Summary
Animals display wide-ranging evolutionary adaptations based on their ecological niche. Octopuses explore the seafloor with their flexible arms using a specialized “taste by touch” system to locally sense and respond to prey-derived chemicals and movement. How the peripherally-distributed octopus nervous system mediates relatively autonomous arm behavior is unknown. Here we report that octopus arms use a family of cephalopod-specific chemotactile receptors (CRs) to detect poorly-soluble natural products, thereby defining a form of contact-dependent, aquatic chemosensation. CRs form discrete ion channel complexes that mediate the detection of diverse stimuli and transduction of specific ionic signals. Furthermore, distinct chemo- and mechanosensory cells exhibit specific receptor expression and electrical activities to support peripheral-information coding and complex chemotactile behaviors. These findings demonstrate that the peripherally-distributed octopus nervous system is a key site for signal processing and highlight how molecular and anatomical features synergistically evolve to suit an animal’s environmental context.
Graphical Abstract

In Brief
The peripherally-distributed octopus nervous system exhibits exceptional signal filtering properties that are mediated by highly specialized, chemotactile sensory receptors that control touch-taste arm behavior.
Introduction
Animals exhibit distinct adaptations depending on their ecological and behavioral context. Octopuses are known for their complex nervous system, most of which is distributed among their eight flexible arms. Octopus arm adaptations support voracious foraging behavior within seafloor environments inaccessible to traditional sense organs to provide a predatory advantage (Hanlon and Messenger, 1996; Hochner, 2012; Young, 1971). This unique octopus chemotactile ‘touch-taste’ sense is mediated by suction cups (suckers) along the arms which sense and manipulate prey utilizing local neural signaling to integrate sensory cues and carry out arm-autonomous behaviors (Fouke and Rhodes, 2020; Hochner, 2012; Sumbre et al., 2001; Wells et al., 1965; Young, 1971). The arms contain a central axial nerve cord with brachial ganglia located at each sucker (Gutfreund et al., 2006; Young, 1971). Arm ganglia process motor and sensory information, enabling local signal processing that allows the arm, and even individual suckers, to perform autonomous behaviours (Grasso, 2008; Sumbre et al., 2001). Cells localized in the sucker rim epithelium are morphologically-similar to receptor cells in other animals (Graziadei, 1964), suggesting the sucker serves as the sensory organ for the octopus chemotactile sense, but the stimuli, and molecular and cellular mechanisms underlying this specialized sensory modality are unknown.
It has been proposed that the evolutionary transition from aquatic to terrestrial life necessitates the detection of hydrophobic airborne ligands instead of water-soluble hydrophilic molecules (Mollo et al., 2014). Indeed, aquatic chemosensation has long been associated with distant waterborne signalling via hydrophilic chemicals while terrestrial sensation utilizes detection of airborne volatile molecules that are poorly soluble in water. Nonetheless, aquatic organisms produce and respond to a variety of hydrophobic compounds, suggesting a distinct form of aquatic ‘taste’ mediated by contact-dependent chemosensation of insoluble molecules which do not readily diffuse in water. For instance, molluscs and other marine animals produce and respond to a variety of hydrophobic terpenoids, among the largest class of natural compounds, which are also widespread in terrestrial organisms (Mollo et al., 2014; Mollo et al., 2017). Thus, numerous marine organisms, including octopus, may detect poorly-soluble chemicals through various forms of contact-dependent chemosensation, but the mechanisms underlying this mode of sensation are unknown.
Here we describe a unique family of chemotactile receptors (CRs) that mediate ‘touch-taste’ sensation in Octopus bimaculoides (Fig. 1A). We identify the stimuli, cells, transduction properties, and related chemotactile behaviors, thereby defining this contact-dependent, aquatic chemosensory modality. We show that suckers contain specialized chemosensory and mechanosensory cells which display discrete electrical properties to transduce specific action potential patterns encoding chemical and touch information, respectively. Mechanosensory cells are enriched for the mechanoreceptor NompC (no mechanoreceptor potential C) which exhibits highly-conserved properties for mediating transient touch signals. Chemosensory cells use a previously uncharacterized family of receptors (CRs) to mediate contact-dependent chemosensation. CRs detect natural product extracts from prey and cephalopod ink and are stimulated by poorly-soluble terpenoid molecules, which are proposed to act as mediators of ‘touch-taste’ sensation in aquatic environments (Mollo et al., 2017). CRs diverge from neurotransmitter receptors, are cephalopod specific, and are highly enriched in the sucker sensory epithelium. They exhibit a combinatorial expression pattern with multiple CRs per chemosensory cell and form homomeric and heteromeric complexes that differentially tune agonist sensitivity and ion permeation properties. Thus, CRs mediate extensive diversity in signal detection and transduction, which is uniquely-suited for sensory coding in the semi-autonomous peripheral nervous system of the octopus. Indeed, we find octopuses explore their environment using stereotypical touch motions that are distinctly modified by contact with different CR terpenoid agonists. Thus, our results demonstrate that the peripherally-distributed octopus nervous system exhibits exceptional signal filtering properties that are mediated by highly specialized, sensory receptors.
Figure 1. Octopus arms contain distinct chemo- and mechanoreceptor cells.

(A) (Left) Octopus bimaculoides. (Middle) Putative nerve tracts from an octopus embryo sucker stained with anti-horseradish peroxidase antibody (HRP, green) and nuclear stain (DAPI, blue). (Right) HRP-positive cells in the sucker epithelium. (B) Morphologically-distinct cell types from the sensory epithelium were defined by their responses to discrete sensory stimuli. 1μm displacement of the dendritic ending activated mechanoreceptor cells, but not chemoreceptor or support cells. (C) Fish extract (<3kDa) elicited inwardly-rectifying currents from chemoreceptor cells, but not mechanoreceptor or support cells. (D) Quantification of responses in the 3 distinct cell types to mechanical and chemical stimulation. See also Figure S1 and Supplemental video 1.
Results
Cellular basis of chemotactile sensation
Similar to descriptions of octopus chemotactile behaviors along the seafloor (Hanlon and Messenger, 1996), tank-housed octopuses used their arms to explore inaccessible spaces for prey. When octopuses were separated from prey (crab) by a barrier with a small hole, octopuses reached through this opening to sample surrounding spaces with sweeping arm motions in which suckers continually probed surfaces. When suckers contacted prey, they quickly attached with arms wrapping around and retrieving prey toward the animal’s body (Fig. S1A and supplementary video 1). By contrast, if suckers touched non-prey objects, arms briefly sampled and continued their search, thus suggesting a role for the sucker in chemotactile sensation (Fig. S1A and supplementary video 1). To ask if suckers contained functional sensory cells akin to other sense organs, we isolated cells from the putative sensory epithelium around the outer rim (Fig. 1A and S1B) and directly tested for sensory responses. We readily obtained cells with three distinctive morphologies, including two with dendritic endings similar to sensory cells observed in previous histological analyses (Graziadei, 1964), and one with an epithelial shape (support) (Fig. 1B). Whole-cell patch clamp recordings revealed that cells with short, rounded dendritic endings (mechanoreceptors) could be defined by intrinsic mechanosensitive responses (Fig. 1B). Mechanoreceptors were insensitive to prey chemicals (<3kDa extract from fish), a stimulus which elicited reaching behaviors in octopuses (Fig. 1B and S1C). Morphologically-distinct chemosensory cells had long, thin dendritic endings and showed a stereotypic inwardly-rectifying current elicited by prey extract (chemoreceptors, Fig.1B – D, and S1D). In contrast to mechanoreceptors, these cells were insensitive to mechanical stimulation (Fig. 1B, D). Thus, the octopus sucker contains discrete populations of sensory cells which can be functionally-defined by their specific sensory modality, thereby establishing a cellular basis for the sucker as a multi-modal sense organ.
How are chemical signals encoded together with mechanical stimuli to elicit specific responses? To address this question, we analyzed the electrical properties of chemoreceptor and mechanoreceptor cells to determine whether they transduce different electrical signals following activation. Such distinct electrical signaling could provide a mechanism for peripheral coding of specific sensory stimuli by the arm’s distributed nervous system. While chemoreceptors and mechanoreceptors had similar resting membrane voltage (Fig. S1E), identical current injection elicited tonic voltage spiking in chemoreceptors versus phasic spiking in mechanoreceptors that was consistent across stimulus strength (Fig. 2A and S1F). Both sensory cell types exhibited similar tetrodotoxin-sensitive voltage-gated inward currents that were absent in non-excitable support cells (Fig. 2B, C and S1G – I). These results suggest similar voltage-dependent activation across sensory cells, prompting us to next analyze K+ currents, which can regulate spike frequency. Mechanoreceptors exhibited small, transient 4-AP-sensitive K+ currents that showed more prominent voltage-dependent inactivation compared with the large, sustained and incompletely-inactivating K+ currents in chemoreceptors (Fig. 2D, E and S1J – L). Considering these results, the large, incompletely-inactivating K+ currents in chemoreceptors could enable repetitive spiking by strong repolarization while transient, inactivation-sensitive K+ currents in mechanoreceptors could facilitate depolarization block and phasic spiking. These findings are important because prolonged touch would initiate the same cellular signal as brief touch, whereas chemoreceptor cell spiking could be tuned by stimulus strength, kinetics, or the effects of permeating ions on downstream signaling cascades.
Figure 2. Chemoreceptor and mechanoreceptor cells transduce distinct electrical signals.

(A) An identical current injection stimulus evoked tonic voltage spiking in chemoreceptor cells and phasic responses in mechanoreceptor cells, respectively. Support cells did not respond to similar stimuli (representative of n = 5). Identical stimuli evoked distinct spike frequencies in chemoreceptor and mechanoreceptor cells. n = 11 – 14, p < 0.0001 for > 20pA, multiple row two-tailed student’s t-test. Data represented as mean ± SEM. (B, C) Chemoreceptor and mechanoreceptor cells had similar voltage-gated inward currents, which were sensitive to tetrodotoxin (TTX, 1μM) and absent in non-excitable support cells. Chemoreceptor Va1/2 = −6.71 ± 0.62mV, Vi1/2 = −37.70 ± 1.5mV, n = 7. Mechanoreceptor Va1/2 = −5.66 ± 0.58mV, Vi1/2 = −43.57 ± 1.2mV, n = 7. (D, E) Chemoreceptor and mechanoreceptor cells had distinct 4-AP (1mM)-sensitive K+ currents: mechanoreceptor currents were activated at more negative voltages, had smaller amplitude, and exhibited more sensitive and complete voltage-dependent inactivation. Chemoreceptor Va1/2 = 13.05 ± 0.99mV, Vi1/2 = −29.80 ± 0.95mV, n = 6. Mechanoreceptor Va1/2 = −4.35 ± 1.4mV, Vi1/2 = −18.95 ± 0.95mV, n = 6. Data represented as mean ± SEM. See also Figure S1.
