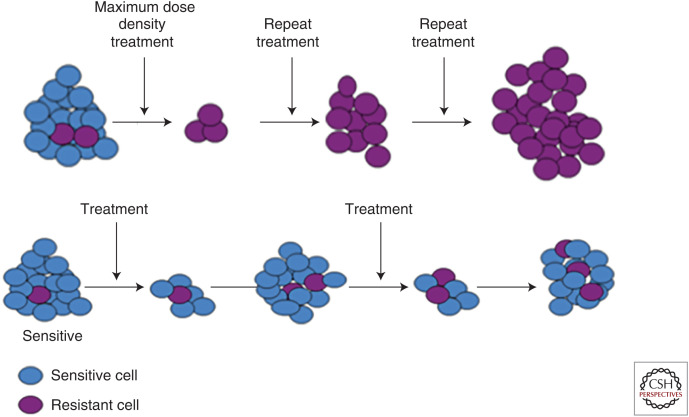
To be successful, AT must identify and exploit the molecular and phenotypic costs of resistance. For the cancer cells, resistance comes with a cost. Within the ecological context of the tumor, cancer phenotypes evolve in accordance with adaptive landscapes that describe fitness as a function of a cancer cell's phenotype. The fitness to a cancer cell of possessing a particular trait, including level of resistance, will depend on its microenvironment, presence or absence of therapy, and the densities and frequencies of the different cell types present in the cancer population. With a cost of resistance, the relative fitness of sensitive and resistant cells to each other will depend upon whether therapy is active or withdrawn (Fig. 1). The success of a cancer cell depends upon its ability to compete successfully with other cancer cells within the context of the presence or absence of therapy. AT uses therapy to suppress the sensitive cells and then withdraws therapy to let the competitively superior sensitive cells (in the absence of therapy) be the “therapy” for suppressing the resistant cells (Fig. 2). The key is to cycle therapy off before the sensitive tumor cells have been driven to extinction or so severely suppressed that they cannot exert any control over the resistant ones. The therapy is cycled on and off in a manner that seesaws the competitive balance back and forth between the two cell types. In the absence of therapy, as the sensitive cancer cells recover at the expense of the resistant ones, drugs can again be used to knock them down. The cycle is complete. Thus, tumor volume is controlled or steadily decreased through the repeated use of small doses of drugs based on the dynamic state of the tumor and its subpopulations of cancer cell types. The goal is to administer a much less than maximum dose while shrinking or maintaining at an acceptable level the overall tumor burden and total population size of cancer cells.
Figure 1.
To mimic growth dynamics of resistant and sensitive cell populations, labeled MCF7 (sensitive) and MCF7/Dox (resistant) cells were cocultured in the absence of doxorubicin with physiological levels of glucose. The phenotypic cost of resistance decreased fitness of the resistant cells and allowed the sensitive population to proliferate at the expense of the resistant phenotype.
Figure 2.
(Top row) Conventional high-dose density therapy explicitly aims to eliminate all cancer cells that are sensitive to therapy. However, this maximally selects for resistant phenotypes and eliminates competitors permitting rapid progression—an evolutionary dynamic termed “competitive release.” (Bottom row) Adaptive therapy explicitly aims to maintain a small population of cells that are sensitive to treatment. While the resistant cells survive, the metabolic cost of the molecular machinery of resistance (Fig. 1) renders them less fit in the absence of therapy. Thus, when therapy is withdrawn, the tumor will regrow, but the fitness advantage of the sensitive cells allows them to proliferate at the expense of the resistant population. At the end of each cycle, the tumor remains sensitive to therapy.
The advantage of AT over continuous therapy depends on the cost of resistance, the competitive interaction between sensitive and resistant cancer cells, and rates of cell turnover (Strobl et al. 2020). Even without a cost of resistance, simply having competition between cell types can render AT superior to continuous therapy, but therapy failure becomes inevitable. The success of AT increases with the rate of cell turnover and the cost of resistance. AT can be expected to be most superior to maximum dose if three conditions are met. First, the therapy is highly effective against sensitive cancer cells. Second, sensitive cells outcompete resistant cells in the absence of therapy. Third, sensitive cells in the absence of therapy can proliferate faster than resistant cells can in the presence of therapy. This leads to either persistent or declining cycles of total tumor burden. Initial therapy rapidly drops the population size of sensitive cells. This results in the evolution and increase of resistant populations of cancer cells. Therapy is then stopped prior to the resistant cells completely replacing the sensitive cells. At this point, the resistant cells by virtue of their faster proliferation rates and greater competitive ability will both increase and suppress the resistant cells. Prior to the sensitive cells returning to threatening population sizes, therapy must be resumed. This sequence represents a complete cycle. Such cycling works because the therapy can decisively crash the sensitive cells, and the recovery of the sensitive cells provide the therapy for the resistant cells. In practice, effective AT will require tailoring the therapy protocol to the nature of the interventions, the characteristics of the particular cancer, the nature and cost of resistance, useful metrics of tumor burden and composition, and an associated mathematical model to integrate tumor metrics to determine and manage optimal timing of therapy dosing.
