SUMMARY
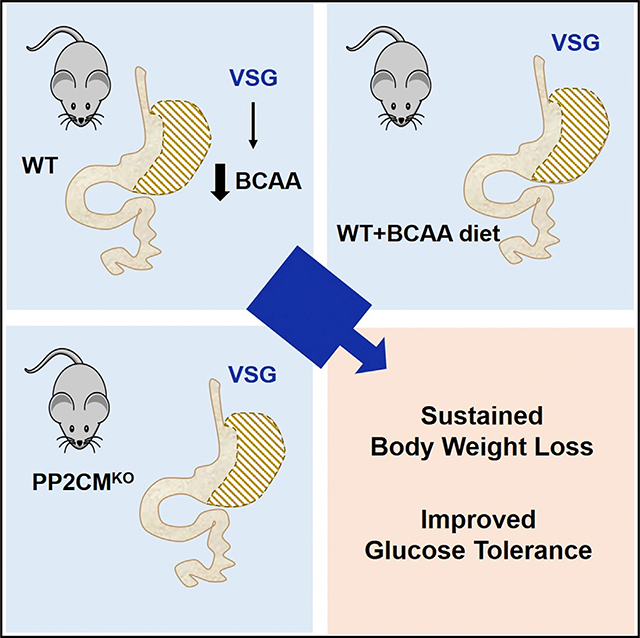
Elevated levels of branched-chain amino acids (BCAAs) and their metabolites are strongly positively associated with obesity, insulin resistance, and type 2 diabetes. Bariatric surgery is among the best treatments for weight loss and associated morbidities. Clinical studies have reported that bariatric surgery decreases the circulating levels of BCAAs. The objective of this study was to test the hypothesis that reduced BCAA levels contribute to the metabolic improvements of sustained weight loss and improved glucose tolerance after vertical sleeve gastrectomy (VSG). We find that, as in humans, circulating BCAAs are significantly lower in VSG rats and mice. To increase circulating BCAAs, we tested mice with either increased dietary intake of BCAAs or impaired BCAA catabolism by total body deletion of mitochondrial phosphatase 2C (Pp2cm). Our results show that a decrease in circulating BCAAs is not necessary for sustained body weight loss and improved glucose tolerance after VSG.
In Brief
Increased branched-chain amino acid (BCAA) levels are biomarkers of metabolic disease, and bariatric surgeries reduce BCAA levels. Bozadjieva Kramer et al. show that both dietary and genetic manipulations can block the surgical effect on BCAAs but do not alter potent, beneficial effects on weight loss and glucose tolerance.
Graphical Abstract
INTRODUCTION
Obesity has become a growing healthcare concern of which associated complications, such as cardiovascular morbidity, type 2 diabetes (T2D), and insulin resistance, pose major health care challenges worldwide (Afshin et al., 2017). Numerous studies have focused on understanding the metabolomics profile associated with obesity and its comorbidities. Consequently, metabolic signatures including increased levels of circulating branched-chain amino acids (BCAAs) have consistently exhibited a strong correlation with obesity and insulin resistance (Guasch-Ferré et al., 2016; Huffman et al., 2009; Newgard et al., 2009; Palmer et al., 2015; Shah et al., 2012; Tai et al., 2010; Walford et al., 2016; Würtz et al., 2013).
Circulating BCAA (leucine, isoleucine, and valine) levels are increased in both humans and rodents with obesity (Newgard et al., 2009; Zhou et al., 2019). Elevated plasma levels of these three essential amino acids are associated with a 5-fold increased risk for the future development of T2D (Wang et al., 2011). Improving BCAA catabolism with a pharmaceutical approach effectively lowers circulating BCAA levels and attenuates insulin resistance in mouse models of obesity, creating novel avenues for potential therapy (Zhou et al., 2019). The strong link between increased BCAA levels and insulin resistance has been demonstrated in humans with metabolic disorders and higher BMIs. However, even in studies in which humans are matched for BMI, those with insulin resistance have higher circulating BCAAs (Huffman et al., 2009; Palmer et al., 2018; Tai et al., 2010). A non-targeted metabolic profile of hyperglycemic/T2D and normoglycemic patients similarly revealed a strong correlation between BCAA and BCAA metabolites with impaired fasting glucose and T2D (Menni et al., 2013). These findings consistently demonstrate that perturbations in BCAA homeostasis are associated with metabolic dysfunction. The mechanisms behind these observations have been posited in several recent reviews on this topic (Arany and Neinast, 2018; Neinast et al., 2019a; Newgard, 2017; White and Newgard, 2019). Importantly, these data have led to the hypothesis that increased BCAA levels directly contribute to metabolic dysfunction in patients with obesity and T2D.
Although invasive, weight loss surgeries such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) have proven to be among the best treatments for weight loss and T2D (Schauer et al., 2017). Recent studies also showed that bariatric surgery significantly reduces circulating BCAA levels post-surgery and BCAA levels remain lower at 2 years post-operatively (Hanvold et al., 2018; Pakiet et al., 2020; Wijayatunga et al., 2018). These effective surgical interventions not only lead to decreased circulating BCAA levels but also have been shown to improve BCAA catabolism, specifically in adipose tissue (Laferrère et al., 2011; She et al., 2007b). Clinical data have also suggested that weight loss surgeries reduce the levels of circulating BCAAs more effectively than conventional weight loss interventions (Laferrère et al., 2011; Lips et al., 2014). However, these studies are difficult to interpret because the diets consumed by the control group and weight-loss surgery patients are likely not the same. In fact, bariatric surgery alters food preference in humans and rodents (le Roux and Bueter, 2014; Wilson-Pérez et al., 2013b). Overall, it remains unclear whether decreasing (or normalizing) circulating BCAA levels in humans with obesity is a direct contributor to the metabolic improvements after these surgical interventions. Moreover, it is important to identify whether a decrease in circulating BCAA levels post-surgery could be a predicative measure of sustained body weight loss and improved glucose homeostasis.
VSG produces sustained weight loss and important weight-independent effects on metabolism, including increased GLP-1 secretion, early-phase insulin secretion, improved glucose tolerance, and increased hepatic insulin sensitivity in rodent models (Chambers et al., 2013; Wilson-Pérez et al., 2013a, 2013b). To answer the question of whether improved BCAA homeostasis plays a role in metabolic improvements after bariatric surgery, we used rodent models of VSG, which have a decrease in BCAA coupled with profound improvement in weight loss and metabolic profiles. We used both dietary and genetic manipulations to prevent the VSG-induced reductions in circulating BCAAs in the context of high-fat-diet (HFD)-induced obesity. First, we show that circulating BCAA levels are indeed lower in VSG rats than in sham rats and mice as soon as 2 weeks after surgery. Subsequently, we supplemented HFD with increased levels of the three BCAAs (leucine, isoleucine, and valine) while maintaining the same total protein content as control HFD. Finally, we used a mouse model of impaired BCAA catabolism, by total body deletion of mitochondrial phosphatase 2C (Pp2cm). Pp2cm is a key enzyme and an activator of the mitochondrial branched-chain α-ketoacid dehydrogenase (BCKD) responsible for the rate-limiting step in BCAA catabolism. Our results showed that a decrease in circulating BCAAs is not necessary for sustained body weight loss and improved glucose tolerance after VSG.