Molecular determinants of chemotactile sensation
To investigate the molecules involved in sensation, we generated transcriptomes from numerous octopus tissues, including a variety of traditional sense organs and reference tissues for comparison with the sucker and the sucker sensory epithelium, which contains primary sensory cells (Fig. 3A). The only previously characterized sensory receptor orthologue enriched in the sucker epithelium was the mechanoreceptor NompC (no mechanoreceptor potential C, Fig. 3B). Indeed, NompC was localized to sensory cells in sucker epithelium (Fig. 3C) and heterologously-expressed channels exhibited similar transient activation and desensitization kinetics and were blocked by Gd3+, as observed in native mechanoreceptor cells (Fig. 3D, E). Furthermore, response kinetics, pressure sensitivity, ion permeation properties of octopus NompC matched the well-characterized Drosophila melanogaster orthologue, consistent with the overall protein conservation (41.6% identity), including amino acids critical for mechanosensitivity (Yan et al., 2013) (Fig. 3E and S2A – D). Thus, we conclude that octopus suckers are mechanosensitive and propose that the conserved mechanoreceptor NompC is involved in mechanotransduction.
Figure 3. NompC is a conserved mechanoreceptor in octopus arms.
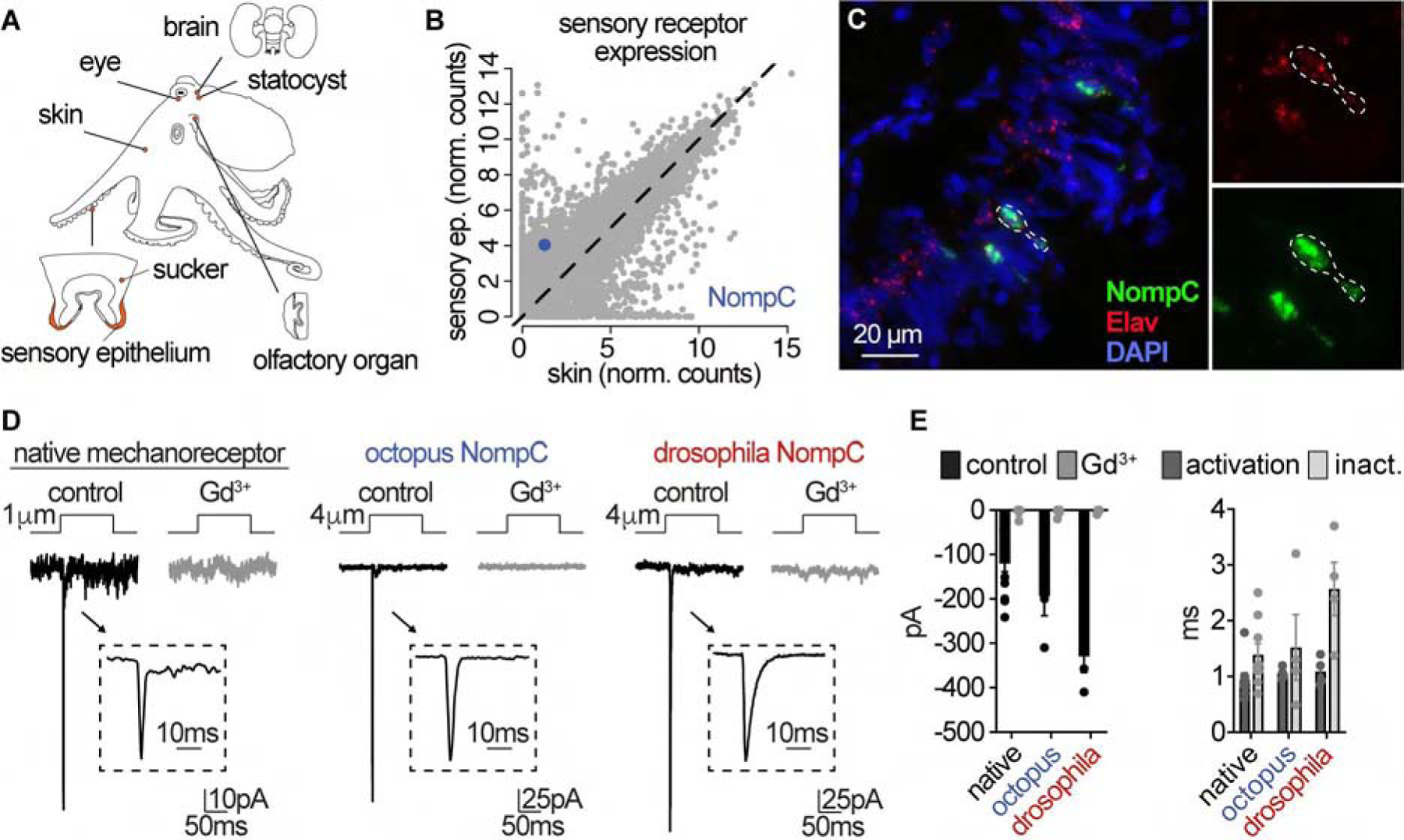
(A) Octopus tissue samples used for comparative transcriptomics. (B) Comparison of mRNA transcripts (normalized counts) revealed NompC (no mechanoreceptor potential C) as the sensory receptor orthologue enriched in the sucker epithelium. (C) Co-localization of NompC and neuronal marker Elav visualized by RNAscope in situ hybridization. (D) Comparison of mechanically-evoked currents (pipette displacement) in native mechanoreceptor cells (1.46 ± 0.18μm, n = 13) to Octopus bimaculoides NompC (4.75 ± 0.75μm, n = 4) and Drosophila melanogaster NompC (4.25 ± 0.47μm, n = 4) expressed in HEK293 cells. Untransfected cells did not respond to similar stimulation (n = 6). (E) Mechanically-evoked currents exhibited similar transient kinetics and gadolinium (Gd3+)-sensitivity (100 μM). n = 4 – 6 cells. Data represented as mean ± SEM. See also Figure S2.
Surprisingly, we did not find any known chemoreceptor orthologues enriched in the sucker epithelium. However, we did observe substantial enrichment of ionotropic receptors described in the Octopus bimaculoides genome and sucker transcriptome (Albertin et al., 2015). These receptors were classified as cephalopod-specific “atypical” acetylcholine receptors because they lack a canonical acetylcholine binding site. We found these receptors (hereinafter referred to as chemotactile receptors, CRs) were specifically expressed in the sensory epithelium of the sucker (Fig. 4A). CRs lack an acetylcholine binding site (Taly et al., 2009), and diverge from traditional acetylcholine receptors across three analyzed octopus species, suggesting a shared evolutionary origin (Fig. 4B, S3A, B, and Supplemental table 1). CR expression was restricted to the distinctive sensory cells of the sucker epithelium and, interestingly, individual CRs were co-expressed in combinatorial patterns across discrete sensory cells. (Fig. 4C and S3C). CRs were typically co-expressed with at least one other CR, a feature which could facilitate increased diversity in signal detection and transduction. Heterologously-expressed CRs robustly responded to fish and crab extract, exhibiting the same inwardly-rectifying current as observed in native chemoreceptor cells (Fig. 4D and S3D). Furthermore, CRs were insensitive to acetylcholine below 100mM, rendering them >1000X less-sensitive than human alpha7 acetylcholine receptors (hα7), which did not respond to prey extract (Fig. 4D, S3D, and S4A). To further substantiate a role for CRs in mediating chemoreceptive currents, we found the antagonist mecamylamine similarly inhibited native chemoreceptor cells and expressed CRs (Fig. S4B, C). In addition to the sensory cell-specific CR expression profile, these distinctive features closely match native chemoreceptor responses, suggesting that CRs form the predominant chemoreceptors in suckers.
Figure 4. Chemotactile Receptors Are Enriched in Sucker Epithelium, Exhibit Combinatorial Expression, and Are Sensitive to Prey-derived Chemicals.

(A) mRNA transcripts encoding CRs were enriched in the sucker sensory epithelium relative to other sampled tissues (brain, eye, statocyst, skin, olfactory organ, sucker, sensory epithelium of sucker cup). Scale: z-scaled normalized counts. (B) Phylogenetic analysis across 3 distinctdifferent octopus species revealed that CRs branch from canonical acetylcholine receptors, suggesting a common ancestor. Scale: branch length, Newick tree format. (C) CRs exhibited combinatorial expression patterns. 3 distinct CRs (CR840, CR518, and CR737) localized to the sensory epithelium, as visualized by RNAscope in situ hybridization. Nuclei stained with DAPI (blue). (D) Xenopus laevis oocytes expressing CRs were insensitive to acetylcholine (ACh, 1mM) but robustly responded to fish or crab extract with an inwardly-rectifying current similar to those observed in native chemoreceptor cells. Fish extract did not elicit currents from ACh-sensitive human α7 acetylcholine receptors (hα7) or uninjected oocytes. n = 4 – 8, p < 0.0001 for fish extract responses in CRs versus hα7, one-way ANOVA with post-hoc Bonferroni test. See also Figure S3 and S4.
CRs mediate diverse signal detection
The ecologically-relevant stimuli important for octopus chemotactile behaviors are unknown. To investigate CR sensitivity to natural chemicals which may guide foraging behavior, we selected CR518 and CR840 as ‘model’ CRs to describe fundamental receptor characteristics. We investigated these CRs because they exhibited robust heterologous expression and responded to fish and crab prey extract (Fig. S3D). Using these receptors, we found that prey-extract-evoked responses in native cells and heterologously-expressed CRs were inhibited by octopus ink (<3kDa extract), which is released as a conspecific alarm or an escape mechanism (Derby, 2007; Hanlon and Messenger, 1996) (Fig. 5A, B). Consistent with this observation, cephalopod ink has been shown to inhibit olfactory responses in squid and other animals (Gilly, 1992; Hanlon and Messenger, 1996; Lucero, 1992). By comparing CRs, we noticed the effects of octopus ink were much stronger on CR518 versus CR840 (Fig. 5B), leading us to ask whether specific CRs detect particular molecules. Indeed, separation of fish extract into hydrophilic (HPL) and hydrophobic (HPB) components evoked specific responses in each CR: CR840 responded to HPL, while CR518 was activated by HPB (Fig. 5C). Furthermore, these results were recapitulated in experiments using native cells, in which subpopulations were responsive to HPB or both fractions (Fig. 5D). Considering these results, we used a patch clamp screen to test CR sensitivity to a panel of 25 compounds from discrete structural or physiochemical classes, including those commonly detected by olfactory or taste systems in other animals (Fig. 5E). To our surprise, CRs were insensitive to most common odorants or tastants, except for the bitter compound chloroquine which potently activated CR840, and to a lesser-extent CR518 (Fig. 5E and S5A – C). Considering the unusual ‘touch-taste’ nature of the octopus chemotactile sense, we also tested poorly-soluble hydrophobic terpenoids which have been proposed as stimuli for aquatic contact-dependent chemosensory modalities (Mollo et al., 2014; Mollo et al., 2017). Intriguingly, CR518 was potently activated by terpenoids, including the physiologically-relevant sesquiterpene polygodial and furanosesquiterpene atractylon, which are defensively secreted by prey molluscs and cnidarians, respectively (Giordano et al., 2017; Long and Hay, 2006) (Fig. 5E and S5A – C). Native cells exhibited similar sensitivity to identified CR agonists, responding with biophysically-similar inwardly-rectifying currents (Fig. 5F, G). Furthermore, distinct native chemosensory cells responded to specific CR agonists, further supporting the observation that individual chemoreceptor cells express different combinations of CRs (Fig. 5F, G). Thus, native cells and CRs are activated by discrete compounds, including those involved in touch-dependent aquatic chemosensation (Mollo et al., 2017).