Models based in catastrophe theory demonstrate why traditional therapies often fail and why evolutionarily informed therapies such as AT can increase progression-free survival or even increase the likelihood of cure (Gatenby and Frieden 2008a). Traditional therapies generally do not anticipate how residual disease will survive. A fixed MTD therapy is most likely to succeed in eliminating all cancer cells when the tumor is small, when there are no disseminated tumors, and when both the cancer cell phenotypes and their tumor ecosystem are homogeneous. An example can be seen in catastrophe models with contagion effects—nearby entities can expect to experience the same event. A tornado or fire sweeping through an area creates a tight spatial autocorrelation between the destruction of adjacent homes or trees, respectively. Yet, a few houses (or trees) will remarkably remain unscathed within an otherwise devastated landscape. These structures survive either because of quirks in the topography or because the structure may be unusually stout. In larger, disseminated, and/or heterogeneous tumors, therapy cannot eliminate all cells. Some cancer cells will survive due to the luck of residing in protective enclaves, and others will survive due to their already possessing resistant phenotypes. Heterogeneity in tumor microenvironments ensures that drug exposures will vary widely within the tumor. Heritable variation within a large population of cancer cells insures that partially or wholly resistant phenotypes already exist. Following therapy, one can expect to see the inescapable evolution and proliferation of resistant cancer cells.
The feasibility of AT and the theoretical model has proceeded in a stepwise fashion. In vitro, a breast cancer cell line was shown to have a high cost of resistance to the drug verapamil. A computational model suggested how this could be exploited to prolong progression-free survival (Silva et al. 2012). AT was then tested in a mouse model using two different breast cancer cell lines (MDA-MB-231 and MCF-7). The therapy of paclitaxel was either given continuously, cycled on or off depending on the tumor's size, or stepped down or up gradually in response to the tumor's size. AT was superior to continuous therapy, and the AT that cycled on and off in response to the state of the tumor worked best (Enriquez-Navas et al. 2016).
The response to AT appeared to have two phases: The first phase was associated with the initial application of therapy when the tumors were growing exponentially. Full, standard-of-care dosing seemed necessary to break the growth curve and drive a reduction in tumor size and cancer cell numbers. The second phase was associated with maintaining the tumor volume within acceptable bounds. Interestingly, the amount of drug necessary to maintain stability during this second phase diminished rapidly. The computational models did not predict or anticipate the need for lower and lower drug concentrations.
Changes in vasculature within the tumor during AT may explain the need for lower drug concentrations. Over time, it appeared that the tumor's blood system equilibrated on a more normal vascularity than the greatly dysregulated angiogenesis seen in the tumors of the control and standard-of-care mice. This observation points to the need to model and appreciate all three dynamics: cancer cell population size and evolution, tumor size and heterogeneity, and cancer cell–tumor ecosystem feedbacks.
Drug Mimicry to Exploit the Cost of Resistance
Operating transporters such as PgP pumps are energetically costly to a cancer cell's whose resistance strategy is to pump the toxic agent out of the cell. In defending against verapamil, perhaps half of the cell's ATP budget goes into bailing out the drug. Targeting these pumps with treatments that reduces their effectiveness has been explored extensively but has not resulted in any clinically effective strategies to prolong treatment response.
In the intratumoral environment of limited resources, this increased ATP demand requiring diversion of substrate from other activities including proliferation and invasion. Thus, an alternative treatment strategy to blocking pump function seeks to exhaust the cancer cell's resources by maximizing pump activity through administration of nontoxic (or minimally toxic) substrate (Kam et al. 2015). As discussed in Enriquez-Navas et al. (2015) as “ersatzdroges” (Kam et al. 2015), the idea is to provide an otherwise harmless chemical agent that acts as a mimic causing the resistant cancer cell to act as if this agent must be pumped out just like the actual drug. The cancer cells waste energy and there is now selection for those cancer cells that remain susceptible to the chemotherapy. In verapamil-resistant breast cancer cells lines, the application of drug mimics increased the cells’ metabolism, reduced proliferation rates and tumor growth, and the rate of metastases in a mouse model (Timcheva and Todorov 1996).
Drug mimicry may provide additional benefits. Even as a given cell wastes energy it may be compounding the problem for other cancer cells in its immediate neighborhood. By using its standard resistant mechanism such as the MDR1 pump to continuously remove the drug compound from its cytoplasm, the cancer cell returns the drug to the interstitial fluids where it may enter another cancer cell. Thus, the fake drug may create a group sellout effect (Brown et al. 2017) where a given cell expends energy to solve its problem while passing the problem onto another cancer cell.
The use of the ersatzdroges (Kam et al. 2015) as chaff exploits ecological and evolutionary dynamics. The resistant cells waste energy bailing the drug mimic. The cells become less competitive relative to other cells with fewer pumps. And evolution should now disfavor the membrane pumps or select for fewer pumps thus maintaining original drug efficacy. This represents a form of evolutionary therapy known as a double-bind therapy (Maley et al. 2004a; Gatenby et al. 2009a; Basanta et al. 2012). A double-bind therapy uses the cancer's adaptive response to one therapy (in this case, a cytotoxin) to make another more effective (forced bailing of the fake drug) and vice versa.