RESULTS
VSG Decreases Circulating Levels of BCAAss
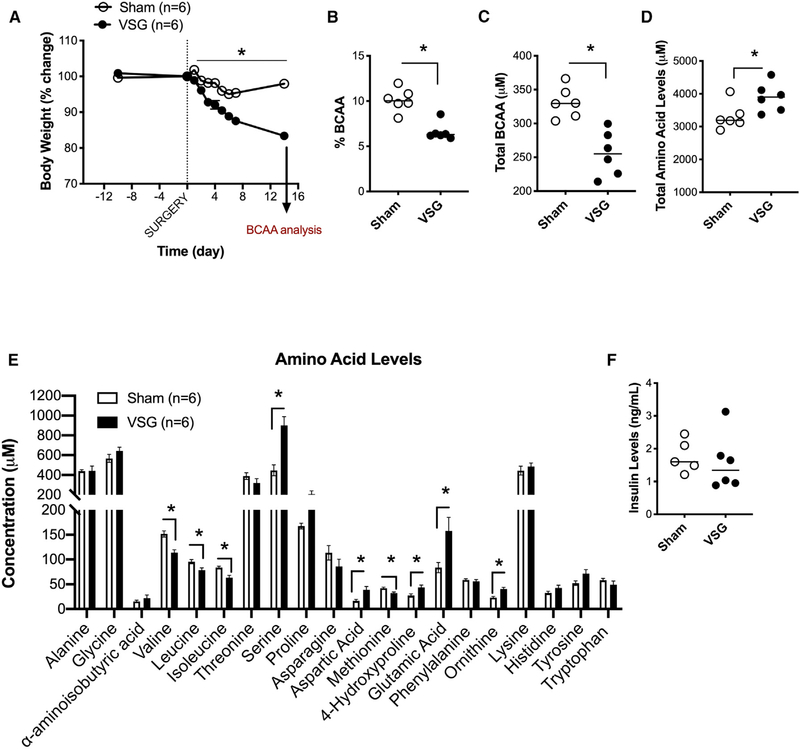
Clinical studies show that weight-loss surgeries reduce the levels of circulating BCAAs (Hanvold et al., 2018; Laferrère et al., 2011; Magkos et al., 2013; Pakiet et al., 2020; Wijayatunga et al., 2018). We first determined whether the decrease in circulating BCAAs observed in humans is also observed in rodent models of VSG. We generated a cohort of Long-Evans rats with ad lib access to 45% Tso’s HFD with butter fat before and after undergoing sham or VSG surgery. Rats that received VSG had a significant decrease in body weight 2–16 days post-surgery compared to rats that received sham surgery (Figure 1A). On day 14, the percentage and total levels of fasting BCAAs were decreased in VSG compared to those of sham animals (Figures 1B and 1C). The decrease in plasma BCAA levels was not attributed to a decrease in total amino acid levels, which were actually increased in the VSG rats (Figure 1D). In addition to a decrease in plasma levels of valine, leucine, and isoleucine in VSG rats, individual amino acid analysis showed that VSG rats had increased levels of glutamic acid (Figure 1E). Glutamic acid, or glutamate, is produced during the transamination reaction, the first step in BCAA catabolism. Finally, the alterations in amino acid levels seen between VSG and sham rats were independent of circulating insulin levels that were not different between the groups at the time of amino acid analysis (Figure 1F). Interestingly, there was a positive correlation between plasma BCAA levels and body weight change (Figure S1A) but no correlation between BCAA levels and HOMA IR (homeostatic model assessment of insulin resistance) (Figure S1B).
Figure 1. VSG Decreases Circulating Levels of BCAA.
(A) Body weight of Long-Evans rats with ad lib access to 45% Tso’s high-fat diet (HFD) with butter fat before and after undergoing sham or VSG surgery.
(B and C) Percent (B) and total levels (C) of fasting plasma branched-chain amino acids (overnight fast) on day 14 post-surgery.
(D) Total plasma amino acid levels on day 14 post-surgery.
(E) Individual plasma amino acid levels on day 14 post-surgery.
(F) Fasting insulin levels (overnight fast) on day 14 post-surgery. Animals n = 6/group. Data are shown as means ± SEM. *p < 0.05; (Student’s two-tailed t test).
Dietary Supplementation of BCAA Impairs Glucose Tolerance in HFD-Fed Mice
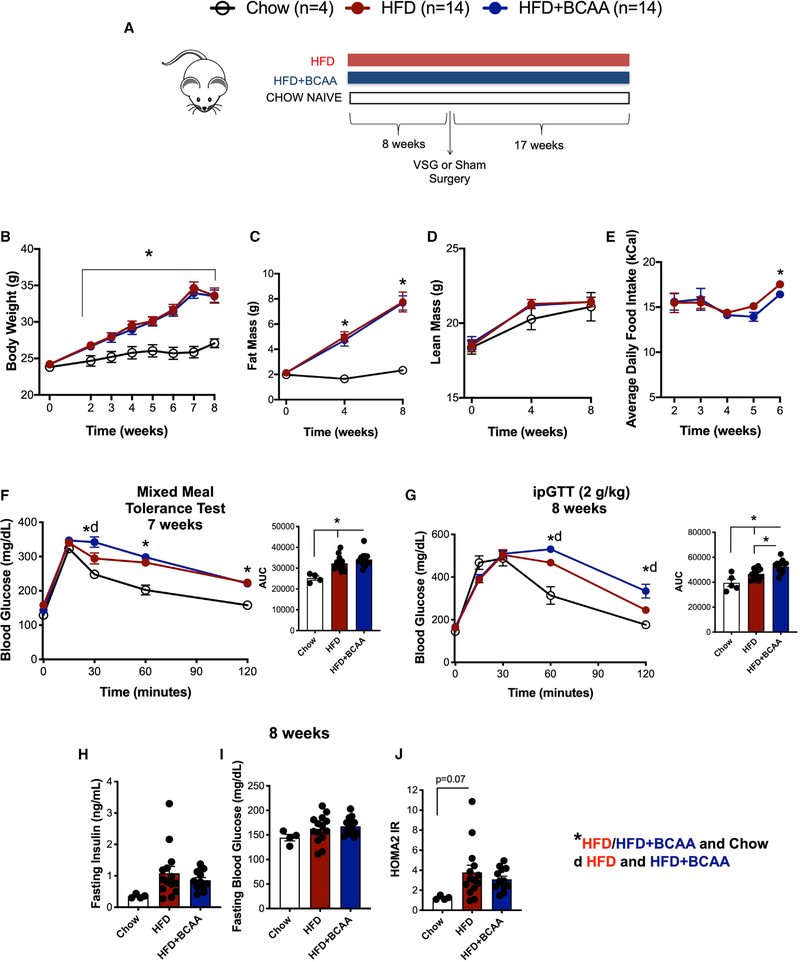
We tested the effect of increased dietary intake of BCAAs on body weight, adiposity, food intake, and glucose tolerance in HFD-fed mice. Wild-type male C57BL/6J were fed control and BCAA-supplemented HFD (Figure 2A). The BCAA-supplemented diet contained four times the levels of BCAA compared to the control diet (Figure 2A; Table S1). The control and BCAA-supplemented diet both contained 60% fat and had the same total amino acid content (Table S1). Neither the control HFD nor the HFD+BCAA diet were deficient in any amino acids and essential fatty acids (Table S2; National Research Council, 1995). We included a small group of standard chow-fed mice as a reference group. Wild-type male C57BL/6J fed HFD and HFD+BCAA diets had comparable body weight gain, with similar adiposity and lean mass (Figures 2B, 2C, and 2D). Mice fed HFD+BCAA showed decreased food intake 5 weeks after the start of the diet (Figure 2E). Additionally, they showed impaired glucose tolerance after a mixed meal tolerance test (Figure 2F) and intraperitoneal tolerance test (ipGTT; Figure 2G) compared to control mice fed HFD. BCAA supplementation did not affect fasting insulin or blood glucose levels (Figures 2H and 2I). The surrogate marker for insulin resistance HOMA2 IR (calculated based on fasting blood glucose and insulin levels) was not different after dietary BCAA supplementation during high-fat feeding compared to the control HFD (Figure 2J).