Figure 5. Discrete CRs and chemoreceptor cells are regulated by distinct compounds.

(A) Native chemoreceptor cells were activated by fish extract and inhibited by octopus ink (<3kDa). n = 6, p < 0.0001, one-way ANOVA with post-hoc Bonferroni test. (B) Fish extract activated oocytes expressing CR518 or CR840, and CR518 was more sensitive to ink. n = 4 – 6, p < 0.0001 for CR518 inhibition, one-way ANOVA with post-hoc Bonferroni test. (C) HEK293 expressing CR840 responded to hydrophilic (HPL) but not hydrophobic (HPB) fish fractions while CR518 was only stimulated by HPB fractions. Responses normalized to total fish extract, n = 8 – 10, p < 0.0001 one-way ANOVA with post-hoc Bonferroni test. (D) Distinct native chemoreceptor cells were sensitive to HPB or both individually-applied HPL or HPB fractions. (E) Patch clamp screen in HEK293 cells expressing CR840 or CR518 quantified as log2 (current amplitude at −110mV), n = 3 – 10, 1mM of tested compound. Both CRs robustly responded to the bitter compound chloroquine and CR518 was sensitive to several terpenoids. (F) Distinct fish-sensitive chemoreceptor cells responded to independently-applied CR-sensitive compounds (25μM). (G) Current-voltage (I-V) relationships from a native chemoreceptor cell and HEK293 cells expressing CR840 or CR518. Similar to extracts, indicated compounds evoked inwardly-rectifying currents and CRs exhibited distinct pharmacological profiles in response to 30μM atractylon and nootkatone, 200μM carvacrol, and 25μM chloroquine. Data represented as mean ± SEM. See also Figure S5.
We next examined whether specific compounds are encoded by discrete mechanisms of receptor activation. First, we used excised outside-out membrane patches to determine that CRs were directly activated by their specific agonists (Fig. S5D). Patches expressing CR518 were activated by the terpenoid atractylon and the bitter compound chloroquine, while CR840 was only stimulated by chloroquine (Fig. S5D). These results were supported by the analysis of chimeric channels in which the extracellular domain of CR840 was swapped with CR518 to confer distinct pharmacological sensitivity (Fig. S5E). Collectively, these findings indicate that the predicted extracellular N-terminus mediates agonist sensitivity and potentially ligand-binding. Voltage dependence also varied among analyzed CRs and agonists. While voltage alone elicited minimal activity, agonist-evoked currents induced during voltage ramps exhibited non-monotonic inward rectification with a characteristic ‘hook’ at very negative voltages that was variable in amplitude. To further investigate voltage-dependence for activation, we analyzed agonist-elicited conductance-voltage relationships using voltage step protocols (Fig. S5F). CR840 had steep voltage dependence with maximal conductance observed in response to the most negative voltages tested (Fig. S5F, G). CR518 activation was more pronounced at negative voltages, but was less voltage-sensitive (Fig. S5F, G). Voltage-dependent activation and deactivation kinetics also differed based on the CR and ligand (Fig. S5H, I). Thus, our results demonstrate that individual CRs can encode numerous features regarding distinct chemical stimuli.
CRs form ion channel complexes to regulate agonist sensitivity and signal transduction
Considering that individual CRs and chemoreceptor cells were selectively activated by specific compounds and that CRs were co-expressed within individual chemoreceptor cells, we wondered whether CR co-expression regulates receptor function. To test this possibility, we screened co-expressed receptors for responses to prey extract and found that some individually-expressed receptors robustly responded, while others were insensitive or did not form functional channels (Fig. 6A and S6). Interestingly, we noticed that responsive receptors exhibited differences in response amplitude and voltage-dependence if co-expressed with insensitive receptors (Fig. 6A and S6). Considering this result, we hypothesized that particular CR subunits may assemble to tune CR properties, similar to canonical acetylcholine receptors (Taly et al., 2009). Indeed, co-immunoprecipitation demonstrated that the insensitive CR828 could physically associate with responsive receptors, such as CR518 or CR840 (Fig. 6B and S7A). We next tested the functional consequence of subunit association by comparing homomeric and heteromeric CR complexes. Similar to homomeric receptors, CR518–828 and CR840–828 remained insensitive to ACh (Fig. S7B), were blocked by mecamylamine (Fig. S7C), and were selectively activated by extract fractions and molecules that affected corresponding homomeric complexes (Fig. S7D, E). While these broad pharmacological profiles were conserved, CR828 association significantly altered agonist sensitivity: Heteromeric CR840–828 exhibited markedly reduced chloroquine sensitivity compared with homomeric CR840, while heteromeric CR518–828 had enhanced nootkatone and atractylon sensitivity compared with homomeric CR518 (Fig. 6C, D and S7F). Thus, we conclude CRs can function as homomeric or heteromeric receptors to facilitate diverse agonist sensitivity. Because each sensory cell expresses combinations of CR subunits, formation of cell-specific ion channel complexes could allow for wide-ranging modes of signal detection and transduction.
Figure 6. CRs form heteromeric channel complexes that influence stimulus detection and transduction.

(A) Fish extract elicited distinct responses from oocytes depending on CR co-expression (n = 2 – 14). (B) Co-immunoprecipitation CRs with indicated epitope tags using anti-FLAG beads showed that CR828 directly interacted with CR518 or CR840 when co-expressed in oocytes. Neither CR was immunoprecipitated on FLAG beads when both co-expressed CRs were HA-tagged (* = IgG) (C) HEK293 cells expressing heteromeric CR840–828 exhibited decreased chloroquine-sensitivity compared with CR840 alone. Currents normalized to maximal currents evoked by maximal concentration in the same cell. n = 6 – 8, p < 0.0001, two-tailed Student’s t-test. (D) CR518–828 expression caused increased nootakatone-sensitivity compared with CR518. n = 8 – 12, p < 0.0001, two-tailed student’s t-test. (E) (Top) In chemoreceptor cells, fish extract evoked inwardly-rectifying currents with two distinct ion selectivity profiles: cell type 1 was permeant to monovalent ions (n = 4) and type 2 also passed Ca2+ (n = 3). (Bottom) Quantification, p < 0.0001 for Ca2+ permeation, two-way ANOVA (n = 7) with post-hoc Bonferroni test, p < 0.001. (F) (Top) HEK293 expressing CR complexes showed distinct ion selectivity. (Bottom) p < 0.05 for differences in Ca2+ permeation in CR518 and CR840 and p < 0.0001 for Ca2+ in homo- and heteromeric CR518, two-way ANOVA with post-hoc Bonferroni test (n =4–5). Data represented as mean ± SEM. See also Figure S6 and S7.
CRs are ionotropic receptors, therefore, we hypothesized that CR diversity, combinatorial co-expression profiles, and heteromeric complex formation could correspond with distinct transduction mechanisms in the form of mediating specific ionic signals. Ion substitution experiments in native cells demonstrated that prey extract evoked inwardly rectifying currents that were selective for small monovalent cations (Na+, Cs+) in approximately half of the sampled cells, while the other half responded with nearly identical inwardly rectifying currents that also passed Ca2+ (Figure 6E). Remarkably, this result was exactly recapitulated by expressed CRs; CR840 was monovalent cation-selective and CR518 permeated both monovalents and Ca2+ (Fig. 6F). Additionally, CR518 had larger single-channel conductance than CR840, meaning that opening fewer channels could activate native cells, thus providing another means of encoding distinct signals (Figure S7G). While CR828 association did not affect single-channel conductance, heteromeric CR518–828 exhibited increased Ca2+ permeation relative to homomeric CR518 (Fig. 6F). This is important because CR complex-dependent properties in ion permeation would affect downstream signal transduction by sensory cells, which in turn may provide a mechanism for encoding specific sensory signals. Collectively, these results demonstrate that CRs exhibit combinatorial expression and can associate in discrete ion channel complexes to mediate distinctive agonist sensitivity and ion permeation. Thus, CRs are capable of extensive signal filtering and coding, well-suited to contribute to peripheral processing in the distributed, semi-autonomous nervous system of the octopus arm.
CR agonists tune chemotactile foraging behavior
How are these complex chemical signals encoded together with mechanical stimulation to elicit specific chemotactile behaviors? To address this question, we developed a behavioral assay in which octopuses could probe identical surfaces infused with different CR agonists. In these experiments, the tank floor was divided into two sides and covered in agarose infused with a CR terpenoid agonist or seawater (Fig. 7A). Over the course of control trials in which both sides of the agarose floor contained only sea water, octopuses exhibited stereotypical exploratory behaviors involving sweeping arm motions in which suckers probed the agarose floor (supplemental video 2). Consistent with receiving similar tactile information, arms responded with a consistent number and duration of touches across either side of the tank floor (Fig. 7B and supplemental video 2). However, when animals touched terpenoid-infused surfaces, these exploratory behaviors were markedly affected in a chemical-dependent manner. The CR agonist polygodial, a defensive sesquiterpene emitted by marine invertebrates (Long and Hay, 2006), elicited high frequency touches with short duration and resulted in arm retraction and general avoidance (Fig. 7C and supplemental video 3). On the control side of the tank, arms exhibited normal exploratory sweeping motions, further emphasizing the requirement for contact-dependent chemosensation (supplemental video 3). Nootkatone, another sesquiterpene CR agonist, also elicited increased touches with a higher proportion exhibiting short duration, however, exposure did not evoke significant avoidance (Fig. 7D). The weak CR agonist carvacrol, a monoterpene, evoked increased touches with short duration, albeit to a lesser extent compared with other terpenoids (Fig. 7E). These results demonstrate stereotypic exploratory tactile arm behavior can be tuned by chemical stimulation. This is consistent with the integration of discrete sensory cell properties: mechanoreceptor cells exhibit narrowly-tuned transient mechanoreceptor responses and phasic action potentials, which would initiate the same signal regardless of touch duration. CRs provide diverse stimulus detection and transduction mechanisms which could modify tonic spiking activity to transmit distinct neural signals in a chemical-dependent manner.
Figure 7. Chemotactile exploration integrates CR agonists and mechanical stimuli.

(A) Octopuses explored agarose floors divided into chemical-containing and control sides. Example quantification of the number and duration of floor-touches over 10 minutes. Blue represents touches on compound-infused agarose and grey represents neutral agarose touches. (B) Control experiment in which agarose sides were independently-prepared and only contained seawater. Exploring octopuses showed no difference in the number or duration of agarose touches. (Left) Number of touches on both sides. (Right) Histogram with overlaid density plot showing the distribution of duration of touches for both sides. (C) Polygodial (100μM, n = 7) elicited increased touches (p < 0.05, paired Student’s t-test) with shorter duration compared to control side in the same experiments (p < 0.0001, Wilcoxon t-test). (D) Similar results were observed for nootkatone (500μM, n = 10, p < 0.05 for number of touches and p < 0.0001 for duration) and (E) carvacrol (500μM, n = 9, p < 0.01 for number of touches and p < 0.001 for duration). Bar graphs display mean ± SEM, histograms display counts for binned duration (boxes), median (dashed lines) and kernel density estimate (solid line). See also Supplemental video 2 and 3.