Targeting the Cancer's Adaptive Strategies
We advocate an evolutionarily informed approach to cancer therapy. Such an approach appreciates and anticipates the ways by which all or some of the cancer cell lineages will evolve phenotypes that subvert therapy efficacy (Stankova et al. 2018). Add wolves, and the elk will respond resulting in a cascade of ecosystem consequences (e.g., wolf reintroduction into Yellowstone National Park (Boyce 2018). Hence, the application of evolution-based therapies will require forward thinking and explicit considerations of temporal dynamics of cell numbers (ecological dynamics) and cancer cell phenotypes (evolutionary dynamics). It requires anticipating and exploiting the longer-term ecological and evolutionary dynamics as new phenotypic properties arise and increase among the cancer cells. Thinking ahead may identify the opportunities for double-bind therapies (Gatenby et al. 2009a) or “sucker's gambits” (Fig. 1; Maley et al. 2004b). In the case of double-bind therapy, the goal is to select a second-line therapy that exploits the cancer cells’ resistance strategy to the first line of therapy. Interestingly, such a therapy has been used for treating bacterial infections of the stomach caused by Heliobacter pylori (Fuentes-Hernandez et al. 2015).
In cancer, an example of a double-bind therapy happened perhaps by serendipity. In rendering an immunotherapy, the cancer cells may evolve to be more susceptible to a chemotherapy. In 29 patients with small-cell lung cancer, Antonia et al. tested for the efficacy of a p53 vaccine (Antonia et al. 2006). The therapy proved mostly ineffective. Only one patient showed even a partial response. The patients subsequently underwent chemotherapy. When used as a first-line therapy, this drug results in a response rate of <5%. However, as a second-line therapy following the vaccine, the chemotherapy produced an astonishing 67% response rate.
To be effective as a double-bind therapy, the paired sets of drugs should be given sequentially rather than together. Timing becomes critical, as the goal is to apply the first drug to not only get an ecological effect (reduced tumor burden) but an evolutionary response toward resistance. By delaying the second drug too long, tumor burdens may recover; by applying it too soon, it may be less effective from lack of any evolutionary change to the first drug (West et al. 2020). Models suggest how the sequential cycling of the two drugs can be used as an AT to prolong progression-free survival or to permanently control the disease. In our clinical example, it is possible that this evolutionary cycle could have been completed by revaccinating the patients after chemotherapy, which may have selected for increased p53 expression.
Controlling Cancer's Complex Dynamics
Most cancer therapies attempt to directly kill cancer cells while sparing normal cells as much as possible. Directly aiming to kill the pest makes sense and should always be part of a pest-management program. However, it does directly promote resistance. What if it is possible to modify the tumor environment instead? The applications of antiangiogenic therapies provide one such example. But targeting properties of the cancer's ecosystem must be done with sufficient understanding of the ecological and evolutionary properties of the cancer. With sufficient knowledge of the cancer's ecological and evolutionary dynamics, it should be possible to target environmental selection forces and steer underlying evolutionary dynamics toward cancer phenotypes that are less proliferative or invasive.
Cancers are open complex dynamical systems. Their complexity emerges from the large and diverse number of interacting components that likely exhibit nonlinear dynamics. The components include the cancer cells (which may be diverse in their phenotypes and subpopulations), the wide range of normal cell types, the nutrients supplied by the blood, and the accumulation of metabolites through cell secretions and cell death. All these components can influence each other and change over time. The system is “open” because cancer cells must interact with the host (for instance, all cancer cells derive their nutrients from the host).
Small perturbations within complex adaptive systems have the potential to become magnified in terms of subsequent changes in both the state and dynamics of the system. We should be able to exploit this property. Modeling suggests how cancer therapy can be framed as an optimal control problem (Swan 1990; Matveev and Savkin 2002), where the goal is to apply therapies in a manner that engineers cure (e.g., extinction therapy) (Gatenby and Frieden 2008b; Sehl et al. 2011; Gatenby et al. 2019, 2020), generates permanent control (the cancer becomes a livable chronic disease) (Orlando et al. 2012), or greatly stalls the emergence of lethal populations of completely resistant cancer cells (Cunningham et al. 2018). The goal is to achieve the best outcome with the least amount of therapy or toxicity to the patient (Wang et al. 2012). Ideally, one has a sufficient level of understanding of the tumor ecosystem to know when interventions will be most effective for steering and perturbing the tumor environment. What small yet decisive biological forces are available? Perturbing pH may prove useful. Cancer cells show higher levels of glycolysis resulting in the production of lactic acid. The acidic conditions of most tumors select for cancer cells that are motile and invasive. Manipulation of tumor pH can reverse these Darwinian dynamics. In a mouse model, small increases (∼0.2 pH units) in tumor pH altered evolutionary dynamics (Ibrahim-Hashim et al. 2017). Not only was cancer therapy more effective at controlling the tumor, the cancer cells themselves evolved different traits that rendered them less metastatic and less proliferative (Ibrahim-Hashim et al. 2012).
