Abstract
Background
An Inferior vena cava filter (IVCF) provides prophylaxis against pulmonary embolism in patients that cannot be anticoagulated. A removable IVCF (R-IVCF) provides prophylaxis during a high-risk period while potentially eliminating long-term complications associated with a permanent IVCF. Factors influencing success of R-IVCF removal are ill-defined.
Methods
The study was a retrospective review of a prospectively maintained patient registry comprising patients who received an R-IVCF (Bard Recovery™ and G2™) at an academic level 1 trauma center. The influence of time in vivo, filter design, and filter head position on computed abdominal tomographic (CAT) scan (touching caval wall vs. free) on removal success was examined.
Results
Ninety-two patients each received an R-IVCF. Thirty-nine patients underwent removal attempt and 30 R-IVCFs were removed. Time in vivo did not affect removal success (success: 228 ± 104 days versus unsuccessful: 289 ± 158 days, p = 0.18). Filter design impacted filter head position (Recovery: 43% touching versus G2: 6% touching, p = 0.023). Position of the filter head influenced removal success (touching: 50% success versus free: 88% success, p = 0.021).
Conclusions
Position of the filter head is the key determinant of removal success. Specific device designs may impact filter head position as was the case with the two designs in this analysis. Time in vivo does not affect removal success.
Introduction
Patients with significant risk of (or established) deep venous thrombosis (DVT) and pulmonary embolism (PE) with contraindications to anticoagulation frequently require an inferior vena cava filter (IVCF) for protection from initial or recurrent thromboembolic events. Permanent IVCF effectively prevent PE (>95% in long-term follow-up) but carry a defined side-effect profile related to their chronicity [1–5]. Recently, several manufacturers introduced removable IVCFs (R-IVCF) to reduce long-term complication rates. These devices function similar to permanent devices but allow percutaneous removal when a patient’s DVT/PE risk subsides and/or anticoagulation becomes possible.
The United States Food and Drug Administration currently approves three commercially available R-IVCFs for use in the United States. Early published experience reveals increased IVCF use with the advent of retrievable devices but a general failure to capitalize on the potential for removal [6–8]. We examined our experience with this technology at a major academic tertiary referral center to specifically analyze factors influencing device removal.
Materials and methods
We retrospectively identified patients in a clinical registry maintained per recommended clinical practice [6] from August 2004 through June 2007 at the University of Wisconsin–Madison Hospital and Clinics, a 465-bed hospital with a level 1 trauma center. The University of Wisconsin–Madison Human Subject Institutional Review Board granted approval for the project.
The review included all patients receiving a Bard Recovery™ or G2™ R-IVCF (Bard Peripheral Vascular, Inc., Tempe, AZ) for PE prophylaxis. The filter was introduced as the Bard Recovery but redesigned as the G2 R-IVCF to reduce the incidence of filter angulation in the vena cava after launching. This extreme angulation resulted in a high rate of filter head/vena cava wall apposition and incorporation of the filter head into the vena cava wall due to an inflammatory response. This incorporation precluded appropriate positioning of the filter removal system over the filter head. Between August 2004 and January 2006 patients received the Recovery R-IVCF; from Februray 2006 to present they received the G2, designed with a longer set of axially arranged struts engineered to minimize angulation.