Discussion
Octopuses have evolved a unique body plan and peripherally-distributed nervous system to support a repertoire of sophisticated behaviors, thereby providing a competitive predatory advantage in their benthic environment. The octopus nervous system is among the most complex of invertebrates with the majority of neurons dedicated to semi-autonomous execution of arm and sucker behaviors during exploratory foraging and prey capture (Hanlon and Messenger, 1996; Hochner, 2012; Young, 1971). Here, we establish CRs as one distinguishing molecular feature of this advanced and unique nervous system. We find that CRs are conserved across octopuses, further suggesting that these receptors are suited to this distinct form of aquatic chemotactile sensation and foraging. Our results indicate that CR diversity, combinatorial expression, and their ability to form heteromeric ion channel complexes mediates substantial diversity in the signals detected and transduced by octopus suckers. Furthermore, multiple CRs and their combinations remain to be characterized as we obtained partial sequences for some receptors, functional properties of most subunits have not been thoroughly investigated, and we have not yet identified stimuli for others. Thus, we have only begun to appreciate the signal filtering and processing conferred by these proteins. Indeed, CRs are related to cys-loop receptors, which form pentameric channels that are regulated by subunit composition and stoichiometry (Walsh et al., 2018), features which could similarly impact pharmacological and biophysical properties of CRs.
Among other animal chemosensory systems, the expression pattern of CRs is in contrast with vertebrate olfactory receptors in which one receptor subtype is expressed per cell (Bargmann, 2006). Insect odorant receptors are also expressed in specific cells, however each subunit co-assembles with a universally-expressed coreceptor (Benton et al., 2006). The expression pattern of evolutionarily-unrelated insect gustatory receptors is perhaps most similar to CRs, as gustatory receptors are expressed in various combinations and form heteromeric complexes (Joseph and Carlson, 2015). Indeed, CR expression appears to be similarly diverse as compared with related nicotinic acetylcholine receptors which exhibit variable expression across tissues to mediate vast functional diversity (Marcovich et al., 2020). Therefore, this cephalopod-specific evolutionary innovation could be driven by expression patterning prior to gene duplication as acetylcholine receptors are often found in the invertebrate peripheral nervous system. Insects exhibit a similar feature in which a family of glutamate receptor-related genes is required for certain sensory modalities (Benton et al., 2009). This family of ionotropic receptors displays combinatorial expression patterns with multiple subtypes required for diverse sensory neuron responses and animal behaviors (van Giesen and Garrity, 2017). While our studies show that CRs exhibit similar combinatorial expression and can form heteromeric complexes in expression systems, biochemical analyses of native CR complex stoichiometries will be essential toward decoding the logic of this octopus chemosensory system. Further analysis of cephalopod genomes will also enhance our understanding of CR evolution.
In addition to highlighting a role for specialized proteins in the extensive signal filtering of the octopus arm nervous system, our study demonstrates discrete cellular pathways by which mechanical and chemical information is processed. Although there are likely additional cellular subtypes based on uncharacterized signaling mechanisms or molecular markers, we find that intrinsic sensory cell properties are well-correlated with their sensory modality:
Mechanoreceptor channels and mechanosensory cell voltage spiking exhibit phasic responses, even to prolonged stimulation. Thus, our results suggest tactile sensation is narrowly-tuned, possibly reflected by octopus arms exhibiting consistent touch responses in the absence of chemicals. This observation is consistent with earlier work on the mechanosensory system of the octopus arm in which interneurons showed fast-adapting, phasic responses to acute or sustained mechanical stimulation of the sucker (Rowell, 1966). Such tuning properties could provide a simple coding mechanism to distinguish sustained touch of inanimate objects from moving prey objects that would result in repetitive release, deformation of suckers, and subsequent stimulation of mechanoreceptors.
Chemosensory cells exhibit tonic firing properties that could be regulated by specifically-expressed CRs with discrete ligand sensitivity, response kinetics, or ion permeation. For example, monovalent cation-selective CRs could elicit tonic firing whereas Ca2+-permeable CRs might mediate distinct electrical signals by regulating downstream Ca2+-activated conductances or signaling cascades. Such dynamic communication between sensory receptors and the voltage-gated conductances of their cognate cells could facilitate transmission of particular electrical signals to the nervous system depending on ligand identity, concentration, duration, or natural product mixtures (food versus ink, for example). These features correspond with our observation that CR agonists differentially affect chemotactile foraging behavior. Investigation of electrical signal integration by arm ganglia will determine how these complementary molecular and cellular components are assembled to mediate sensorimotor behavior. Our results demonstrate general principles by which single proteins and cells provide immensely flexible signal processing and highlight the importance in considering each functional component of a neural system toward understanding information coding and behavior.
Aquatic chemosensation is poorly understood compared with its terrestrial counterpart. Aquatic chemical sensing has been associated with waterborne hydrophilic molecules, however poorly-soluble terpenoids associated with terrestrial olfaction have been demonstrated to elicit contact-dependent behavioral responses in aquatic organisms (Giordano et al., 2017; Long and Hay, 2006). Our results demonstrate that several terpenoids act on CRs and chemosensory cells and elicit distinct chemotactile behaviors in octopuses, thus establishing a molecular basis for this aquatic tactile form of chemosensation or ‘taste’. Octopuses are voracious opportunistic predators and marine invertebrates comprise a major source of their diet (Hanlon and Messenger, 1996). Indeed, terpenoids are produced by marine invertebrates as a defense mechanism (Cimino et al., 1983), and therefore these chemicals could serve as warning signals indicating toxic prey (Long and Hay, 2006). Considering that CRs are sensitive to bitter compounds and defensive terpenoids, and that chemotactile behavior facilitates ‘blind feeding’ involving searching for food in seafloor crevices (Hanlon and Messenger, 1996), chemotactile sensation might serve to abort search behavior in response to repulsive signals. However, a myriad of unknown natural compounds might act as chemotactile stimuli to mediate distinct behaviors depending on specific habitats, behavioral context or species variation. Distinct species of octopuses or other coleoid cephalopods may have evolved CRs to detect natural products from their specific ecological niches or to facilitate particular behaviors. Identification of additional natural compounds will further our understanding of CR evolution, chemotactile sensation across aquatic organisms and environments, and how animals adapt to ecological variation.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nicholas Bellono (nbellono@harvard.edu).
Materials Availability
All unique reagents generated in this study are available from the Lead Contact.
Data and code availability
Deep sequencing data are archived at Gene Expression Omnibus under accession number GSE156748.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Adult female California two-spot octopuses (Octopus bimaculoides) were wild-caught (Aquatic Research Consultants, San Pedro, CA), fed daily with fiddler crabs (Uca pugnax, Northeast Brine Shrimp, Oak Hill, FL), and kept on a 12hr light/dark cycle in natural sea water. Sensory cells were isolated from suckers following tissue extraction and sucker epithelium dissection from animals that were sedated using step-wise increases in ethanol (ending at 3%, (Butler-Struben et al., 2018)). Prior to electrophysiology and sequencing experiments, cells were isolated from the sucker epithelium by treatment with protease (10mg/mL Sigma type XIV, #P5147) at 37°C for 20min and mechanical dissociation in divalent free solution (mM): 430 NaCl, 150 Sucrose, 10 KCl, 10 HEPES, pH 7.6. Sensory cells were identified by their distinct morphology (Fig. 1). Animal protocols were approved by the Harvard University Animal Care and Use Committee (protocol ID 18-05-325) with further guidance from the Marine Biological Laboratories Marine Resource Center.
HEK293T cells (ATCC, authenticated and validated as negative for mycoplasma by vendor) were grown in DMEM, 10% fetal calf serum, and 1% penicillin/streptomycin at 37°C, 5% CO2. Cells were transfected using lipofectamine 2000 (Invitrogen/Thermo fisher scientific, #11668019) according to the manufacturer’s protocol. For octopus chemotactile receptor (CR) expression, cells were transfected with 1μg of acetylcholine receptor trafficking proteins (TMEM35 and Ric3) (Gu et al., 2016; Lansdell et al., 2005), 0.5μg CR subunit, and 0.3μg GFP. Trafficking proteins, particularly TMEM35, improved expression. Mechanosensitive proteins were assayed using HEK293 cells transfected with 1μg of either Drosophila melanogaster NompC or Octopus bimaculoides NompC. To enhance channel expression, cells were transfected for 6–8hr, plated on coverslips, and then incubated at 30°C for 1–4 days before experiments. Oocytes were received from Ecocyte and kept at 17°C in Barth’s medium (88mM NaCl, 1mM KCl, 0.33mM Ca(NO3)2, 0.41mM CaCl2, 0.82mM MgSO4, 2.4mM NaHCO3, 5mM HEPES, and 0.1mg/mL gentamycin, pH 7.6 with NaOH) and injection was performed in subsequent days. Oocytes were injected using Nanoject III (Drummond scientific) with equal concentrations of receptors and accessory proteins for a total of 50ng RNA. Injections were carried out 24–36hr prior to recording and kept in Barth’s medium at 17°C until recording.
METHOD DETAILS
Molecular biology
Octopus bimaculoides NompC (no mechanoreceptor potential C) full sequence was obtained using SMARTer 3’ RACE (Takara, #634858). Briefly, RNA was extracted from octopus suckers using standard Trizol extraction, purified with Zymo Clean and Concentrator Kit (Zymo research #R1013), and diluted in 10–20μL RNAse free H2O without DNAase treatment. A NompC-specific 5’ primer (TCGCCTCCGCCGATCAACCTATTC) was used to amplify the 3’ end and PCR products were cloned as suggested by the company and verified by sequencing. Consensus from sequenced products including a stop codon was used for vector design for obNompC-GFP that was codon-optimized and synthesized by Genscript (Piscataway, NJ). All CR constructs were codon-optimized and synthesized by Genscript (Piscataway, NJ). Gene numbers are (NCBI): CR829: LOC106880829, CR110: LOC106875110, CR986: LOC106869986, CR840: LOC106875775, CR828: LOC106880828, CR737: LOC106876737, CR989: LOC106869989, CR111: LOC106875111, CR918: LOC106870918, CR518: OCBIM_22006518mg. Chimeric channels were designed based on transmembrane prediction and synthesized by Genscript. Human TMEM35 and Ric3 were from Genscript. Drosophila NompC-GFP was a gift from YN Jan. For oocyte expression, vectors were linearized using PfoI (Thermo Fisher #FERER1751) or BamHI (NEB #R3136T) for 2hr at 37°C. Linearized DNA was purified using a PCR purification Kit (Quiagen #28104) and eluted in 30μL RNAse free water. RNA synthesis was performed with approximately 1μg DNA using mMessage mMachine T7 Transcription Kit including 15min of DNAse treatment (Ambion #AM1344). RNA was treated with a Zymo Clean & Concentrator Kit and aliquoted at a concentration of approximately 1μg/μL for injection.