Clinical Applications
The heritable variation, struggle for existence (competitive interactions), and the influence of heritable phenotypes on fitness lead to the evolutionary and ecological dynamics characteristic of natural selection. As first principles for cancer therapy, they can and must be built into mathematical models of cancer's complex dynamics (Wang et al. 2012) in the presence and absence of therapy. The models become an essential tool for successfully understanding and managing the cancer. To test, apply, and update such models requires data of sufficient quality and of the correct type.
Currently, the paucity of relevant and usable data may be the greatest hindrance to evolutionarily informed cancer therapy. Of course, gene-expression and some other forms of molecular data exist. Much of current oncology is based on such big data. But absent from these molecular data and current analyses are the levels of spatial and temporal resolution required of evolutionary models. Purely molecular data may not fully represent the cancer cells’ phenotypes that are subjected to natural selection and represent the actual means by which cancer cells become resistant. The system is dynamic and so time series data are best, but often challenging to obtain based on cost, practicality, and patient health and comfort. Blood draws, imaging (CT and MRI), and biopsies, in that order, are increasingly difficult to obtain frequently. The models must be tailored toward the available data (Gatenby et al. 2013), and the emerging models should inform the type and frequency of data collected from the patient, within the aforementioned constraints.
We suggest that the applications of evolutionary concepts and therapies can allow us to make quantum strides by simply changing our use of existing therapies, patient and tumor metrics, and data analysis and modeling. Such results may be possible at a fraction of the cost of novel drug discovery and development.
Preliminary data from the initial clinical trial in abiraterone therapy for metastatic castrate-sensitive prostate cancer was encouraging (Zhang et al. 2017). This trial has achieved accrual goals and outcomes are slightly better than those reported in the preliminary analysis (unpubl.). Other trials in castrate-sensitive prostate cancer are underway. Reed et al. (2020) have proposed that these dynamics are applicable to treatment of metastatic pediatric sarcomas, and a multi-institutional trial (NCT04343387) testing this hypothesis is underway.
SUMMARY AND PROSPECTUS
As soon as the therapist begins treating a patient's cancer, a kind of predator–prey game of coevolution begins. If all cancer cells are not killed, then the remaining populations will begin to evolve resistance strategies. Furthermore, these resistance strategies will evolve within the complex dynamics of the tumor and its constituent cancer and normal cells.
Current practice establishes fixed schedules for administering the drugs and their doses. Therapy continues until some level of remission is achieved or until the tumor progresses. While therapists know that the drugs may induce a cascade of ecological and evolutionary dynamics, such dynamics are not necessarily measured nor used to modify therapy with the aim of controlling these eco-evolutionary dynamics. A therapy that achieves a large precipitous drop in tumor burden (perhaps as a partial or complete response) and a lengthy period of remission prior to progression can seem sensible. Yet, it is during the period of remission not at the point of progression that the game has been forfeited. Progression is the ecological manifestation of the evolutionary emergence of resistance that happened much earlier. Permitting resistant populations to triumph portends disaster for the patient even as results seem momentarily promising.
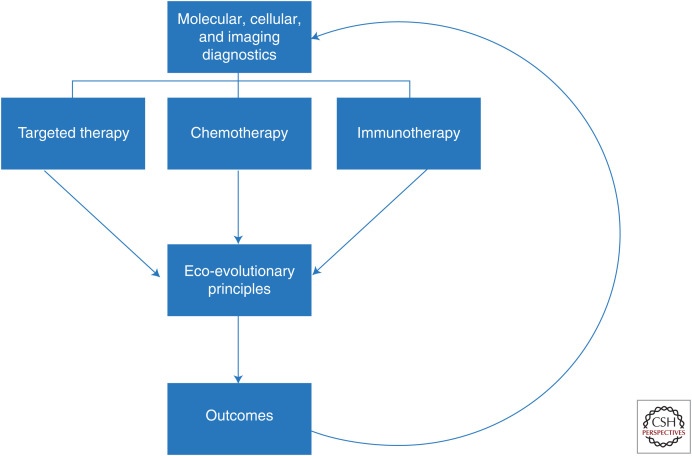
With this in mind, we advocate evolution-based therapy (Fig. 3). The therapy regimen itself should be as responsive and flexible as the cancer populations under treatment. In AT, for example, therapy might be withdrawn or switched to a new strategy based on some observable tumor response. Realizing that evolutionary failure precedes the ecological consequences of rising tumor burden, therapies should be cycled on and off (AT) (Gatenby et al. 2009b; Silva et al. 2012; Kam et al. 2015), switched (double-bind therapy), or sequenced as first and second strikes (extinction therapy).
Figure 3.
Current “personalized medicine” paradigms almost exclusively focus on defining predictive biomarkers that can identify effective treatments. This approach, however, fails to recognize that even highly effective therapies are almost always defeated by evolution of resistance. Here, we propose that “precision medicine” in cancer care requires both identification of optimal treatment modality and understanding of the Darwinian dynamics that govern response and resistance to therapy and, thus, ultimate patient outcome.