Figure 2. Dietary Supplementation of BCAA Impairs Glucose Tolerance in HFD-Fed Mice.
(A) WT male C57BL/6J were fed control and BCAA-supplemented HFD. BCAA-supplemented diet contained 4 times the levels of BCAA compared to the control diet. The control and BCAA-supplemented diet both contained 60% fat and had the same total protein content (Table S1). We included a small group of standard chow-fed mice as a reference group.
(B–E) Body weight (B), fat mass (C), lean mass (D), and average daily food intake (E) of male C57BL/6J fed HFD and HFD+BCAA diet.
(F) Mixed meal tolerance test was performed 7 weeks post-initiation of diet. Shown as blood glucose response and area under the curve (AUC).
(G) Intraperitoneal tolerance test (ipGTT; 2 g/kg) was performed 8 weeks post-initiation of diet. Shown as blood glucose response and AUC.
(H–J) Fasting insulin (6 h) (H), fasting blood glucose levels (6 h) (I), and HOMA2 IR (calculated based on fasting blood glucose and insulin levels) (J) 8 weeks after start of respective diet. Animals, n = 14/group. Data are shown as means ± SEM. *p < 0.05 (one-way ANOVA with Tukey’s post-test) with legends: *p < 0.05 HFD/HFD+BCAA compared to chow; d, p < 0.05 HFD compared to HFD+BCAA.
Dietary Supplementation of BCAA Does Not Impede Sustained Weight Loss and Improved Glucose Tolerance after VSG
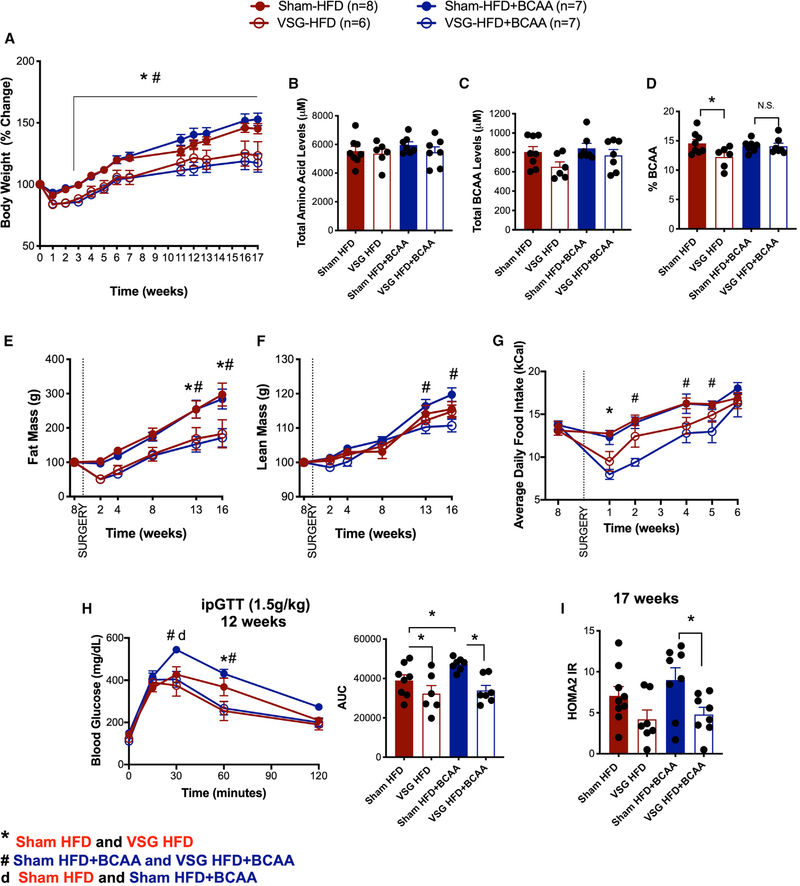
Our results show that dietary BCAA supplementation along with high-fat feeding results in impaired glucose tolerance. Next, we asked whether the decrease in BCAA levels after VSG contributed to the surgery-induced improvements in body weight loss and glucose tolerance. After 8 weeks of HFD or HFD+BCAA diet, mice were divided into four groups and underwent sham or VSG surgery. Mice were returned to their pre-operative diet 3 days after sham/VSG surgery. BCAA supplementation did not affect the sustained weight loss after VSG surgery (Figure 3A). We measured the levels of circulating amino acids after an overnight fast, 17 weeks after surgery. All four groups had comparable levels of fasting total plasma amino acid levels (Figure 3B). Although the plasma BCAA levels (leucine, isoleucine, and valine) showed only a trend toward being decreased in the VSG-HFD mice (Figure 3C), the percentage of BCAA levels in plasma (relative to total amount of amino acid levels) was significantly lower in VSG-HFD mice than in sham-HFD mice (Figure 3D). This effect of VSG to lower BCAA levels was blocked in VSG-HFD+BCAA mice compared to sham-HFD+BCAA mice (Figures 3C and 3D). BCAA supplementation did not affect the loss of fat mass following VSG surgery (Figure 3E). However, lean mass in VSG-HFD+BCAA mice was significantly lower than that in sham-HFD+BCAA mice 13–16 weeks post-surgery (Figure 3F). Daily food intake decreased in both VSG groups after surgery. VSG-HFD mice had decreased daily food intake for 2 weeks after surgery, consistent with previous observations in wild-type (WT) mice after VSG (Ryan et al., 2014; Figure 3G). VSG-HFD+BCAA mice had decreased daily food intake for 5 weeks post-surgery (Figure 3G).
Figure 3. Dietary Supplementation of BCAA Does Not Impede Sustained Weight Loss and Improved Glucose Tolerance after VSG.
(A) Body weight (% change) following VSG and sham surgery.
(B–D) Total plasma amino acid levels (B), total BCAA (C), and percent BCAA levels (relative to total amino acid levels) (D) in plasma after overnight fast.
(E and F) Fat mass (E) and lean mass (F) after VSG and sham surgery.