Transcriptomics
Samples were prepared using standard Trizol extraction combined with Zymo Clean and Concentrator Kit, diluted in 10–20μl RNAse free H2O without DNAseI treatment. Resulting RNA was used to build libraries with the Kapa mRNA HyperPlus kit. Samples were sequenced using HiSeq Rapid Run (2×150 bp). For raw sequences no new codes were used, in brief, adapter trimming was performed using trim galore and, after subsequent quality control, a reference transcriptome was de novo assembled using Trinity (Haas et al., 2013) and open reading frames were determined using transdecoder. Gene expression levels were estimated using two approaches: (1) Reads were pseudo-aligned and transcript abundance was estimated using Kallisto using novel transcriptome assembly as reference (Bray et al., 2016). (2) Reads were aligned to reference NCBI genome using the Rsubread package (Liao et al., 2019). The Octopus bimaculoides genome ((Albertin et al., 2015), NCBI PRJNA305125) and de novo assembled transcriptome were used as a reference. Proteins later studied were selected based on expression level and corresponding full-length sequence using both templates.
Phylogenetics
For phylogenetic trees, sequences were selected from available proteomes (PRJNA305125, O. bimaculoides), (PRJNA492973, O. vulgaris) and (http://gigadb.org/dataset/100503, O. Minor). Sequences were either sorted by annotation (NCBI) or acetylcholine receptor-like sequences identified using Jackhmmer (sequence analysis software HMMER v.3.3 (http://hmmer.org/) (Eddy, 2009) and subsequent pBlast to confirm similarity with acetylcholine receptor-like proteins in other octopus species. Sequences were selected based on length and trees made with PhyML standard protocol (Castresana, 2000; Chevenet et al., 2006; Dereeper et al., 2010; Dereeper et al., 2008; Edgar, 2004; Guindon and Gascuel, 2003). This analysis was performed on the Phylogeny.fr platform and comprised the following steps: First, sequences were aligned with MUSCLE (v3.8.31) configured for highest accuracy (MUSCLE with default settings). After alignment, ambiguous regions (i.e. containing gaps and/or poorly aligned) were removed with Gblocks (v0.91b) using the following parameters: 1. minimum length of a block after cleaning 2. positions with a gap in less than 50% of the sequence were selected in the final alignment if they were within an appropriate block. 3. all segments with contiguous non-conserved positions bigger than 8 were rejected. 4. minimum number of sequences for a flank position: 55%. The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.1/3.0 aLRT). The default substitution model was selected assuming an estimated proportion of invariant sites (of 0.000) and 4 gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data (gamma = 0.976). Reliability for internal branch was assessed using the aLRT test (SH-Like). Graphical representation and edition of the phylogenetic tree were performed with TreeDyn (v198.3). All other sequences were aligned using clustalW.
Immunohistochemistry
Arms of Octopus bimaculoides were fixed in 4% paraformaldehyde (PFA) in PBS for approximately 16–24hr on a rocker. Tissues were stored in PFA at 4°C until use. Suckers were dissected, washed with PBST (TritonX 0.1%, 2–3 times), and incubated in 30% sucrose in PBST at 4°C on ice. After embedding in OCT (optimal cutting temperature compound), samples were frozen and sectioned using a cryostat (Leica CM3050S) at 18μm sections. Sections were dried 30–60min and fixed again for 15min in 4% PFA in PBS. After subsequent washes in PBST, samples were blocked for 1hr in 10% normal goat serum (NGS) in PBST and antibody solution (Anti-HRP Alexa 488, Jackson Immuno # 123-545-021) was applied for 2–4hr at room temperature in NGS. Finally, the samples were washed 3–5 times in PBST, mounted in Vectashield with DAPI (Vector Laboratories), and imaged with a Zeiss LSM880 confocal. Images were processed using FIJI and Adobe Photoshop. For whole mount staining of embryos, tissue was fixed, washed in PBST and incubated with antibody solution overnight.
In situ hybridization (RNAscope)
Tissues were collected from live specimens and suckers were immediately frozen in OCT. Sections (18 μM) were performed on fresh frozen tissue using a cryostat (Leica CM3050S). Probes were designed by ACD Inc. and the manufacturer-recommend protocol was followed as described for fresh frozen tissues. Pretreat 3 was used for 30min and fluorescent probes used included TSA-FITC, TSA-Cy3 and TSA-Cy5 (Perkin Elmer # NEL744E001KT and #NEL754001KT), samples were mounted in ProLong Gold (Thermo Fisher # P36931) with DAPI, and imaged with a Zeiss LSM880 confocal. Images were processed using FIJI and Adobe Photoshop. Co-expression was determined using Zen Blue (Zeiss) colocalization tool.
Electrophysiology
Patch clamp recordings were carried out at room temperature using a MultiClamp 700B amplifier (Axon Instruments) and digitized using a Digidata 1550B (Axon Instruments) interface and pClamp software (Axon Instruments). Whole-cell recording data were filtered at 1kHz and sampled at 10kHz. For single-channel recordings, data were filtered at 2kHz and sampled at 20kHz. Voltage-gated currents were leak-subtracted online using a p/4 protocol, and membrane potentials were corrected for liquid junction potentials. For octopus cell recordings, borosilicate glass pipettes were polished to 8–10MΩ. The standard extracellular solution contained (in mM): 430 NaCl, 10 KCl, 10 HEPES, 10 CaCl2, 50 MgCl2, pH 7.6. Two intracellular solutions were used for recording. Most experiments used a Cs+-based solution to reduce K+ currents (mM): 500 Cs+ methanesulfonate, 4 MgCl2, 10 HEPES, 30 sucrose, 10 CsEGTA, pH 7.6. To measure K+ currents and membrane voltage, we used (mM): 500 K+ gluconate, 10 HEPES, 10 sucrose, 4 MgCl2, 10 KEGTA, pH 7.6. For whole-cell recordings in HEK293 cells, pipettes were 3–5MΩ. The standard extracellular solution contained (in mM): 140 NaCl, 5 KCl, 10 HEPES, 2 CaCl2, 2 MgCl2, pH 7.4. The intracellular solution contained (mM): 140 Cs+ methanesulfonate, 1 MgCl2, 5 NaCl, 10 CsEGTA, 10 HEPES, 10 sucrose, pH 7.2. In compound screening experiments, EGTA was substituted for equimolar BAPTA (rapid Ca2+ chelator) to further test direct action of active compounds. Single-channel recordings from HEK293 patches used extracellular solution containing (mM): 140 NaCl, 10 HEPES, 1 NaEGTA, pH 7.4. The pipette solution was (mM): 140 CsCl, 10 HEPES, 1 CsEGTA, pH 7.4. Single-channel recordings from oocyte patches were made with 3–5MΩ a pipette solution containing (mM): 104 NaCl, 10 HEPES, pH 7.4.
The following pharmacological agents were used: 4-Aminopyridine (Tocris, #0940), acetylcholine (Sigma, #A6625), mecamylamine (Tocris, #2843), GdCl3 (Sigma, #7532), BAPTA (Tocris, #2786), Cd2+ (Sigma, #202908), tetrodotoxin (Tocris, #1078), nootkatone (Sigma, #W316620), polygodial (Cayman Chemicals, #14979), atractylon (Carbosynth, #FA74011), threonine, arginine, alanine, serine, leucine, glutamine, histidine, lysine (all Sigma), benzaldehyde (Sigma, #418099), 2-(Diethylamino)ethanol (Sigma, #471321), Isoamyl acetate (Sigma, #112674), Sucrose (Sigma, #S7903), Pyridine (Fisher scientific, #13178), 3-Aminopyrrolidine (Sigma, #540781), triethylamine (Sigma, #471283), 2,5-dimethylpyrazine (Sigma, W327204), tyramine (Sigma), chloroquine (Sigma, #C6628), denatonium benzoate (Sigma, #D5765), carvacrol (Sigma, #W224502), eucalyptol (Fluka, #46090), limonene (Sigma, #183164). Compounds were dissolved in water, ethanol (<1%), or DMSO (<1%). Natural products (fish, crab, ink) were flash-frozen, ground with mortar and pestle, and filtered with 3kDa ultracentrifugal filters (Amicon UFC500324). Fractions were generated with C18 filters (Restek, #24051) using methanol and water to collect hydrophilic and hydrophobic fractions, respectively. Inhibitory effects were quantified as differences in normalized peak current from the same cell following bath application of the drug (Itreatment/Icontrol) and agonists were quantified by increases in peak current versus basal (Itreatment/Ibasal). For CRs and chemoreceptor cells, effects were quantified using currents measured at −110mV. Estimated EC50 and 95% confidence intervals were calculated from sigmoidal dose-response relationships established from normalizing agonist-evoked currents to those elicited by maximal concentrations in the same cell. Desensitization was measured by normalizing current at the end of agonist application to peak transient currents. Whole-cell recordings were used to assess mechanical sensation together with a piezoelectric-driven (Physik Instrumente) fire-polished glass pipette (tip diameter 1μm). Mechanical steps in 1μm increment were applied every 5s while cells were voltage-clamped at −90mV.
In native cells, 100ms voltage ramps from −120 to 80mV were applied every 1s used to assess agonist-elicited currents, which were quantified at −110mV. Time courses displayed currents measured at −110mV during successive ramps. Agonist-evoked currents were measured at −110mV during 500ms ramps from −120 to 100mV in HEK293 and 200ms ramps from −120 to 120mV in oocytes. Voltage-gated currents were measured in response to a 200ms voltage pulse in 10mV increments from a −110mV holding potential. G-V relationships were derived from I-V curves by calculating G: G=ICaV/(Vm-Erev) and fit with a Boltzmann equation. Voltage-dependent inactivation was measured using test pulses to −10mV (inward currents) or +60mV (K+ currents) voltage pulses following a series of 1s pre-pulses ranging from −110mV to +60mV. Voltage-dependent inactivation was quantified as I/Imax, with Imax occurring at the voltage pulse following a −110mV prepulse. Current kinetics were quantified using single exponential fits or the time to reach peak amplitude from activation. Voltage-dependent K+ currents were isolated by including 1μM TTX and 500μM Cd2+ in the extracellular solution. In current-clamp recordings, resting membrane potential was measured without injecting current (I=0). 1s depolarizing current steps of various amplitudes were injected to measure spikes which were quantified by frequency (spikes/second) or amplitude. CR G-V relationships were established from normalized tail currents measured at −40 mV following 1s voltage pulses in 10mV increments from a −150mV to 50mV from a 0mV holding potential. Activation kinetics were determined by fitting the initial rising phase of currents activated with a single exponential. Deactivation kinetics were quantified with a single exponential fit upon steps to −40 mV following more negative voltage pulses.