To apply evolutionarily informed therapies, physicians will need continuous or repeated measures of how the tumor and the constituent cancer cells are changing over time and space. Regular measurements of serum markers and clinical imaging provide some of the best time series of tumor dynamics. There is a need to convert clinical data into a dynamical understanding of the impact of therapy on the tumor ecosystem and the distribution, abundance, and phenotypes of the resident cancer cells. The analysis and understanding of diverse sources of time series data invite patient-specific computational models.
That this can be done is now evidenced by a successful AT trial of metastatic castrate-resistant prostate cancer (Zhang et al. 2017, 2019) that has inspired an ongoing trial with castrate-sensitive metastatic prostate cancer. A multi-institutional clinical trial on metastatic, fusion-positive rhabdomyosarcoma has just begun. It emerged from a combination of mathematical modeling, assessments of clinical outcomes, and a national meeting of pediatric oncologists (Reed et al. 2020). An evolutionary tumor board (ETB) was recently approved as a clinical trial at the Moffitt Cancer Center. Physicians can present patients who have run out of therapeutic options to the ETB, which includes evolutionary biologists, mathematical oncologists, clinical trial coordinators, cancer biologists, and physicians. A team than analyzes the unique patient's data as well as historical outcomes data. The team then offers evolution-based treatment options. The physician is then free to consider the options and institute treatment felt to be optimal based on his/her clinical judgment and consultation with the patient. The ETB is formed within a clinical trial (NCT04343365) so that every patient is followed, and his/her clinical course compared to the model predictions allowing an “n-of-1” trial.
ACKNOWLEDGMENTS
Through a sequence of unfortunate errors, the original published version of this article inadvertently substituted an early submission rather than the final, revised version of the manuscript. We thank the editors at Cold Spring Harbor Laboratory Press for their assistance in bringing this to our attention and providing the opportunity to publish the correct version along with updates. We thank Drs. Enriquez-Navas and Wojtkowski for their help on the original manuscript. This work is supported by NIH grants U54CA143970-1 and RO1CA170595 and a grant from the James S. McDonnell Foundation.
Footnotes
Editors: Charles Swanton, Alberto Bardelli, Kornelia Polyak, Sohrab Shah, and Trevor A. Graham
Additional Perspectives on Cancer Evolution available at www.perspectivesinmedicine.org
REFERENCES
- Aktipis CA, Kwan VS, Johnson KA, Neuberg SL, Maley CC. 2011. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS ONE 6: e26100 10.1371/journal.pone.0026100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amend SR, Torga G, Lin KC, Kostecka LG, de Marzo A, Austin RH, Pienta KJ. 2019. Polyploid giant cancer cells: unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate 79: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee J-H, et al. 2006. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12: 878–887. 10.1158/1078-0432.CCR-05-2013 [DOI] [PubMed] [Google Scholar]
- Axtell RC, Arends JJ. 1990. Ecology and management of arthropod pests of poultry. Annu Rev Entomol 35: 101–126. 10.1146/annurev.en.35.010190.000533 [DOI] [PubMed] [Google Scholar]
- Bakker R. 1983. The deer flees, the wolf pursues: incongruencies in predator–prey coevolution. In Coevolution (ed. Slatkin M, Futuyma DJ). Sinauer, Sunderland, MA. [Google Scholar]
- Basanta D, Gatenby RA, Anderson AR. 2012. Exploiting evolution to treat drug resistance: combination therapy and the double bind. Mol Pharm 9: 914–921. 10.1021/mp200458e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. 2017. Evolutionary rescue. Annu Rev Ecol Evol Syst 48: 605–627. 10.1146/annurev-ecolsys-110316-023011 [DOI] [Google Scholar]
- Benveniste R, Davies J. 1973. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem 42: 471–506. 10.1146/annurev.bi.42.070173.002351 [DOI] [PubMed] [Google Scholar]
- Bosch I, Croop J. 1996. P-glycoprotein multidrug resistance and cancer. Biochim Biophys Acta 1288: F37–F54. [DOI] [PubMed] [Google Scholar]
- Boyce MS. 2018. Wolves for Yellowstone: dynamics in time and space. J Mammal 99: 1021–1031. 10.1093/jmammal/gyy115 [DOI] [Google Scholar]
- Brown JS, Laundré JW, Gurung M. 1999. The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80: 385–399. 10.2307/1383287 [DOI] [Google Scholar]
- Brown JS, Cunningham JJ, Gatenby RA. 2017. Aggregation effects and population-based dynamics as a source of therapy resistance in cancer. IEEE Trans Biomed Eng 64: 512–518. 10.1109/TBME.2016.2623564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KG, Sikic BI. 2012. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res 18: 1863–1869. 10.1158/1078-0432.CCR-11-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Yu AM. 2014. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des 20: 793–807. 10.2174/138161282005140214165212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LM, Saccheri IJ. 2013. The peppered moth and industrial melanism: evolution of a natural selection case study. Heredity (Edinb) 110: 207–212. 10.1038/hdy.2012.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JJ. 2019. A call for integrated metastatic management. Nat Ecol Evol 3: 996–998. 10.1038/s41559-019-0927-x [DOI] [PubMed] [Google Scholar]