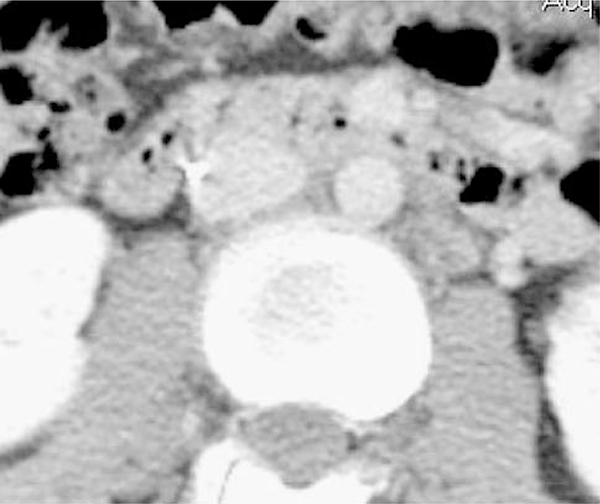
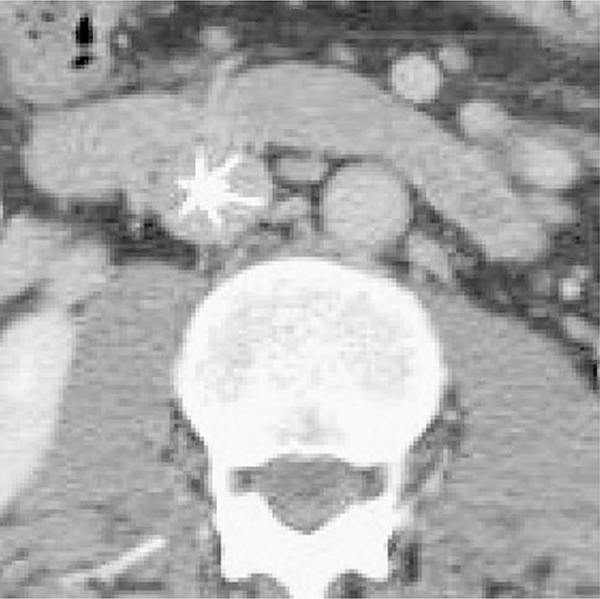
Clinical evaluation for device removal occurred when patients were no longer at high risk for DVT/PE, had recovered completely from their injuries, and/or were able to be anticoagulated if indicated. Workup consisted of a preprocedure outpatient computed abdominal tomographic (CAT) scan of the abdomen and pelvis with intravenous contrast to evaluate for the presence of an in-device clot, determine device position, and ascertain other pathology that could influence the decision for retrieval. Filter head position on CAT (touching versus free) was determined prior to the attempt to remove the filter. If no contrast was visible between the IVC wall and the filter head, the device was considered touching (Fig. 1). If contrast was visible between the filter head and IVC wall (even if angulated), the IVCF was considered free (Fig. 2).
Fig. 1.
CT scan demonstrating “touching” R-IVCF
Fig. 2.
CT scan demonstrating “free” R-IVCF
Surgeons performed all removal attempts (except one device removed by a cardiologist) in an inpatient operating room utilizing the full-sterile technique. The right internal jugular vein was accessed under ultrasound guidance. Fluoroscopy verified guidewire placement through the superior and inferior vena cava and the device. Confirmation by three views (anteroposterior, 30° right, and left anterior oblique) using a C-arm assured retrieval-cone positioning over the device. Inspection of the devices confirmed the presence or absence of fracture, clot, and overall integrity. All patients were discharged home the same day following standard postanesthesia care unit observation. No patients underwent more than one removal attempt.
The following factors were analyzed to determine their impact on removability: device time in vivo, filter device design, and filter head position (touching vs. free). Continuous variables were analyzed by Student’s t-test and categorical variables were analyzed by two-tailed Fisher’s exact test. A p < 0.05 was considered statistically significant.
Results
The registry contained 92 patients with an R-IVCF. All filters were placed at our institution except for one placed at an outside institution and referred to our institution for removal. Trauma surgeons (KAK, LDF, and MJS) placed 86 of the 92 R-IVCF devices and peripheral vascular surgeons placed 5. One surgeon (KAK) performed all but one removal attempt.
Table 1 lists the indications for all filters placed. The majority of patients (75 devices, 81%) who received an R-IVCF had sustained trauma. Nontrauma indications for R-IVCF placement included preoperative placement in seven high-risk bariatric patients (8%) and seven general surgical patients (8%) with known DVT or previous PE and contraindications to anticoagulation, and one patient undergoing multiple, complicated, lower-extremity orthopedic operations for an infected total knee joint. Two postoperative patients (3%) had known DVT and bleeding complications precluding anticoagulation.
Table 1.
Indications for R-IVCF placement
| Indication | No. of R-IVCF placed | Total (%) |
|---|---|---|
| Trauma | 75 | 81 |
| Preoperative DVT | 7 | 8 |
| Bariatric surgery | 7 | 8 |
| Postoperative bleed + DVT | 2 | 2 |
| Planned multiple lower-extremity operations | 1 | 1 |
Trauma demographics and disposition are listed in Table 2. For these patients, the average time from injury to R-IVCF placement was 5 days (median = 3, range = 1–26).
Table 2.