In ion substitution experiments, relative permeability was determined by measuring the shift in Erev after the substitution of equimolar monovalent cations. For native cells, the extracellular solution contained (mM) 500 Na+, Cs+, NMDG+ or 300 Ca2+ and intracellular solution contained 490 CsCl and 10 CsEGTA. For HEK293, the extracellular solution contained (mM) 150 Na+, Cs+, NMDG+ or 100 Ca2+, Mg2+ and intracellular solution contained 150 CsCl and 1 CsEGTA. Solutions were buffered with 10mM HEPES. Permeability ratios were estimated using the Goldman-Hodgkin-Katz (GHK) equation: PX/PNa = ([Na+]Luminal/[X]Cytoplasmic)(exp(ErevF/RT)), PCa/PNa = (4[Ca2+]Luminal/[Na+]Cytoplasmic)(exp(ErevF/RT)). Single-channel currents were measured from the middle of the noise band between closed and open states or derived from all-points amplitude histograms fit with Gaussian relationships at closed and open peaks for each excised patch record. Conductance was calculated from the linear slope of I–V relationships. CR single-channel currents were measured in membrane attached patches at the indicated voltage pulses. NompC N(PO) was calculated during pressure steps while voltage was held at −80mV. Single mechanosensitive channels were studied using excised outside-out patches exposed to pressure applied via a High-Speed Pressure Clamp system (HSPC, ALA-scientific). Pressure-response relationships were established using pressure steps in 10mmHg increments while patches were voltage-clamped at −80mV. Voltage-dependence of currents was measured from −100mV to 100mV in 20mV increments while applying repetitive 60mmHg pressure pulses. Excised patch recordings of macroscopic CR currents were carried out at −60mV.
Two-electrode voltage recordings were carried out at room temperature with an Oocyte Clamp OC-725C amplifier (Warner Instruments) and digitized using a Digidata 1550B (Axon Instruments) interface and pClamp 11 software. Data were filtered at 1kHz and sampled at 10kHz. Recordings were performed using borosilicate glass pipettes with resistances of ~1MΩ when filled with 3M KCl. All chemicals were diluted in ND96 extracellular solution (96mM NaCl, 2mM KCl, 5mM HEPES, 1mM MgCl2, 2mM CaCl2 adjusted to pH 7.4 with NaOH). Stimulus-evoked currents were obtained using 200 ms voltage ramps from −120mV to 120mV applied every 500ms with an inter-stimulus holding potential of −80mV. Dose-response relationships were calculated using peak currents measured at −110mV.
Biochemistry
X. laevis oocytes were prepared and injected as described above. Oocytes expressing CR channels were incubated for 2 days at 17°C prior to use. Membrane enrichment was performed using a protocol adapted from (Chang et al., 2004). All steps are performed at 4°C in a cold room or on ice. First, crude oocyte lysates were prepared by homogenizing 20 oocytes in 200μL of homogenization buffer (lysate sample) (20 mM Tris-HCl (pH 7.4) 5mM MgCl2, 5mM NaH2PO4, 1mM EDTA, 80mM sucrose, 1 cOmplete tablet (Sigma, #11697498001)) with a small mortar and pestle. Membranes were then washed by centrifuging the lysate at 100xg for 10min at 4°C, and the cytosol-containing supernatant (cytosolic fraction sample) was carefully removed, and the membrane-containing pellet was then resuspended in 200μL lysis buffer. Membranes were washed a second time. Membranes were solubilized by resuspending the pellet in 200μL solubilization buffer (lysis buffer + 1% TritonX-100, membrane fraction sample). anti-FLAG M2 magnetic beads (20μL/sample) (Millipore Sigma, #M8823) were washed 3X in 1mL solubilization buffer for 15min at 4°C before use, and the membrane fraction was added to beads and incubated at 4°C for 1hr with constant rotation. Beads were pelleted with a magnet-rack, and washed 5X with 1mL solubilization suffer, removing the supernatant after each wash. Beads were then boiled in western blot sample buffer (2X: 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.01% bromphenol blue and 0.125M Tris HCl, pH approx. 6.8, 1mM PMSF, and 2 protease inhibitor tablets/50mL) for 5min at 99°C. Lysate, cystosol, and membrane Fraction samples were mixed 1:1 in sample buffer and boiled for 5min at 99°C. Samples were used immediately for western blotting or stored frozen at −80°C until use.
Western blot samples were loaded onto Any-kD precast gels (Biorad #4569034) and electrophoresis was run at a constant 150V for 1hr. Proteins were then transferred to nitrocellulose for blotting using mixed-molecular weight settings on a Turbo Blot transfer system (Biorad #1704150). Gels were blocked in Odyssey Blocking Solution (LI-COR Biosciences #927–60001) for 30min, incubated in primary antibody (mouse anti-FLAG M2 (Millipore-Sigma #F1804) or mouse anti-HA (Thermo Fischer Scientific #26183)) diluted 1:5,000 in Intercept T-20 Antibody Diluent (LI-COR Biosciences #927–65001) overnight at 4°C, washed 3X in TBS, incubated for 1 hour at 25°C in secondary antibody (IRDye 800CW goat anti-Mouse #926–32210) diluted 1:10,000 in Intercept T-20 Antibody Diluent, and finally washed 3 times in TBS before imaging. Western blots were imaged using an Odyssey CLx imaging system (LI-COR Biosciences) and processed using Image Studio (LI-COR Biosciences).
Behavior
Animals used for behavior experiments were fasted for 24 hours before trials. Behavioral tests were performed using an acrylic tank wrapped with a white adhesive vinyl sheet and covered in paneling to leaving a viewing window on one side of the tank. For reaching behaviors, octopuses were separated from prey (crabs) using an acrylic barrier with a small hole for reaching. Crabs were attached to a suction cup and a separate cup was inserted as a control object. To test the effects of chemicals on chemotactcile sensation, 400mL of 1.5% concentration agarose was added and solidified on the bottom of the tank floor. The agar floor was bisected widthwise, and one side was removed (alternated across trials). 200mL of 1.5% agarose infused with the indicated compounds was then added to the empty half of the tank, resulting in a uniform depth of agar across the tank. The tank was added to the same aquatic system for housing the animals, animals were added to freely explore the tank, and the entire 10-minute trial was monitored with a GoPro HERO7 camera (GoPro Inc.). Afterward, the animal was returned to its home tank for at least 24 hours.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data were analyzed with Clampfit (Axon Instruments), Prism (Graphpad), R (R Core Development Team), or Behavioral Observation Research Interactive Software (Oliver Friard, 2016) and represented as mean ± SEM. n represented independent experiments for the number of cells/patches or behavioral trials. Data were considered significant if p < 0.05 using paired or unpaired two-tailed Student’s t-tests, Wilcox test or one- or two-way ANOVAs. All significance tests were justified considering the experimental design and we assumed normal distribution and variance, as is common for similar experiments. Sample sizes were chosen based on the number of independent experiments required for statistical significance and technical feasibility.
Supplementary Material
Figure S1. Related to Figures 1 and 2. Octopus sensory cell characterization (A) Overhead view of behavioral assay in which octopuses reached arms through a barrier to explore an isolated area containing a stationary crab or control object. Also shown in Supplementary video 1. Octopuses spent significantly more time probing crabs than the control object, n = 4 trials, p < 0.01, two-tailed Studenťs t-test. (B) Cross-section of octopus sucker stained with anti-horseradish peroxidase antibody (HRP, green) and nuclear stain (DAPI, blue). (C) Octopus reaching behavior in response to seawater (control) or fish or crab extract (< 3kDa) presented to arms. Pie charts indicate number of times octopus reacted (dark gray) or did not react (light gray) to the stimulus. Number of trials indicated on right. (D) Representative chemoreceptor cell currents in response to the indicated voltage ramps. This protocol demonstrates that relatively short ramps could be used to inactivate the majority of voltage-gated inward currents, thereby allowing for analysis of sensory responses with less contamination from voltage-gated currents. (E) Chemoreceptor and mechanoreceptor cells had similar resting potentials that were more negative than support cells. n = 5 – 6, p < 0.0001, one-way ANOVA with post-hoc Tukey test. (F) Voltage spike amplitude was larger in chemoreceptors versus mechanoreceptors. n = 5 per cell type, p < 0.0001, two-tailed studenťs t-test. (G) Current-voltage relationships demonstrated voltage-gated inward currents were similar in chemoreceptors and mechanoreceptors, and absent in support cells. n = 7 per cell type, p < 0.0001 for support cells versus others, two-way ANOVA with post-hoc Bonferroni test. (H) 1μM tetrodotoxin (TTX) blocked voltage-gated inward currents in chemoreceptors and mechanoreceptors. n = 4 per cell type, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. (I) Voltage-gated inward current kinetics were similar in chemoreceptor and mechanoreceptor cells (n = 6 – 8). (J) Current-voltage relationships of K+ currents demonstrated that mechanoreceptors had significantly smaller K+ current amplitude compared with chemoreceptors and support cells. n = 4 – 6, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (K) Chemoreceptor and mechanoreceptor K+ currents exhibited different sensitivity to 1mM 4-AP-mediated block. n = 4 per cell type, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. (L) Mechanoreceptor K+ currents had faster activation and inactivation kinetics. n = 6 per cell type, p < 0.0001, two-tailed studenťs t-test. Data represented as mean ± SEM.
Figure S3. Related to Figure 4. Octopus chemotactile receptors (CRs) are modified acetylcholine receptors (A) (Left) CR phylogenetic relationships compared with acetylcholine receptor-like sequences in Octopus bimaculoides. (Right) Comparison of CR518 orthologues from different octopus species. Branch support values in red. Scale: branch length, Newick tree format. (B) Alignment of predicted A, B and C loops from analyzed Octopus bimaculoides CRs and the human a7 acetylcholine receptor (hα7) demonstrated that CRs lack most residues which contribute to the canonical acetylcholine (ACh) binding side (highlighted in red). (C) In situ hybridization from sucker epithelium (represented in Fig. 4C) showed that CRs exhibited a combinatorial expression pattern with certain subtypes coexpressed in the same cells (n = 11 – 32, % of cells expressing indicated subtypes). (D) Quantification of currents elicited by prey chemicals in two CRs (CR840 and CR518) and hα7 from Fig 4D. Data represented as mean ± SEM.
Figure S4. Related to Figure 4. CRs are insensitive to acetylcholine (A) CR840 and CR518 were insensitive to ACh (1mM, grey arrow indicates basal current overlap). ACh dose-response relationship for CRs compared with hα7 showed that hα7 was 1000-fold more sensitive and CRs only consistently responded to 100mM ACh (n = 3 – 5). (B, C) CRs were inhibited by mecamylamine (1mM in representative traces), similar to native chemoreceptor cell responses. n = 3 – 5 for expressed CRs, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. n = 4 for native chemoreceptors, p < 0.0001, two-tailed studenťs t-test. Data represented as mean ± SEM.