- Cunningham JJ, Brown JS, Gatenby RA, Staňková K. 2018. Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J Theor Biol 459: 67–78. 10.1016/j.jtbi.2018.09.022 [DOI] [PubMed] [Google Scholar]
- Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA. 2015. Application of evolutionary principles to cancer therapy. Cancer Res 75: 4675–4680. 10.1158/0008-5472.CAN-15-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Navas PM, Kam Y, Das T, Hassan S, Silva A, Foroutan P, Ruiz E, Martinez G, Minton S, Gillies RJ, et al. 2016. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med 8: a324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Vázquez-García I, Mustonen V. 2015. The value of monitoring to control evolving populations. Proc Natl Acad Sci 112: 1007–1012. 10.1073/pnas.1409403112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. 2010. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10: 147–156. 10.1038/nrc2789 [DOI] [PubMed] [Google Scholar]
- Fuentes-Hernandez A, Plucain J, Gori F, Pena-Miller R, Reding C, Jansen G, Schulenburg H, Gudelj I, Beardmore R. 2015. Using a sequential regimen to eliminate bacteria at sublethal antibiotic dosages. PLoS Biol 13: e1002104 10.1371/journal.pbio.1002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D. 2007. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17: 157–162. 10.1016/j.gde.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Gatenby RA. 2009. A change of strategy in the war on cancer. Nature 459: 508–509. 10.1038/459508a [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Frieden BR. 2008a. Inducing catastrophe in malignant growth. Math Med Biol 25: 267–283. 10.1093/imammb/dqn014 [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Frieden BR. 2008b. Inducing catastrophe in malignant growth. Math Med Biol 25: 267–283. 10.1093/imammb/dqn014 [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Brown JS. 2020. Integrating evolutionary dynamics into cancer therapy. Nat Rev Clin Oncol 10.1038/s41571-020-0411-1 [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Brown J, Vincent T. 2009a. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res 69: 7499–7502. 10.1158/0008-5472.CAN-09-1354 [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Silva AS, Gillies RJ, Frieden BR. 2009b. Adaptive therapy. Cancer Res 69: 4894–4903. 10.1158/0008-5472.CAN-08-3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Grove O, Gillies RJ. 2013. Quantitative imaging in cancer evolution and ecology. Radiology 269: 8–14. 10.1148/radiol.13122697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby R, Zhang J, Brown J. 2019. First strike–second strike strategies in metastatic cancer: lessons from the evolutionary dynamics of extinction. Cancer Res 79: 3174–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Artzy-Randrup Y, Epstein T, Reed DR, Brown JS. 2020. Eradicating metastatic cancer and the eco-evolutionary dynamics of Anthropocene extinctions. Cancer Res 80: 613–623. 10.1158/0008-5472.CAN-19-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georghiou GP, Taylor CE. 1986. Factors influencing the evolution of resistance. In Pesticide resistance: strategies and tactics for management (ed. National Research Council), pp. 157–169. National Academies Press, Washington, DC. [Google Scholar]
- Gnoni A, Silvestris N, Licchetta A, Santini D, Scartozzi M, Ria R, Pisconti S, Petrelli F, Vacca A, Lorusso V. 2015. Metronomic chemotherapy from rationale to clinical studies: a dream or reality? Crit Rev Oncol Hematol 95: 46–61. 10.1016/j.critrevonc.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Heim S, Lage H. 2005. Transcriptome analysis of different multidrug-resistant gastric carcinoma cells. In Vivo (Brooklyn) 19: 583–590. [PubMed] [Google Scholar]
- Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, Gatenby RA. 2012. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol 188: 624–631. 10.1016/j.juro.2012.03.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim-Hashim A, Robertson-Tessi M, Enriquez-Navas PM, Damaghi M, Balagurunathan Y, Wojtkowiak JW, Russell S, Yoonseok K, Lloyd MC, Bui MM, et al. 2017. Defining cancer subpopulations by adaptive strategies rather than molecular properties provides novel insights into intratumoral evolution. Cancer Res 77: 2242–2254. 10.1158/0008-5472.CAN-16-2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Gatenby R, Aktipis CA. 2015. Opinion: control vs. eradication: applying infectious disease treatment strategies to cancer. Proc Natl Acad Sci 112: 937–938. 10.1073/pnas.1420297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadkol H, Jain V, Patil A. 2019. Multi drug resistance in cancer therapy—an overview. J Crit Rev 1–6. 10.22159/jcr.2019v6i6.35673 [DOI] [Google Scholar]