Characteristics of trauma patients receiving R-IVCF
| Age (years) | 38.4 ± 14.6 |
| Sex (% M) | 68 |
| ISS | 32 ± 13 |
| Mechanism | 100% Blunt |
| ICU LOS | 8 ± 7 |
| Hospital LOS | 23 ± 15 |
N = 74; one patient with initial hospitalization and R-IVCF placed for trauma at different institution
ISS, injury severity score; LOS, length of stay in days
Of the 92 patients with filters, four patients (three trauma, one nontrauma) died of nonfilter-related causes prior to evaluation for removal, including one due to aspiration, one from multisystem organ failure, and two from unknown cause of death after discharge (Table 3). Of the 88 survivors at the time of this analysis, 43 were not candidates for removal because they had not completely recovered from their injuries and remained at high risk for DVT/VTE due to continued immobilization, rehabilitation, or need for further orthopedic or other operative procedures. They remain in the registry for eventual removal when fully recovered from their injury or illness. The remaining 45 patients were evaluated for removal (Table 3). There was no attempt to retrieve the filter after CT evaluation due to patient refusal (3), presence of clot in the device documented by CAT (1), newly diagnosed malignant ovarian mass on CAT (1), and clinical weakness with failure to thrive (1), leaving 39 for analysis. These 39 eligible patients underwent attempted removal with successful removal in 30 (77%). One device was removed during catheterization for an unrelated heart condition without first obtaining a CT scan. The filter retrieval device could not be positioned over the filter head in eight cases and one failure occurred due to retrieval device fracture despite excellent positioning.
Table 3.
R-IVCF accountability
| Total R-IVCF placed | 92 |
| Death prior to evaluation | 4 |
| Not yet candidate for removal | 43 |
| Patient refusal | 3 |
| Clot in device | 1 |
| New malignancy | 1 |
| Failure to thrive | 1 |
| Total | 53 |
| Attempted filter removal | 39 |
| Successful removal | 30 |
Thirty-eight patients who underwent attempted removal had preremoval CT scans (one scan was performed at an outside institution and was not available for review). Table 4 describes the patients who underwent removal attempt at our institution and the 37 scans for which data were available.
Table 4.
Analysis of factors influencing R-IVCF removal
| Factor | Outcome | p-Value | |
|---|---|---|---|
| Time in vivo (days) | (+) removed | (−) removed | 0.18 |
| 228 ± 104 | 289 ± 158 | ||
| Filter design | Touching | Free | 0.023 |
| Recovery™ | 9/21 (43%) | 12/21 (57%) | |
| G2™ | 1/16 (6%) | 15/16 (94%) | |
| Filter head position | (+) removed | (−) removed | 0.021 |
| Touching | 5/10 (50%) | 5/10 (50%) | |
| Free | 24/27 (89%) | 3/27 (11%) | |
Time in vivo had no effect on retrieval of the filter during the period of the current study. Devices removed successfully had been in place 228 ± 104 days (median = 209 days, range = 33–441 days) compared with 289 ± 158 days (median = 303 days, range = 80–548 days) for devices not removed (p = 0.18). Filter design affected filter position. Nine of 21 (43%) first-generation Recovery filters touched the vena cava wall compared with 1 of 16 (6%, p = 0.023) G2 filters. Filter positioning and touching, in turn, affected retrievability. Successful retrieval occurred for only five of ten IVCFs touching the vena cava wall compared with 22 of 25 nontouching IVCFs (p = 0.027). Failure to remove one nontouching filter occurred because of separation of the shaft of the retrieval device resulting in an extremely angulated device being left in place. Not surprisingly, procedure times were shorter for successful versus unsuccessful removal attempts (19 ± 9 vs. 48 ± 23 min, p < 0.0001).
Of clinical note, one device produced intractable abdominal pain because an R-IVCF strut was penetrating the duodenum as shown on CT scan. Removal produced pain relief which was immediately noted in the recovery room without further complication. One patient experienced atrial fibrillation postremoval requiring cardioversion with propafenone with discharge home the same day. Failure to remove one patient’s device occurred because the shaft of the removal device fractured. These were the only device- and removal-associated complications.
Three (4%) patients experienced a nonfatal PE with an R-IVCF in place that was diagnosed 5, 9, and 41 days postinjury and 3, 5, and 39 days after filter placement, respectively. Axial images of the device at the time of PE diagnosis were available for only one patient whose device was well centered within the vena cava. There were no other complications.
Discussion
Deep venous thrombosis and thromboembolic disease remain frequent and challenging problems in caring for severely injured and high-risk surgical patients [3]. In the trauma population, clinical conditions, including associated injuries, orthopedic devices, and hemorrhagic risks, often preclude early diagnosis and use of mechanical and/or pharmacologic DVT prophylaxis [9]. In such situations, an IVCF may provide the only safe prophylactic option against PE. Major risks and complications attributed to permanent IVCFs include caval occlusion causing symptomatic edema, device fracture, migration, or complication with other procedures, e.g., central venous catheterization [4, 10]. Removable IVCFs provide the advantage of prophylaxis during time of risk with potential for elimination of long-term risk.