(G) Average daily food intake after VSG and sham surgery.
(H) ipGTT (2 g/kg) was performed 12 weeks after VSG and sham surgery. Shown as blood glucose response and AUC.
(I) HOMA2 IR, calculated based on fasting blood glucose and insulin levels, 17 weeks after surgery. Animals, n = 6–8/group. Data are shown as means ± SEM. *p < 0.05 (two-way ANOVA with Tukey’s post-test) with legends: *p < 0.05 sham HFD compared to VSG HFD; d, p < 0.05 sham HFD compared to sham HFD+BCAA; #p < 0.05 sham HFD+BCAA compared to VSG HFD+BCAA.
Sham mice fed HFD+BCAA (20-week duration of diet, 12 weeks post-surgery) showed significant glucose intolerance after ipGTT (2 g/kg) when compared to control mice fed HFD (Figure 3H). Importantly, both VSG-HFD and VSG-HFD+BCAA mice had a significant improvement in glucose clearance compared to their respective sham controls (Figure 3H). The surrogate marker for insulin resistance HOMA2 IR (calculated based on fasting blood glucose and insulin levels after a 6-h fast) shows that VSG decreased insulin resistance independent of dietary BCAA supplementation (Figure 3I).
Indirect gas calorimetry measurements showed that dietary supplementation with BCAA lowered the respiratory exchange ratio (RER) after VSG. VSG-HFD+BCAA mice had decreased RER during dark phase compared to VSG-HFD mice (Figures S2A–S2C). A trend of increased energy expenditure was seen in both VSG groups, with no differences between the two diets (Figure S2D). No differences were seen in locomotor activity or food or water intake between the surgical groups and diets (Figures S2E, S2F, and S2G). Additionally, dietary supplementation with BCAA did not alter pancreas weight, pancreatic beta cell mass, and liver and epididymal white adipose tissue (eWAT) (Figures S3A–S3D). A trend of decreased pancreatic beta cell mass was seen in both VSG groups, with no differences between the two diets (Figure S3B).
Orally Administered Valine and Glucose Are Absorbed into Circulation Faster in VSG Than in Sham Rats
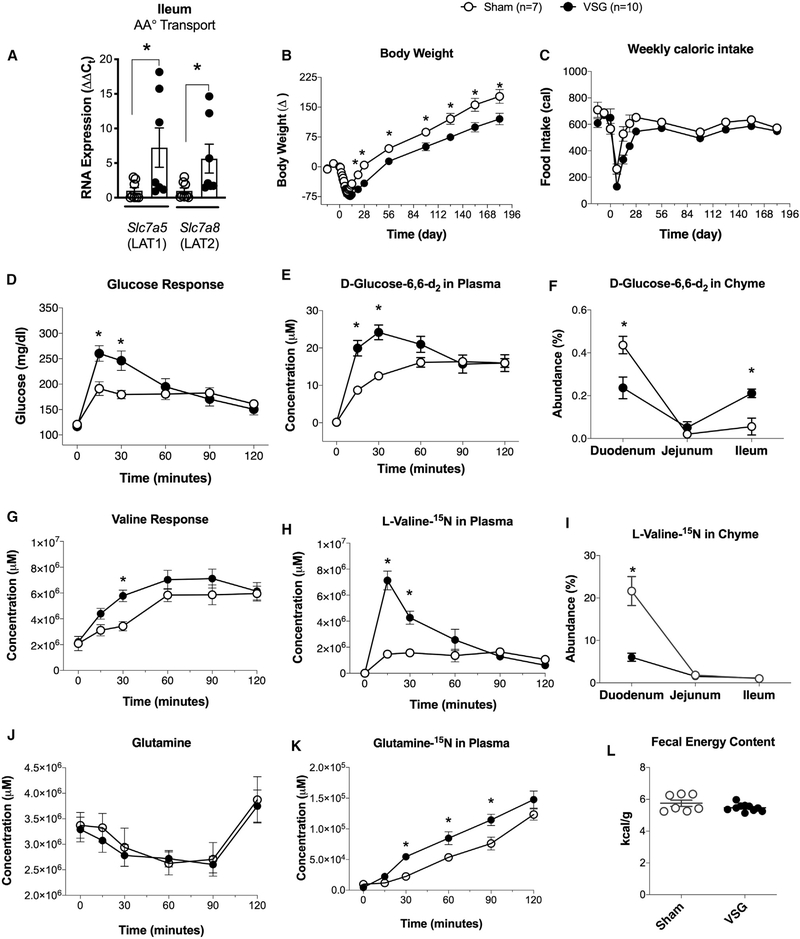
Our data show that increasing the dietary intake of BCAAs does not impede the sustained body weight loss and improvements in glucose tolerance after VSG. Subsequently, we tested the hypothesis that VSG decreases the absorbance of BCAA levels in the gut, thus leading to lower plasma BCAA levels. This hypothesis was supported by increased RNA expression of neutral amino acid transporters in ileal mucosal scrapes in VSG compared to sham mice (Figure 4A). System L (Slc7a5/LAT1 and Slc7a8/LAT2) transporters release basolaterally smaller intracellular neutral amino acids in exchange for larger extracellular neutral amino acids (BCAAs) (Bröer, 2008; Dave et al., 2004). These data suggested that the gut could also play a role in BCAA uptake through expressional regulation of amino acid transporters after VSG.
Figure 4. Orally Administered Valine and Glucose Are Absorbed Faster into Circulation in VSG Than Sham Rats.
(A) RNA expression of amino acid transporters in ileal mucosal scrapes from WT sham and VSG mice (n = 6).
(B and C) Body weight (B) and weekly caloric intake (C) in VSG and sham Long-Evans rats (n = 7–10).
(D–F) Total glucose response (D), circulating D-glucose-1,6-d2 (E), and luminal isotopic glucose (F) in VSG and sham Long-Evans rats (n = 7–10).
(G–I) Total circulating valine levels (G), circulating levels of L-valine-15N (H), and luminal levels of L-valine-15N (I) in VSG and sham Long-Evans rats (n = 7–10).
(J and K) Total circulating glutamine levels (J) and (Circulating levels of glutamine-15N (K) in VSG and sham Long-Evans rats (n = 7–10).
(L) Fecal matter was collected from the period of 185–191 days post-surgery for energy quantification using bom-calorimetry (n = 7–10) in VSG and sham Long-Evans rats (n = 7–10). Data are shown as means ± SEM. *p < 0.05; (Student’s two-tailed t test).