- Kam Y, Das T, Tian H, Foroutan P, Ruiz E, Martinez G, Minton S, Gillies RJ, Gatenby RA. 2015. Sweat but no gain: inhibiting proliferation of multidrug resistant cancer cells with “ersatzdroges.” Int J Cancer 136: E188–E196. 10.1002/ijc.29158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S. 2003. Complex adaptive systems: exploring the known, the unknown, and the unknowable. Bull Am Math Soc 40: 3–19. 10.1090/S0273-0979-02-00965-5 [DOI] [Google Scholar]
- Li G, Piampongsant S, Faria NR, Voet A, Pineda-Peña AC, Khouri R, Lemey P, Vandamme AM, Theys K. 2015a. An integrated map of HIV genome-wide variation from a population perspective. Retrovirology 12: 18 10.1186/s12977-015-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Hu SQ, Xiao L. 2015b. The cancer-associated fibroblasts and drug resistance. Eur Rev Med Pharmacol Sci 19: 2112–2119. [PubMed] [Google Scholar]
- Lin KC, Torga G, Sun Y, Axelrod R, Pienta KJ, Sturm JC, Austin RH. 2019. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin Exp Metastasis 36: 97–108. 10.1007/s10585-019-09958-1 [DOI] [PubMed] [Google Scholar]
- Lloyd MC, Cunningham JJ, Bui MM, Gillies RJ, Brown JS, Gatenby RA. 2016. Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer Res 76: 3136–3144. 10.1158/0008-5472.CAN-15-2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang L, Neitzel LR, Loganathan SN, Tang N, Qin L, Crispi EE, Guo Y, Knapp S, Beauchamp RD, et al. 2017. The MAPK pathway regulates intrinsic resistance to BET inhibitors in colorectal cancer. Clin Cancer Res 23: 2027–2037. 10.1158/1078-0432.CCR-16-0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley CC, Reid BJ, Forrest S. 2004a. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol Biomarkers Prev 13: 1375–1384. [PubMed] [Google Scholar]
- Maley CC, Reid BJ, Forrest S. 2004b. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol Biomarkers Prev 13: 1375–1384. [PubMed] [Google Scholar]
- Maley CC, Aktipis A, Graham TA, Sottoriva A, Boddy AM, Janiszewska M, Silva AS, Gerlinger M, Yuan Y, Pienta KJ, et al. 2017. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer 17: 605–619. 10.1038/nrc.2017.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev AS, Savkin AV. 2002. Application of optimal control theory to analysis of cancer chemotherapy regimens. Syst Control Lett 46: 311–321. 10.1016/S0167-6911(02)00134-2 [DOI] [Google Scholar]
- McDermott M, Eustace AJ, Busschots S, Breen L, Crown J, Clynes M, O'Donovan N, Stordal B. 2014. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front Oncol 4: 40 10.3389/fonc.2014.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander A. 1914. Can insects become resistant to sprays. J Econ Entomol 7: 167–173. 10.1093/jee/7.2.167 [DOI] [Google Scholar]
- Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, et al. 2013. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497: 108–112. 10.1038/nature12065 [DOI] [PubMed] [Google Scholar]
- Neve P, Vila-Aiub M, Roux F. 2009. Evolutionary-thinking in agricultural weed management. New Phytol 184: 783–793. 10.1111/j.1469-8137.2009.03034.x [DOI] [PubMed] [Google Scholar]
- Newton PK, Ma Y. 2019. Nonlinear adaptive control of competitive release and chemotherapeutic resistance. Phys Rev E 99: 022404 10.1103/PhysRevE.99.022404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooter K, Herweijer H. 1991. Multidrug resistance (MDR) genes in human cancer. Br J Cancer 63: 663–669. 10.1038/bjc.1991.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L, Simon R. 1986. The Norton–Simon hypothesis revisited. Cancer Treat Rep 70: 163–169. [PubMed] [Google Scholar]
- Oliveira EE, Guedes RNC, Tótola MR, De Marco P Jr. 2007. Competition between insecticide-susceptible and -resistant populations of the maize weevil, Sitophilus zeamais. Chemosphere 69: 17–24. 10.1016/j.chemosphere.2007.04.077 [DOI] [PubMed] [Google Scholar]
- Orlando PA, Gatenby RA, Brown JS. 2012. Cancer treatment as a game: integrating evolutionary game theory into the optimal control of chemotherapy. Phys Biol 9: 065007 10.1088/1478-3975/9/6/065007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta KJ, Hammarlund EU, Axelrod R, Amend SR, Brown JS. 2020. Convergent evolution, evolving evolvability, and the origins of lethal cancer. Mol Cancer Res 18: 801–810. 10.1158/1541-7786.MCR-19-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M, Rajaram S, Steininger RJ, Osipchuk D, Roth MA, Morinishi LS, Evans L, Ji W, Hsu CH, Thurley K, et al. 2016. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat Commun 7: 10690 10.1038/ncomms10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Metts J, Pressley M, Fridley BL, Hayashi M, Isakoff MS, Loeb DM, Makanji R, Roberts RD, Trucco M, et al. 2020. An evolutionary framework for treating pediatric sarcomas. Cancer 126: 2577–2587. 10.1002/cncr.32777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton M, Busi R, Neve P, Thornby D, Vila-Aiub M. 2014. Herbicide resistance modelling: past, present and future. Pest Manag Sci 70: 1394–1404. 10.1002/ps.3773 [DOI] [PubMed] [Google Scholar]
- Rodrigues DS, de Arruda Mancera PF. 2013. Mathematical analysis and simulations involving chemotherapy and surgery on large human tumours under a suitable cell-kill functional response. Math Biosci 10: 221–234. 10.3934/mbe.2013.10.221 [DOI] [PubMed] [Google Scholar]