A recently published study on a large multicenter experience highlighted a major shortcoming of the current use of this new technology in practice, namely, there was no attempt to remove the device in the majority (72%) of cases [6]. This unfortunate shortcoming limited the value of a high technical success rate (87%) for removal. The authors concluded that loss of follow-up was the major barrier to IVCF retrieval. They implied that the major impediment to gaining maximal benefit from the device was the variety of providers (trauma/vascular/cardiothoracic surgeons and interventional radiologists) who placed the devices but who did not track the patients long term for removal.
Our data represent devices placed exclusively by surgeons into high-risk patients often at the time of other surgical and orthopedic procedures. The trauma staff placed more than 90% of the R-IVCFs, but one surgeon (KAK) assumed primary responsibility and performed all but one removal attempt using fluoroscopy with a C-arm in the surgical suite on an outpatient basis. Since 50% or more of pulmonary emboli occur within the first week after trauma [11], the average time to placement was 5 ± 5 days, similar to reports from other centers [6]. The trauma division prospectively maintained a clinical registry of all R-IVCF patients with periodic proactive follow-up and individual evaluation for device removal when clinically appropriate. Some patients were lost to follow-up, moved, or died; this is expected in a trauma population. However, the trauma nurse practitioner assessed for removal 42 of 52 (81%) devices placed during the first 2 years of our experience (for which we have 10 months or more of follow-up time). The nurse practitioner spends approximately 10% of her full-time position maintaining the database, reviewing the clinical progress, and evaluating these patients in the outpatient clinic.
Our clinical practice used exclusively the Bard Recovery and G2 devices for several reasons. Initial work with the Gunther Tulip™ R-IVCF studied patients with filters in place for very short times. Initial FDA approval was based on a study of 41 patients with a mean in vivo time of 11 days. The OptEase product has increased risk of caval occlusion [2, 6, 7]. The original clinical trial that examined Bard Recovery devices evaluated 58 devices, 45 of which were removed an average of 60 days later (range = 1–161 days). This time frame for removal appeared more appropriate for the trauma population who often experience substantial rehabilitation, limited mobility, and presumed risk of thromboembolism for prolonged periods of time. At the time of this analysis, 43 patients remain in the registry for eventual evaluation and assessment for removal of the filters.
Not all filters could be removed and any number of factors could influence successful removal. Duration in vivo is likely a minor factor (if a factor at all). Within the narrow time frame represented by this difference (228 ± 104 – 289 ± 158 days), time in vivo was not a statistically significant factor (Table 4). It is unknown whether an upper time limit for removal exists because the longest duration in vivo of a successfully removed filter was 441 days in our series. Although the number of nontrauma patients was small, trauma vs. nontrauma did not appear relevant to successful removal. Subjectively, no other factors, including body habitus, played a role in removability. BMI is not routinely recorded in our trauma database but only three trauma patients weighed more than 250 pounds. In these three patients, one removal was successful, a second was not evaluated for removal due to continued recovery, and the third patient died. Within the bariatric surgery cohort, removal was attempted in three patients and was successful in one. Both of the bariatric surgery patients with failed retrieval had first-generation Recovery devices and were early (first 9 months) in our experience. The filter head was touching the vena cava in one of these failed attempts. The successful attempt occurred in the second year of our experience and involved a G2 device that was not touching the vena cava.
The most important factor for successful retrieval appeared to be device design and relates to positioning of the filter in the vena cava after launch. In early 2006 a redesigned G2 R-IVCF with a longer set of struts replaced the original Recovery device. This alteration reduced the incidence of extreme angulation of the filter when initially placed. This reduced the incidence of the head of the filter touching the vena cava, a factor that leads to adherence of the head to the vena cava wall in an inflammatory, fibrotic response and inability to position the retrieval cone over the filter. We analyzed the position of the filter head on all CAT scans obtained and classified patients into two groups: “touching,” i.e., no contrast between the filter head and the vena cava wall on CAT, or “free,” where contrast could be seen between them. Table 4 shows touching occurred significantly more often with Recovery filters, precluding their removal. Our data indicate that the design change positively impacted device positioning. If the filter head touched the caval wall, the chance of removal success was only 50%; if the filter head was free (even if severely angulated), success improved significantly, approaching nearly 90% (Table 4).