To test the hypothesis that VSG leads to decreased absorbance of BCAAs into circulation, we generated a cohort of VSG and sham Long-Evans rats. Body weight and weekly caloric intake in VSG and sham Long-Evans rats show sustained body weight loss for 196 days after surgery, without differences in caloric intake (Figures 4B and 4C). At day 196, animals were fasted overnight, and on the following morning, they received a mixture of liquid meal (Ensure Plus) containing 125 mg/ml glucose + 125 mg/ml 6,6-[2H]D-glucose + 12.5mg/ml [15N]L-valine. VSG rats had a higher glucose and isotope-glucose excursion at 15 and 30 min after the liquid meal, which is consistent with an increased absorbance of glucose due to increased gastric emptying as a result of VSG (Chambers et al., 2013; Evers et al., 2020; Figures 4D and 4E) and increased absorption capacity due to upregulation of the transporters Slc7a5 and Slc7a8 (Figure 4A). Labeled glucose levels were lower in duodenum chyme but higher in the ileum chyme of VSG rats (Figure 4F). Similarly, VSG rats had a higher valine and isotope-valine excursion 15 and 30 min after the liquid meal (Figures 4G and 4H). Labeled valine levels were lower in duodenum chyme of VSG rats than in sham rats (Figure 4I), consistent with increased efficiency of absorption. Glutamine levels were not different between sham and VSG rats before (overnight fast) and after a liquid meal (Ensure Plus) containing 125 mg/ml glucose + 125 mg/ml 6,6-[2H] D-glucose + 12.5mg/ml [15N]L-valine (Figure 4J). However, labeled glutamine-15N, produced during the transamination reaction of valine, was increased in the plasma of VSG rats compared to sham rats (Figure 4K). Finally, fecal energy content was similar between sham and VSG rats, demonstrating no malabsorption in VSG compared to sham rats (Figure 4L). Overall, the data suggest that VSG leads to an increased rate of valine absorption due to increased gastric emptying and an increased rate of transamination.
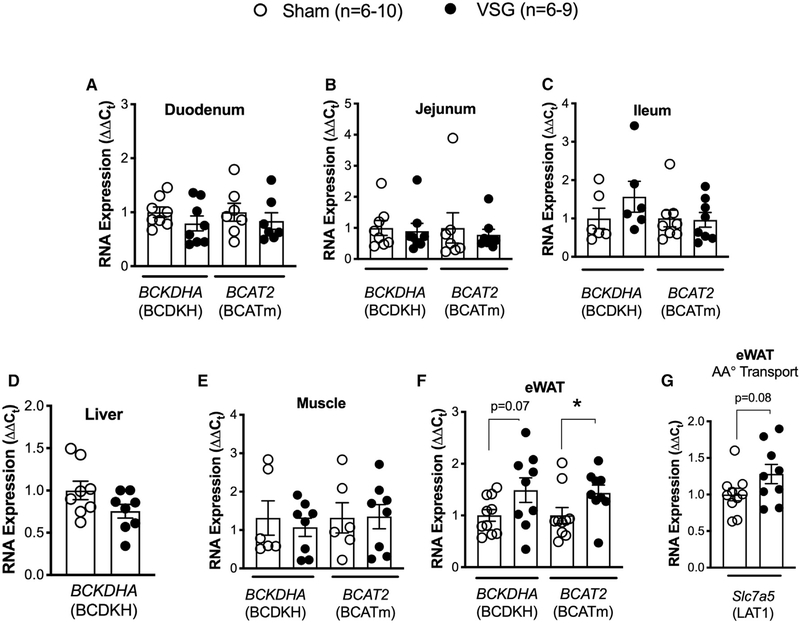
BCAA Catabolism Genes and Amino Acid Transporter LAT1 Expression Are Increased in eWAT of VSG Mice
Our data show that labeled glutamine-15N was increased in plasma of VSG rats compared to sham rats after receiving a mixture of liquid meal (Ensure Plus) containing 125 mg/ml glucose + 125 mg/ml 6,6-[2H]D-glucose + 12.5mg/ml [15N]L-valine (Figure 4K). Glutamine-15N is produced during the transamination reaction of valine, and increased plasma levels of glutamine-15N suggested that VSG mice have increased BCAA catabolism. Therefore, we examined the expression of BCAA catabolism genes BCDKHA (BCDKH) and BCAT2 (BCATm) in intestinal segments (duodenum, jejunum, and ileum), liver (liver does not express BCAT2), soleus skeletal muscle, and eWAT of WT VSG and sham mice (Figures 5A–5F). White adipose tissue was the only site of increased expression of BCAT2 (BCATm) and a trend toward increased expression of BCDKHA (BCDKH) in VSG mice, suggesting that VSG increases BCAA catabolism specifically in the eWAT. Additionally, expression of the amino acid transporter Slc7a5 (LAT1) also showed a trend of increased expression in the eWAT of VSG mice compared to sham mice (Figure 5G). These data suggest that white adipose tissue has increased uptake and catabolism of BCAA after VSG.
Figure 5. BCAA Catabolism Genes and Amino Acid Transporter LAT1 Expression Are Increased in Epididymal White Adipose Tissue (eWAT) of VSG Mice.
(A–F) RNA expression (ΔΔCt) of BCAA catabolism genes BCDKHA (BCDKH) and BCAT2 (BCATm) in duodenum (A), jejunum (B), ileum (C), liver (D) (liver does not express BCAT2), muscle (soleus) (E), and eWAT (F).
(G) RNA expression (ΔΔCt) of amino acid transporter Slc7a5 (LAT1) in the eWAT of WT male sham (n = 6–10) and VSG mice (n = 6–9). Data are shown as means ± SEM, *p < 0.05 (Student’s two-tailed t test).
Impaired BCAA Catabolism, by Total Body Ablation of Pp2cm, Does Not Impede Sustained Body Weight Loss and Improved Glucose Tolerance after VSG
Our data suggest that VSG mice have increased absorption but also increased catabolism of BCAAs after VSG. Thus, we hypothesized that mice with decreased catabolism of BCAA will have impaired metabolic responses to VSG. To test this hypothesis, we utilized the Pp2cmKO mouse model with a total body ablation of Ppm1k expression. Ppm1k encodes mitochondrial phosphatase, an activator of the mitochondrial BCKD responsible for the rate-limiting step in BCAA catabolism (Lu et al., 2009). Consequently, genetically disrupted Ppm1k expression leads to partially impaired BCAA catabolism and elevated plasma levels of BCAA (Lu et al., 2009).
Pp2cmKO mice at 6 weeks of age and fed a standard chow diet showed a trend of lower lean mass, with no differences in body weight and fat mass (Figures S4A–S4C). ipGTTs (2 g/kg glucose) were performed in 6-, 9-, 12-, and 30-week-old chow-fed Pp2cmKO and WT mice and did not show differences in glucose tolerance between the two genotypes (Figures S4D–S4G).