Figure S5. Related to Figure 5. CRs have distinct properties (A) HEK293 cells expressing CR840 were only responsive to chloroquine among sampled compounds (30μM atractylon and nootkatone, 200μM carvacrol, 25μM chloroquine). Chloroquine dose-response relationship for CR840 with an estimated half-maximal effective concentration (EC50): 10.32μM, 95% confidence interval (CI) 9.29 – 11.50μM, n = 7 – 9. (B) CR518 responded to various terpenoids and chloroquine, albeit with decreased chloroquine sensitivity compared to CR840. CR518 dose-response relationships: atractylon EC50 = 29.43μM, 95% CI = 25.94 – 32.99μM, nootkatone EC50 = 24.70μM, 95% CI = 20.27 – 29.62μM, carvacrol EC50 = 204.41μM, 95% CI = 163.5 – 251.3μM, chloroquine EC50 = 92.29μM, 95% CI = 75.21 – 106.50μM, n = 5 – 13. (C) CR840 exhibited dose-dependent responses to chloroquine and CR518 responded to increasing concentrations of atractylon or nootkatone (representative of n = 7 – 13). (D) Outside-out patches from HEK293 cells expressing CR840 or CR518 were sensitive to the same chemicals (30μM) identified in whole-cell recordings, suggesting membrane-delimited action. n = 4 – 5, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. Scale bars: 10pA, 10s. (E) Substitution of CR840 N-terminus conferred its pharmacological profile to CR518, n = 4 – 5, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (F) CR currents elicited by voltage protocol to obtain G-V relationship in the presence of the listed agonists. Arrows indicate when current amplitude was measured at −40mV following activating prepulses from −150mV to 50mV in 10mV increments. (G) G-V relationships for CR840 in response to 10μM chloroquine and CR518 in response to 35μM atractylon or nootkatone. p < 0.0001 for differences in CR840 versus CR518 across voltages, two-way ANOVA with post-hoc Tukey test n = 6 – 9. (H) Deactivation kinetics at −40 mV following voltage pulses from −150mV to 50mV in 10mV increments. Arrows indicate when deactivation rates were measured. (I) τ values from single exponential fits of activation at −120mV and deactivation at −40mV after an activating prepulse of −150mV. n = 6 – 8, p < 0.0001 for one-way ANOVA with post-hoc Tukey test. Data represented as mean ± SEM.
Figure S6. Related to Figure 6. Specific combinations of CRs confer distinct chemosensory responses Fish extract evoked distinct currents which varied in amplitude and voltage-dependent rectification based on the combinatorial expression of CRs in oocytes. Quantification in Fig 6A.
Figure S7. Related to Figure 6. Heteromeric CR properties (A) Immunoblotting for FLAG or HA from oocyte membrane fractions used as input for co-immunoprecipitation in Fig 6B. (B) Comparison of ACh dose-response relationship for CR840–828 and CR518–828 with hα7 (from Fig. S4), n = 3 – 7. (C) CR518–828 and CR840–828 were inhibited by mecamylamine. n = 3 – 6, p < 0.0001, one-way ANOVA with post-hoc Bonferroni test. (D) CR840–828 responded to hydrophilic (HPL) fish fractions while CR518–828 was stimulated by hydrophobic (HPB) but not HPL fractions. Responses normalized to total fish extract, n = 4 – 6. (E) HEK293 cells expressing CR840–828 were only responsive to chloroquine (25μM) among sampled compounds (30μM atractylon and nootkatone, 200μM carvacrol) while CR518–828 responded to various terpenes (30μM atractylon and nootkatone, 200μM carvacrol) and 25μM chloroquine. n = 6 – 7, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (F) Comparison of dose-response relationships between homomeric and heteromeric CRs demonstrate changes in compound sensitivity (n = 6 – 14). (G) Single channel records from oocyte patches expressing the indicated CRs. CR840 and CR518 had distinct conductances that were not altered by CR828. Slope conductance: CR840 = 54.9 ± 1 pS, CR840–828 = 53.1 ± 1 pS, CR518 = 63.7 ± 0.7 pS, CR518–828 = 62.5 ± 0.9 pS, n = 4 – 6.
Supplemental table 1, related to Figure 4: Octopus phylogenetic information. Protein identification numbers and associated databases from Fig 4B.
Supplemental video 1, related to Figure 1: Octopus reaching behavior. Representative octopus separated from prey by a barrier which allows arm reaching and exploration. Arms probed secured crabs significantly more than control objects (suction cup without crab). 1.75X speed.
Supplemental video 2, related to Figure 7: Octopus chemotactile behavior - control. Representative octopus exhibited stereotypic exploratory behavior in a tank with each side of the floor covered in independently poured seawater-infused 1.5% agarose. 4X speed.
Supplemental video 3, related to Figure 7: Octopus chemotactile behavior - polygodial. Representative octopus exhibited brief, increased touches with fast arm retraction and avoidance in response to contacting 100μM polygodial-infused 1.5% agarose versus control. 4X speed.
Figure S2. Related to Figure 3. Octopus NompC is a well-conserved mechanoreceptor (A) Alignment of Octopus bimaculoides (obNompC) and Drosophila melanogaster (dmNompC) sequences revealed high conservation. Red indicates residues important for mechanosensitivity, * indicates identifical residues, : indicates similar residues. (B) 60mmHg elicited Gd3+-sensitive currents in excised patches expressing obNompC or dmNompC. Representative of n = 4. (C, D) obNompC and dmNompC channels were similarly sensitive to mechanical stimulation and exhibited similar conductance. 95% confidence interval for pressure required to induce half-maximal NPO: obNompC = 51 – 58.25mmHg, dmNompC = 47.11 – 56.79mmHg. n = 5. Patches from untransfected cells did not respond to similar stimuli (n = 6). Slope conductance: obNompC = 165.5 ± 4 pS, dmNompC = 154.0 ± 4 pS. n = 4. Data represented as mean ± SEM.
Highlights.
The distributed octopus arm nervous system uses unique chemotactile receptors (CRs)
CRs detect poorly-soluble natural products mediating touch-taste arm behavior
CRs form ion channel complexes to mediate diverse signal detection and filtering
Signals transduced by specific sensory cells regulate complex exploratory behavior
Acknowledgments
We thank B. Walsh for establishing our octopus housing system, C. Winkler for providing animals; J. Rosenthal, B. Grasse, and the Marine Resource Center at the Marine Biological Laboratory for assistance with animals, J. Davila-Velderrain for help with analyses, and B. Bean, C. Dulac, D. Julius, R. Losick, and E. Pollina for critical reading of the manuscript. This research was supported by grants to NWB from the New York Stem Cell Foundation, Searle Scholars Program, Sloan Foundation, Klingenstein-Simons Fellowship, and the NIH (R00DK115879), as well as the Swiss National Science Foundation (P400PB-180894) to LVG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing financial interests.
References
- Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, Brenner S, Ragsdale CW, and Rokhsar DS (2015). The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI (2006). Comparative chemosensation from receptors to ecology. Nature 444, 295–301. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, and Vosshall LB (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4, e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, and Vosshall LB (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. [DOI] [PubMed] [Google Scholar]
- Butler-Struben HM, Brophy SM, Johnson NA, and Crook RJ (2018). In Vivo Recording of Neural and Behavioral Correlates of Anesthesia Induction, Reversal, and Euthanasia in Cephalopod Molluscs. Front Physiol 9, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17, 540–552. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, and Hoenderop JG (2004). Molecular determinants in TRPV5 channel assembly. J Biol Chem 279, 54304–54311. [DOI] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, and Christen R (2006). TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G, S DER, S DES, Sodano G, and Villani G (1983). Dorid nudibranch elaborates its own chemical defense. Science 219, 1237–1238. [DOI] [PubMed] [Google Scholar]
- Derby CD (2007). Escape by inking and secreting: marine molluscs avoid predators through a rich array of chemicals and mechanisms. Biol Bull 213, 274–289. [DOI] [PubMed] [Google Scholar]
- Dereeper A, Audic S, Claverie JM, and Blanc G (2010). BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, et al. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36, W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR (2009). A new generation of homology search tools based on probabilistic inference. Genome Inform 23, 205–211. [PubMed] [Google Scholar]
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouke KE, and Rhodes HJ (2020). Electrophysiological and Motor Responses to Chemosensory Stimuli in Isolated Cephalopod Arms. Biol Bull 238, 1–11. [DOI] [PubMed] [Google Scholar]
- Gilly WF L. MT (1992). Behavioral Responses to Chemical Stimulation of the Olfactory Organ in the Squid Loligo Opalescens. Journal of Experimental Biology 162, 209–229. [Google Scholar]
- Giordano G, Carbone M, Ciavatta ML, Silvano E, Gavagnin M, Garson MJ, Cheney KL, Mudianta IW, Russo GF, Villani G, et al. (2017). Volatile secondary metabolites as aposematic olfactory signals and defensive weapons in aquatic environments. Proc Natl Acad Sci U S A 114, 3451–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso FW (2008). Octopus sucker-arm coordination in grasping and manipulation. American Malacological Bulletin 24(1):13–23. 2008. [Google Scholar]
- Graziadei P (1964). Electron Microscopy of Some Primary Receptors in the Sucker of Octopus Vulgaris. Z Zellforsch Mikrosk Anat 64, 510–522. [DOI] [PubMed] [Google Scholar]
- Gu S, Matta JA, Lord B, Harrington AW, Sutton SW, Davini WB, and Bredt DS (2016). Brain alpha7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 89, 948–955. [DOI] [PubMed] [Google Scholar]
- Guindon S, and Gascuel O (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52, 696–704. [DOI] [PubMed] [Google Scholar]
- Gutfreund Y, Matzner H, Flash T, and Hochner B (2006). Patterns of motor activity in the isolated nerve cord of the octopus arm. Biol Bull 211, 212–222. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. (2013). De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8, 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon RT, and Messenger JB (1996). Cephalopod behaviour (Cambridge, England; New York: University of Cambridge; ). [Google Scholar]
- Hochner B (2012). An embodied view of octopus neurobiology. Curr Biol 22, R887–892. [DOI] [PubMed] [Google Scholar]
- Joseph RM, and Carlson JR (2015). Drosophila Chemoreceptors: A Molecular Interface Between the Chemical World and the Brain. Trends Genet 31, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, and Millar NS (2005). RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol 68, 1431–1438. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2019). The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 47, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JD, and Hay ME (2006). Fishes learn aversions to a nudibranch’s chemical defense. Mar Ecol Prog Ser 307, 199–208. [Google Scholar]
- Lucero MT H. FT; Gilly MF (1992). Electrical responses to Chemical Stimulation of Squid Olfactory Receptor Cells. Journal of Experimental Biology 162, 231–249. [Google Scholar]
- Marcovich I, Moglie MJ, Carpaneto Freixas AE, Trigila AP, Franchini LF, Plazas PV, Lipovsek M, and Elgoyhen AB (2020). Distinct Evolutionary Trajectories of Neuronal and Hair Cell Nicotinic Acetylcholine Receptors. Mol Biol Evol 37, 1070–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo E, Fontana A, Roussis V, Polese G, Amodeo P, and Ghiselin MT (2014). Sensing marine biomolecules: smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front Chem 2, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo E, Garson MJ, Polese G, Amodeo P, and Ghiselin MT (2017). Taste and smell in aquatic and terrestrial environments. Nat Prod Rep 34, 496–513. [DOI] [PubMed] [Google Scholar]