- Rosa R, Monteleone F, Zambrano N, Bianco R. 2014. In vitro and in vivo models for analysis of resistance to anticancer molecular therapies. Curr Med Chem 21: 1595–1606. 10.2174/09298673113209990226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento-Ribeiro AB, Scorilas A, Gonçalves AC, Efferth T, Trougakos IP. 2019. The emergence of drug resistance to targeted cancer therapies: clinical evidence. Drug Resist Updat 47: 100646 10.1016/j.drup.2019.100646 [DOI] [PubMed] [Google Scholar]
- Sartorius UA, Krammer PH. 2002. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 97: 584–592. 10.1002/ijc.10096 [DOI] [PubMed] [Google Scholar]
- Schneider J, Bak M, Efferth T, Kaufmann M, Mattern J, Volm M. 1989. P-glycoprotein expression in treated and untreated human breast cancer. Br J Cancer 60: 815–818. 10.1038/bjc.1989.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ED, Pienta KJ. 1996. Cancer as a complex adaptive system. Med Hypotheses 47: 235–241. 10.1016/S0306-9877(96)90086-9 [DOI] [PubMed] [Google Scholar]
- Sehl M, Zhou H, Sinsheimer JS, Lange KL. 2011. Extinction models for cancer stem cell therapy. Math Biosci 234: 132–146. 10.1016/j.mbs.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. 2017. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 6: e1261779 10.1080/2162402X.2016.1261779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AS, Kam Y, Khin ZP, Minton SE, Gillies RJ, Gatenby RA. 2012. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res 72: 6362–6370. 10.1158/0008-5472.CAN-12-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankova K, Brown JS, Dalton WS, Gatenby RA. 2018. Optimizing cancer treatment using game theory: a review. JAMA Oncol 5: 96–103. 10.1001/jamaoncol.2018.3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl M, West J, Viossat Y, Damaghi M, Robertson-Tessi M, Brown J, Gatenby R, Maini P, Anderson A. 2020. Turnover modulates the need for a cost of resistance in adaptive therapy. bioRxiv 2020.2001.2022.914366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T, Munn LL, Jain RK. 2018. Reengineering the tumor vasculature: improving drug delivery and efficacy. Trends Cancer 4: 258–259. 10.1016/j.trecan.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Hu Z, Sottoriva A, Graham TA, Harpak A, Ma Z, Fischer JM, Shibata D, Curtis C. 2017. Between-region genetic divergence reflects the mode and tempo of tumor evolution. Nat Genet 49: 1015–1024. 10.1038/ng.3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GW. 1990. Role of optimal control theory in cancer chemotherapy. Math Biosci 101: 237–284. 10.1016/0025-5564(90)90021-P [DOI] [PubMed] [Google Scholar]
- Szakács G, Hall MD, Gottesman MM, Boumendjel A, Kachadourian R, Day BJ, Baubichon-Cortay H, Di Pietro A. 2014. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem Rev 114: 5753–5774. 10.1021/cr4006236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik BE, Carrière Y, Gassmann A. 2019. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J Econ Entomol 112: 2513–2523. 10.1093/jee/toz173 [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang Y, Kiani MF, Wang B. 2016. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin Breast Cancer 16: 335–343. 10.1016/j.clbc.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yamada T, Wang R, Tanimura K, Adachi Y, Nishiyama A, Tanimoto A, Takeuchi S, Araujo LH, Boroni M, et al. 2019. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun 10: 259 10.1038/s41467-018-08074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timcheva CV, Todorov DK. 1996. Does verapamil help overcome multidrug resistance in tumor cell lines and cancer patients? J Chemother 8: 295–299. 10.1179/joc.1996.8.4.295 [DOI] [PubMed] [Google Scholar]
- Wang W-X, Ni X, Lai Y-C, Grebogi C. 2012. Optimizing controllability of complex networks by minimum structural perturbations. Phys Rev E Stat Nonlin Soft Matter Phys 85: 026115 10.1103/PhysRevE.85.026115 [DOI] [PubMed] [Google Scholar]
- West J, You L, Zhang J, Gatenby RA, Brown JS, Newton PK, Anderson ARA. 2020. Towards multidrug adaptive therapy. Cancer Res 80: 1578–1589. 10.1158/0008-5472.CAN-19-2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind NS, Holen I. 2011. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int J Breast Cancer 2011: 967419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, Wang Z, Xu CR, Huang YS, Wang BC, et al. 2013. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 79: 33–39. 10.1016/j.lungcan.2012.09.016 [DOI] [PubMed] [Google Scholar]
- Zhang J, Cunningham JJ, Brown JS, Gatenby RA. 2017. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun 8: 1816 10.1038/s41467-017-01968-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Fishman MN, Brown J, Gatenby RA. 2019. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer (mCRPC): updated analysis of the adaptive abiraterone (abi) study (NCT02415621). J Clin Oncol 37: 5041. [Google Scholar]
- Zolfaghari M, Ghadamyari M, Hassan Sajedi R. 2019. Resistance mechanisms of a field population of diamond back moth, Plutella xylostella (Lepidoptera: Plutellidae) to current organophosphate pesticides. J Crop Prot 8: 403–416. [Google Scholar]