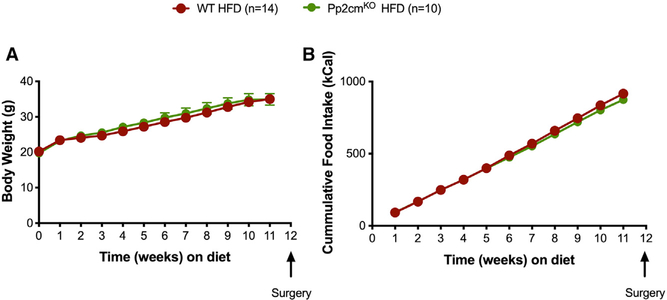
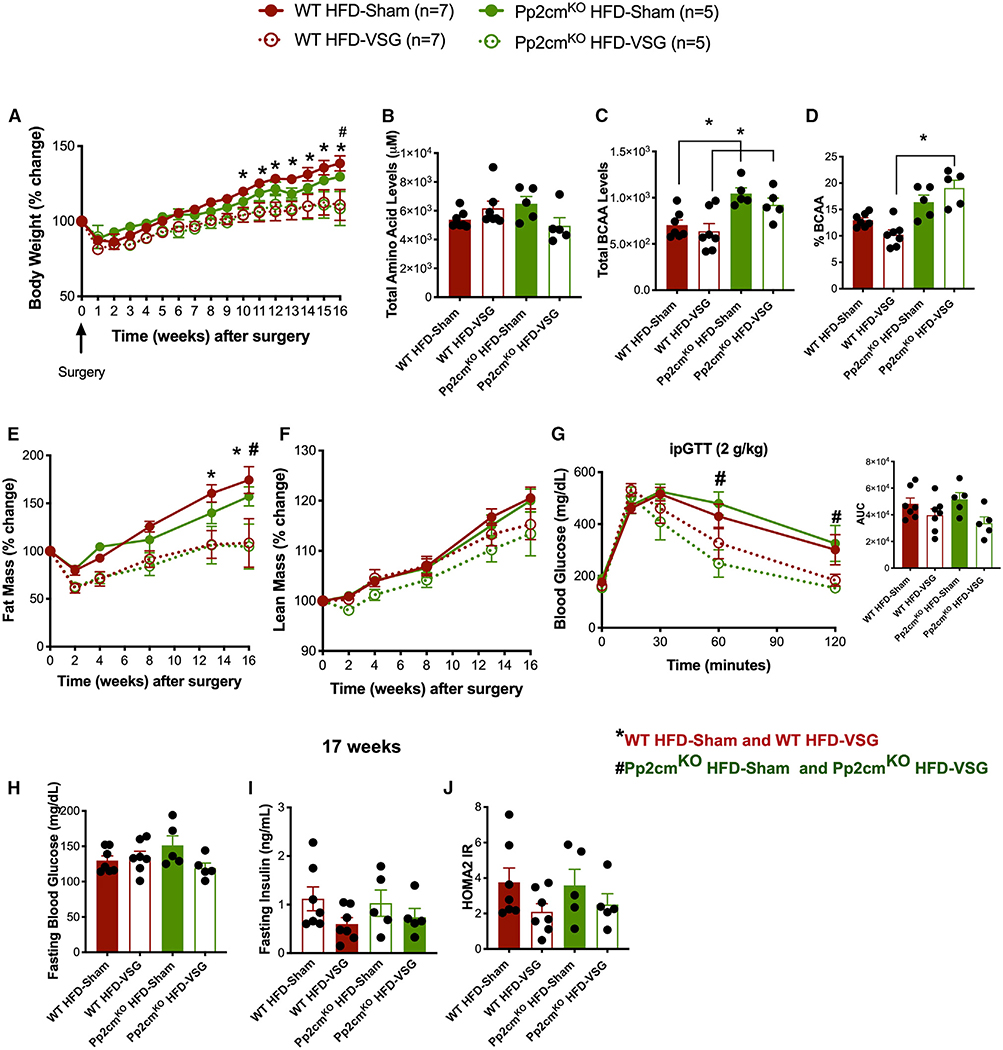
Pp2cmKO and WT littermates gained a comparable amount of body weight when challenged with 60% HFD for 8 weeks (Figure 6A). There were no genotype-specific differences in cumulative food intake over the 8-week span (Figure 6B). To examine the effects of defective BCAA catabolism on body weight loss and glucose tolerance following VSG, we divided the mice into four groups that underwent sham or VSG surgery. Mice were returned to their pre-operative HFD diet 3 days after sham/VSG surgery. Impaired BCAA catabolism by Pp2cm ablation did not affect the sustained weight loss after VSG surgery (Figure 7A). All four groups had comparable levels of total plasma amino acid levels (Figure 7B). Pp2cmKO HFD-sham mice had increased plasma levels of BCAA, and these levels did not decrease after VSG (Figure 7C). As expected, circulating BCAA levels were higher in Pp2cmKO HFD-sham mice than in WT HFD-sham mice (Figure 7C). Plasma BCAA levels and percent BCAA levels (relative to total amino acid levels) were also higher in Pp2cmKO HFD-VSG mice than in WT HFD-VSG mice (Figures 7C and 7D).
Figure 6. Impaired BCAA Catabolism, by Total Body Ablation of Pp2cm, Does Not Lead to Changes in Body Weight or Food Intake under Standard Chow or HFD Conditions.
Body weight (A) and cumulative food intake (B) in WT HFD (n = 14) and Pp2cmKO HFD (n = 10) mice fed 60% HFD for 12 weeks. Data are shown as means ± SEM, *p < 0.05 (Student’s two-tailed t test).
Figure 7. Impaired BCAA Catabolism, by Total Body Ablation of Pp2cm, Does Not Impede Sustained Body Weight Loss and Improved Glucose Tolerance after VSG.
(A) Body weight (% change) following VSG and sham surgery.
(B–D) Total plasma amino acid levels (B), total BCAA (C), and percent BCAA levels (relative to total amino acid levels) (D) in plasma after overnight fast.
(E and F) Fat mass (E) and lean mass (F) after VSG and sham surgery.
(G) ipGTT (2 g/kg) was performed 16 weeks after VSG and sham surgery. Shown as blood glucose response and AUC.
(H–J) Fasting blood glucose levels (6 h) (H), fasting insulin levels (6 h) (I), and HOMA2 IR (J), calculated based on fasting blood glucose and insulin levels, 17 weeks after surgery. Animals, n = 5–7/group. Data are shown as means ± SEM. *p < 0.05 (two-way ANOVA with Tukey’s post-test) with legends: *p < 0.05 WT HFD-sham compared to WT HFD-VSG; #p < 0.05 Pp2cmKO HFD-sham compared to Pp2cmKO HFD-VSG.
Defective BCAA catabolism did not affect the loss of fat mass after VSG surgery (Figure 7E). We observed a trend of decreased lean mass in Pp2cmKO HFD-VSG mice, similar to what we saw in VSG-HFD+BCAA (Figures 3F and 7F). Pp2cmKO HFD-VSG mice showed improved glucose tolerance compared to their sham controls, measured by ipGTT (2 g/kg) 16 weeks after surgery (Figure 7G). Fasting blood glucose levels were comparable between the four groups (Figure 7H). Although, not significant, fasting insulin levels were lower in both VSG groups than in their respective sham controls (Figure 7I). The surrogate marker for insulin resistance HOMA2 IR (calculated based on fasting blood glucose and insulin levels after 6-h fast) shows that VSG mice trended toward decreased insulin resistance after VSG independent of impairment in BCAA catabolism (Figure 7J). Pp2cmKO HFD-sham mice and HFD-VSG mice had similar meal size, meal number, food intake, and locomotor activity compared to WT HFD-sham and WT HFD-VSG mice (Figure S5). These data suggest that impaired BCAA catabolism does not prevent the sustained body weight loss and improved glucose clearance after VSG.