- Oliver Friard MG (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observation. Methods in Ecology and Evolution. [Google Scholar]
- Rowell CH (1966). Activity of interneurones in the arm of Octopus in response to tactile stimulation. J Exp Biol 44, 589–605. [DOI] [PubMed] [Google Scholar]
- Sumbre G, Gutfreund Y, Fiorito G, Flash T, and Hochner B (2001). Control of octopus arm extension by a peripheral motor program. Science 293, 1845–1848. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, and Changeux JP (2009). Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8, 733–750. [DOI] [PubMed] [Google Scholar]
- van Giesen L, and Garrity PA (2017). More than meets the IR: the expanding roles of variant Ionotropic Glutamate Receptors in sensing odor, taste, temperature and moisture. F1000Res 6, 1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RM Jr., Roh SH, Gharpure A, Morales-Perez CL, Teng J, and Hibbs RE (2018). Structural principles of distinct assemblies of the human alpha4beta2 nicotinic receptor. Nature 557, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MJ, Freeman NH, and Ashburner M (1965). Some experiments on the chemotactile sense of octopuses. J Exp Biol 43, 553–563. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, and Jan YN (2013). Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ (1971). The anatomy of the nervous system of Octopus vulgaris (Oxford,: Clarendon Press; ). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Related to Figures 1 and 2. Octopus sensory cell characterization (A) Overhead view of behavioral assay in which octopuses reached arms through a barrier to explore an isolated area containing a stationary crab or control object. Also shown in Supplementary video 1. Octopuses spent significantly more time probing crabs than the control object, n = 4 trials, p < 0.01, two-tailed Studenťs t-test. (B) Cross-section of octopus sucker stained with anti-horseradish peroxidase antibody (HRP, green) and nuclear stain (DAPI, blue). (C) Octopus reaching behavior in response to seawater (control) or fish or crab extract (< 3kDa) presented to arms. Pie charts indicate number of times octopus reacted (dark gray) or did not react (light gray) to the stimulus. Number of trials indicated on right. (D) Representative chemoreceptor cell currents in response to the indicated voltage ramps. This protocol demonstrates that relatively short ramps could be used to inactivate the majority of voltage-gated inward currents, thereby allowing for analysis of sensory responses with less contamination from voltage-gated currents. (E) Chemoreceptor and mechanoreceptor cells had similar resting potentials that were more negative than support cells. n = 5 – 6, p < 0.0001, one-way ANOVA with post-hoc Tukey test. (F) Voltage spike amplitude was larger in chemoreceptors versus mechanoreceptors. n = 5 per cell type, p < 0.0001, two-tailed studenťs t-test. (G) Current-voltage relationships demonstrated voltage-gated inward currents were similar in chemoreceptors and mechanoreceptors, and absent in support cells. n = 7 per cell type, p < 0.0001 for support cells versus others, two-way ANOVA with post-hoc Bonferroni test. (H) 1μM tetrodotoxin (TTX) blocked voltage-gated inward currents in chemoreceptors and mechanoreceptors. n = 4 per cell type, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. (I) Voltage-gated inward current kinetics were similar in chemoreceptor and mechanoreceptor cells (n = 6 – 8). (J) Current-voltage relationships of K+ currents demonstrated that mechanoreceptors had significantly smaller K+ current amplitude compared with chemoreceptors and support cells. n = 4 – 6, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (K) Chemoreceptor and mechanoreceptor K+ currents exhibited different sensitivity to 1mM 4-AP-mediated block. n = 4 per cell type, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. (L) Mechanoreceptor K+ currents had faster activation and inactivation kinetics. n = 6 per cell type, p < 0.0001, two-tailed studenťs t-test. Data represented as mean ± SEM.
Figure S3. Related to Figure 4. Octopus chemotactile receptors (CRs) are modified acetylcholine receptors (A) (Left) CR phylogenetic relationships compared with acetylcholine receptor-like sequences in Octopus bimaculoides. (Right) Comparison of CR518 orthologues from different octopus species. Branch support values in red. Scale: branch length, Newick tree format. (B) Alignment of predicted A, B and C loops from analyzed Octopus bimaculoides CRs and the human a7 acetylcholine receptor (hα7) demonstrated that CRs lack most residues which contribute to the canonical acetylcholine (ACh) binding side (highlighted in red). (C) In situ hybridization from sucker epithelium (represented in Fig. 4C) showed that CRs exhibited a combinatorial expression pattern with certain subtypes coexpressed in the same cells (n = 11 – 32, % of cells expressing indicated subtypes). (D) Quantification of currents elicited by prey chemicals in two CRs (CR840 and CR518) and hα7 from Fig 4D. Data represented as mean ± SEM.
Figure S4. Related to Figure 4. CRs are insensitive to acetylcholine (A) CR840 and CR518 were insensitive to ACh (1mM, grey arrow indicates basal current overlap). ACh dose-response relationship for CRs compared with hα7 showed that hα7 was 1000-fold more sensitive and CRs only consistently responded to 100mM ACh (n = 3 – 5). (B, C) CRs were inhibited by mecamylamine (1mM in representative traces), similar to native chemoreceptor cell responses. n = 3 – 5 for expressed CRs, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. n = 4 for native chemoreceptors, p < 0.0001, two-tailed studenťs t-test. Data represented as mean ± SEM.
Figure S5. Related to Figure 5. CRs have distinct properties (A) HEK293 cells expressing CR840 were only responsive to chloroquine among sampled compounds (30μM atractylon and nootkatone, 200μM carvacrol, 25μM chloroquine). Chloroquine dose-response relationship for CR840 with an estimated half-maximal effective concentration (EC50): 10.32μM, 95% confidence interval (CI) 9.29 – 11.50μM, n = 7 – 9. (B) CR518 responded to various terpenoids and chloroquine, albeit with decreased chloroquine sensitivity compared to CR840. CR518 dose-response relationships: atractylon EC50 = 29.43μM, 95% CI = 25.94 – 32.99μM, nootkatone EC50 = 24.70μM, 95% CI = 20.27 – 29.62μM, carvacrol EC50 = 204.41μM, 95% CI = 163.5 – 251.3μM, chloroquine EC50 = 92.29μM, 95% CI = 75.21 – 106.50μM, n = 5 – 13. (C) CR840 exhibited dose-dependent responses to chloroquine and CR518 responded to increasing concentrations of atractylon or nootkatone (representative of n = 7 – 13). (D) Outside-out patches from HEK293 cells expressing CR840 or CR518 were sensitive to the same chemicals (30μM) identified in whole-cell recordings, suggesting membrane-delimited action. n = 4 – 5, p < 0.0001, two-way ANOVA with post-hoc Bonferroni test. Scale bars: 10pA, 10s. (E) Substitution of CR840 N-terminus conferred its pharmacological profile to CR518, n = 4 – 5, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (F) CR currents elicited by voltage protocol to obtain G-V relationship in the presence of the listed agonists. Arrows indicate when current amplitude was measured at −40mV following activating prepulses from −150mV to 50mV in 10mV increments. (G) G-V relationships for CR840 in response to 10μM chloroquine and CR518 in response to 35μM atractylon or nootkatone. p < 0.0001 for differences in CR840 versus CR518 across voltages, two-way ANOVA with post-hoc Tukey test n = 6 – 9. (H) Deactivation kinetics at −40 mV following voltage pulses from −150mV to 50mV in 10mV increments. Arrows indicate when deactivation rates were measured. (I) τ values from single exponential fits of activation at −120mV and deactivation at −40mV after an activating prepulse of −150mV. n = 6 – 8, p < 0.0001 for one-way ANOVA with post-hoc Tukey test. Data represented as mean ± SEM.
Figure S6. Related to Figure 6. Specific combinations of CRs confer distinct chemosensory responses Fish extract evoked distinct currents which varied in amplitude and voltage-dependent rectification based on the combinatorial expression of CRs in oocytes. Quantification in Fig 6A.
Figure S7. Related to Figure 6. Heteromeric CR properties (A) Immunoblotting for FLAG or HA from oocyte membrane fractions used as input for co-immunoprecipitation in Fig 6B. (B) Comparison of ACh dose-response relationship for CR840–828 and CR518–828 with hα7 (from Fig. S4), n = 3 – 7. (C) CR518–828 and CR840–828 were inhibited by mecamylamine. n = 3 – 6, p < 0.0001, one-way ANOVA with post-hoc Bonferroni test. (D) CR840–828 responded to hydrophilic (HPL) fish fractions while CR518–828 was stimulated by hydrophobic (HPB) but not HPL fractions. Responses normalized to total fish extract, n = 4 – 6. (E) HEK293 cells expressing CR840–828 were only responsive to chloroquine (25μM) among sampled compounds (30μM atractylon and nootkatone, 200μM carvacrol) while CR518–828 responded to various terpenes (30μM atractylon and nootkatone, 200μM carvacrol) and 25μM chloroquine. n = 6 – 7, p < 0.0001, two-way ANOVA with post-hoc Tukey test. (F) Comparison of dose-response relationships between homomeric and heteromeric CRs demonstrate changes in compound sensitivity (n = 6 – 14). (G) Single channel records from oocyte patches expressing the indicated CRs. CR840 and CR518 had distinct conductances that were not altered by CR828. Slope conductance: CR840 = 54.9 ± 1 pS, CR840–828 = 53.1 ± 1 pS, CR518 = 63.7 ± 0.7 pS, CR518–828 = 62.5 ± 0.9 pS, n = 4 – 6.
Supplemental table 1, related to Figure 4: Octopus phylogenetic information. Protein identification numbers and associated databases from Fig 4B.
Supplemental video 1, related to Figure 1: Octopus reaching behavior. Representative octopus separated from prey by a barrier which allows arm reaching and exploration. Arms probed secured crabs significantly more than control objects (suction cup without crab). 1.75X speed.
Supplemental video 2, related to Figure 7: Octopus chemotactile behavior - control. Representative octopus exhibited stereotypic exploratory behavior in a tank with each side of the floor covered in independently poured seawater-infused 1.5% agarose. 4X speed.
Supplemental video 3, related to Figure 7: Octopus chemotactile behavior - polygodial. Representative octopus exhibited brief, increased touches with fast arm retraction and avoidance in response to contacting 100μM polygodial-infused 1.5% agarose versus control. 4X speed.
Figure S2. Related to Figure 3. Octopus NompC is a well-conserved mechanoreceptor (A) Alignment of Octopus bimaculoides (obNompC) and Drosophila melanogaster (dmNompC) sequences revealed high conservation. Red indicates residues important for mechanosensitivity, * indicates identifical residues, : indicates similar residues. (B) 60mmHg elicited Gd3+-sensitive currents in excised patches expressing obNompC or dmNompC. Representative of n = 4. (C, D) obNompC and dmNompC channels were similarly sensitive to mechanical stimulation and exhibited similar conductance. 95% confidence interval for pressure required to induce half-maximal NPO: obNompC = 51 – 58.25mmHg, dmNompC = 47.11 – 56.79mmHg. n = 5. Patches from untransfected cells did not respond to similar stimuli (n = 6). Slope conductance: obNompC = 165.5 ± 4 pS, dmNompC = 154.0 ± 4 pS. n = 4. Data represented as mean ± SEM.
Data Availability Statement
Deep sequencing data are archived at Gene Expression Omnibus under accession number GSE156748.