In our experience removable IVCFs provide safe, effective prophylaxis against fatal PE in a predominantly trauma patient population. These patients require close re-evaluation after discharge because many patients forget or do not realize that the device is present or intended to be temporary. A registry of removable IVCFsprovides, assumption of responsibility for removal by the trauma staff, and assignment for follow-up to a specific practitioner an effective service to the patient. Preoperative examination with contrast-enhanced axial images prior to removal to evaluate for clot (which would make removal at that time unsafe) and device position allows fully informed conversation with and consent of the patient regarding the removal procedure and probable outcomes.
The CAT study could be eliminated by performing a contrast study or intravenous ultrasound (IVUS) at the time of removal. IVUS is an effective method for renal vein localization during device placement [12, 13], eliminates the use of intravenous contrast, and may be a way to evaluate the device for removal. However, such an approach may lead to wasted OR time if clot is discovered and obviates the ability to have a fully informed preoperative conversation with the patient regarding chances for successful removal.
Patients in whom removal is not performed should be followed periodically to monitor for complications and to follow the natural history of temporary devices that remain in vivo permanently.
Acknowledgment
This work was supported by NIH Grant R01 GM53439 (KAK).
Contributor Information
Joshua L. Hermsen, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA
Anna R. Ibele, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA
Lee D. Faucher, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA
Jennifer K. Nale, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA
Michael J. Schurr, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA
Kenneth A. Kudsk, Department of Surgery, University of Wisconsin–Madison, School of Medicine and Public Health, Madison, WI 53792, USA Veterans Administration Surgical Services, William S. Middleton Memorial Veterans Hospital, Madison, WI 53705, USA.
References
- 1.Becker DM, Philbrick JT, Selby JB (1992) Inferior vena cava filters: Indications, safety, effectiveness. Arch Intern Med 152:1985–1994 [PubMed] [Google Scholar]
- 2.Corriere MA, Sauve KJ, Ayerdi J et al. (2007) Vena cava filters and inferior vena cava thrombosis. J Vasc Surg 45:789–794 [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Code KI, Jay RM et al. (1994) A prospective study of venous thromboembolism after major trauma. N Engl J Med 331:1601–1606 [DOI] [PubMed] [Google Scholar]
- 4.Giannoudis PV, Pountos I, Pape HC et al. (2007) Safety and efficacy of vena cava filters in trauma patients. Injury 38:7–18 [DOI] [PubMed] [Google Scholar]
- 5.Greenfield LJ, Proctor MC (1995) Twenty-year clinical experience with the Greenfield filter. Cardiovasc Surg 3:199–205 [DOI] [PubMed] [Google Scholar]
- 6.Karmy-Jones R, Jurkovich GJ, Velmahos GC et al. (2007) Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study. J Trauma 62:17–24 [DOI] [PubMed] [Google Scholar]
- 7.Meier C, Keller IS, Pfiffner R et al. (2006) Early experience with the retrievable OptEase vena cava filter in high-risk trauma patients. Eur J Vasc Endovasc Surg 32:589–595 [DOI] [PubMed] [Google Scholar]
- 8.Quirke TE, Ritota PC, Swan KG (1997) Inferior vena caval filter use in US trauma centers: a practitioner survey. J Trauma 43:333–337 [DOI] [PubMed] [Google Scholar]
- 9.Rogers FB, Cipolle MD, Velmahos G et al. (2002) Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma 53:142–164 [DOI] [PubMed] [Google Scholar]
- 10.Sing RF, Adrales G, Baek S et al. (2001) Guidewire incidents with inferior vena cava filters. J Am Osteopath Assoc 101: 231–233 [PubMed] [Google Scholar]
- 11.Sing RF, Camp SM, Heniford BT et al. (2006) Timing of pulmonary emboli after trauma: implications for retrievable vena cava filters. J Trauma 60:732–734 [DOI] [PubMed] [Google Scholar]
- 12.Ashley DW, Gamblin TC, Burch ST et al. (2001) Accurate deployment of vena cava filters: comparison of intravascular ultrasound and contrast venography. J Trauma 50:975–981 [DOI] [PubMed] [Google Scholar]
- 13.Ashley DW, Gamblin TC, McCampbell BL et al. (2004) Bedside insertion of vena cava filters in the intensive care unit using intravascular ultrasound to locate renal veins. J Trauma 57:26–31 [DOI] [PubMed] [Google Scholar]