DISCUSSION
Metabolomic profiling of humans with obesity compared to that of lean humans has identified BCAAs as a reliable biomarker of insulin resistance (Guasch-Ferré et al., 2016; Huffman et al., 2009; Newgard et al., 2009; Palmer et al., 2015; Shah et al., 2012; Tai et al., 2010; Walford et al., 2016; Würtz et al., 2013). Numerous clinical studies have shown that bariatric surgery reduces the circulating BCAA levels (Hanvold et al., 2018; Laferrère et al., 2011; Magkos et al., 2013; Pakiet et al., 2020; Wijayatunga et al., 2018). The objective of our study was to answer whether improved BCAA homeostasis is necessary for the sustained body weight loss and improved glucose tolerance after VSG. First, we tested whether the decrease in circulating BCAAs observed in humans is also observed in rodent models of VSG. Rats and mice that received VSG had a decrease in fasting BCAA levels as early as 2 weeks post-surgery (rat; Figure 1) and 17 weeks post-surgery (mouse; Figure 3). This decrease in plasma BCAA levels was not attributed to a decrease in total amino acid levels. Also, at 2 weeks post-surgery, decreased levels of fasting plasma BCAAs were not due to reduced insulin levels, which were comparable between sham and VSG rats.
Next, we tested the effect of increased dietary intake of BCAAs on body weight, adiposity, food intake, and glucose tolerance in HFD-fed mice. WT male C57BL/6J were fed control and BCAA-supplemented HFDs, which contained four times the levels of BCAAs compared to the control diet (Table S1). The control and BCAA-supplemented diets both contained 60% fat and had the same total protein and carbohydrate content. WT male C57BL/6J fed HFD and HFD+BCAA diets had comparable body weight gain, with similar adiposity and lean mass. Mice fed HFD+BCAA showed decreased food intake 5 weeks after the start of diet. Additionally, they showed mild glucose intolerance after a mixed meal tolerance test and ipGTT compared to control mice fed HFD.
We sought to determine whether the decrease in BCAA levels after VSG contributed to the surgery-induced improvements in body weight loss or glucose tolerance. BCAA supplementation did not reduce the effectiveness of VSG to reduce food intake and produce sustained weight loss following VSG (Figure 3). The percent BCAA plasma levels (relative to total levels of amino acids) decreased in VSG-HFD mice compared to that in sham-HFD mice. However, in VSG mice exposed to increased BCAA dietary supplementation, this decrease in circulating BCAA levels was greatly attenuated. The changes in plasma BCAA levels were not due to alterations in total plasma amino acid levels. All four groups had comparable levels of total plasma amino acid levels. Sham mice fed HFD+BCAA (20-week duration of diet, 12 weeks post-surgery) showed significant glucose intolerance compared to control mice fed HFD (Figure 3). Importantly, both VSG-HFD and VSG-HFD+BCAA mice had a significant improvement in glucose clearance compared to their respective sham controls (Figure 3). These data support the conclusion that dietary supplementation of BCAA does not impede sustained weight loss and improved glucose tolerance after VSG.
Indirect calorimetry measurements showed that dietary supplementation with BCAA lowers RER after VSG. VSG-HFD+BCAA mice had decreased RER during dark phase compared to VSG-HFD mice (Figure S2). These data suggest that VSG increases the preference for carbohydrates as metabolic substrates, but dietary supplementation with BCAA after VSG shifts the preference toward fats. Previous studies from our lab and others have shown that intracerebroventricular (ICV) infusion of leucine caused a decreased food intake in mice in an mTORC1-dependent manner (Blouet et al., 2008; Cavanaugh et al., 2015; Cota et al., 2006). We did detect a decreased food intake in HFD+BCAA mice starting at around 5 weeks after BCAA diet initiation (Figure 2). Interestingly, after surgery, the VSG-HFD+BCAA mice required more time to return their average daily food intake to levels comparable to those of their sham-HFD+BCAA mice (Figure 3).
We did not observe increased fasting plasma BCAA levels in sham-HFD+BCAA mice compared to sham-HFD mice (Figure 3). Plasma BCAA levels spike but decline back to baseline levels within 3 h after consuming a protein-rich meal (Dangin et al., 2001). We chose to measure the levels of BCAA under static conditions, such as overnight fast, due to the differences in gastric volume and increased gastric emptying in VSG animals, which could have influence on the concentrations of plasma BCAA levels after feeding (Chambers et al., 2013; Evers et al., 2020). Consequently, we may have underestimated BCAA levels in the BCAA-supplemented mice. Studies that have used diets with increased BCAA content have not reported plasma levels of BCAA after diet (Sadagurski et al., 2019). Two recent studies also reported a lack of increase in the plasma BCAA levels after supplementing rodent diet with an increased amount of BCAAs and when compared to the standard diet (Haba et al., 2019; Zhou et al., 2019). This discrepancy makes it difficult to interpret if the impairment in glucose tolerance we and others see after dietary BCAA plus HFD is due to (1) increased plasma levels of BCAA, (2) due to impaired BCAA catabolism resulting from high-fat feeding and increased BCAA diet load, or (3) due to insulin resistance that may impair BCAA catabolism (Lynch and Adams, 2014). Indeed, a recent Mendelian randomization study suggested that BCAA levels do not have a causal effect on insulin resistance in humans (Mahendran et al., 2017). Nevertheless, the current data support the hypothesis that the impairment in glucose tolerance in sham-HFD+BCAA mice may be due in part to impaired catabolism of BCAAs as a result of chronic exposure of increased BCAA content in the context of a HFD.
Increasing dietary intake of BCAAs does not impede the sustained body weight loss and improved glucose tolerance after VSG. VSG does not decrease the absorbance of BCAA levels in the gut, and we find no evidence of overall malabsorption given unchanged levels of fecal energy content. There is instead an increased BCAA absorption, likely as a result of increased gastric emptying and increased RNA expression of neutral amino acid transporters in ileal mucosal scrapes in VSG compared to that in sham mice (Figure 4). The L-type amino acid transporter (LAT) family, and in particular LAT1 (Slc7a5), is responsible for the transport and absorption of most cellular BCAAs (Wang and Holst, 2015). System L (Slc7a5/LAT1 and Slc7a8/LAT2) transporters basolaterally release smaller intracellular neutral amino acids in exchange for larger extracellular neutral amino acids (BCAA) (Bröer, 2008; Dave et al., 2004). Specifically, glutamine enters the cells in a Na+-dependent manner and imports the BCAA leucine into the cell by the Slc7a5-SLC3A2 transporter (Nicklin et al., 2009). These amino acid transporters (Slc7a5/LAT1 and Slc7a8/LAT2) were increased in the ileal mucosa of VSG mice (Figure 4). To test this hypothesis, we administered a liquid meal containing 125 mg/ml glucose + 125 mg/ml D-glucose-6,6-d2 + 12.5mg/ml L-valine-15N to sham and VSG rats. VSG rats had a higher glucose and valine uptake, as well as higher isotope-glucose and isotope valine uptake, at 15 and 30 min after a liquid meal. These data are consistent with increased gastric emptying rate observed after VSG (Figure 4; Chambers et al., 2013; Evers et al., 2020). Potentially, the exposure of the small intestine to higher concentrations of BCAA due to increased gastric emptying may lead to the increased expression of the transporters, similar to that seen with increased dietary exposure to amino acids and other substrates (Ferraris and Diamond, 1989).
Our results show that plasma BCAA levels are decreased in VSG rats and mice, and these changes are not due to decreased absorbance of BCAAs. Therefore, we assessed whether VSG improved BCAA catabolism in tissues, resulting in decreased plasma BCAA levels. A recent study showed that BCAA homeostasis is determined largely by the BCAA catabolic activities in tissues (Neinast et al., 2019b). Heavy isotope steady-state infusions in obese and insulin-resistant db/db mice showed a shift in tissue-specific BCAA catabolism away from fat and liver and toward skeletal muscle (Neinast et al., 2019b). BCAA catabolism begins with the initial transamination step, catalyzed by BCAA transaminase (BCAT), to produce branched-chain ketoacids. The second step of oxidative decarboxylation is catalyzed by BCKD complex, yielding coenzyme A (CoA) esters. The BCKD complex is tightly regulated by the phosphorylation/inhibition by branched-chain α-ketoacid dehydrogenase kinase (BCKDHK) and the dephosphorylation/activation by mitochondrial Pp2cm.
Clinical data have shown that weight loss surgical interventions not only lead to decreased circulating BCAA levels but also have been shown to improve BCAA catabolism specifically in adipose tissue (Laferrère et al., 2011; She et al., 2007b). In agreement with these observations, VSG mice showed increased expression of key catabolic genes (BCAT2 and a trend of increased BCDKHA) in eWAT. Clinical studies have supported these data, showing that patients who have undergone bariatric surgery have increased protein expression of BCATm in omental and subcutaneous fat (She et al., 2007b). Additionally, expression of the amino acid transporter Slc7a5 (LAT1) had a trend toward increased expression in the eWAT after VSG (Figure 5F). These data lead us to speculate that white adipose tissue has increased uptake and catabolism of BCAA following VSG.
Consequently, we tested whether mice with impaired catabolism of BCAA will have impaired metabolic improvements after VSG by whole-body knockout of mitochondrial Pp2cm. We chose this mouse model for several reasons. The Ppm1k gene (coding for Pp2cm) was recently identified as a genomic region strongly associated with BCAA levels and T2D (Lotta et al., 2016). A lack of Pp2cm only partially impairs BCAA catabolism but does result in increased levels of circulating BCAAs (Lu et al., 2009). Overexpression of Ppm1k (gene coding for Pp2cm) lowers circulating BCAA levels, reduces hepatic steatosis, and improves glucose tolerance in the absence of weight loss in Zucker fatty rats (White et al., 2018; White and Newgard, 2019). We chose to use a total body knockout model instead of an adipocyte-specific knockout to prevent a compensatory shift of increased BCAA catabolism by other tissues. Finally, Pp2cmKO mice have a normal lifespan and are not resistant to HFD-induced weight gain as are other mouse models of impaired BCAA catabolism (She et al., 2007a). Chow-fed Pp2cmKO mice showed a trend toward increased fat mass and lower lean mass, without differences in body weight (Figure S4). Intraperitoneal tolerance tests in chow-fed Pp2cmKO and WT mice did not show differences in glucose clearance between the two genotypes (Figure S4). These findings differ from recently published studies by Wang et al. (2019) showing that chow-fed Pp2cmKO mice have improved glucose tolerance and insulin sensitivity. Wang et al. (2019) used WT C57BL/6 mice as controls, and although the authors state that the controls are the same genetic background and aged matched, it is unclear that these controls came from the same litters as Pp2cmKO mice. The control mice used in our studies are littermates of Pp2cmKO mice.
Pp2cmKO and WT littermates gained a comparable amount of body weight, consuming comparable amount of food when challenged with 60% HFD for 8 weeks (Figure 6). After these mice were given either VSG or sham surgery, Pp2cmKO did not show the expected reduction in BCAAs typically seen after VSG (Figure 7). Despite the fact that BCAAs remained high in Pp2cmKO given a VSG, Pp2cmKO showed the same sustained weight/fat loss after VSG surgery (Figure 7). Furthermore, Pp2cmKO showed the same effect of VSG to improve glucose tolerance measured by an ipGTT 16 weeks after surgery. These data support the conclusions that impaired BCAA catabolism does not prevent weight loss and improvements in glucose caused by VSG.
BCAA supplementation or impaired BCAA catabolism by total body deletion of Pp2cm did not affect the loss of fat mass after VSG surgery. However, lean mass in VSG-HFD+BCAA mice was significantly lower than that in sham-HFD+BCAA mice 13–16 weeks post-surgery (Figure 3). We also observed a trend toward decreased lean mass in Pp2cmKO HFD-VSG mice compared to their Pp2cmKO HFD-sham controls (Figure 7F). Muscle loss is a common side effect of rapid weight loss interventions, including bariatric surgery in humans (Davidson et al., 2018; Friedrich et al., 2013; Kenngott et al., 2019; Moizèet al., 2013). BCAA supplementation with HFD induces a chronic activation of mTORC1 in skeletal muscle in rats (Newgard et al., 2009). Additionally, infusions of amino acids in humans activate mTORC1 and consequently decrease insulin sensitivity in muscle and liver (Bevington et al., 2002; Tremblay et al., 2005). However, Magkos et al. (2013) found that mTOR phosphorylation in skeletal muscle was not altered following gastric bypass surgery in individuals with obesity, despite alleviation of BCAA levels, supporting a dissociation between BCAA levels and mTOR activation. It is important to note that we did not see differences in muscle mass between sham-HFD and sham-HFD+BCAA mice, nor Pp2cmKO HFD-sham and WT HFD-sham mice (Figure 3). In the context of rapid weight loss after VSG, we speculate that excess circulating BCAA levels in these mouse models (VSG-HFD+BCAA and Pp2cmKO HFD-VSG) may lead to chronic activation of mTORC1 in muscle (and the resultant inhibition of insulin receptor signaling), which may impair the ability to maintain muscle mass in response to rapid weight loss caused by VSG but while these mice are still fed diets high in fat.
Although we observed impaired glucose tolerance in mice fed HFD+BCAA as reported by others (Cota et al., 2006; Newgard, 2017; Newgard et al., 2009), we did not observe changes in markers of insulin sensitivity. The surrogate marker for insulin resistance HOMA2 IR shows that insulin resistance improves after VSG independent of dietary BCAA supplementation or impaired catabolism. It is possible that a longer exposure to BCAA-supplemented HFD would have induced significant insulin resistance. A direct measurement of insulin sensitivity by a hyperglycemic-euglycemic clamp would provide a more sensitive test of this hypothesis.
In summary, our results show that plasma BCAA levels are decreased in VSG rats and mice and that these changes are not due to decreased absorption or improved total body catabolism of BCAAs. Although it is clear that circulating levels of BCAAs are excellent biomarkers for metabolic status, the current data do not support a causal role for determining sustained body weight loss and glycemic improvements after VSG. The key question is whether there are common mechanisms that mediate both the alterations in BCAA regulation and improved glucose regulation that occur after bariatric surgical procedures. Answering how surgical manipulation of the gut can alter BCAA metabolism in non-gastrointestinal tissues is an important research goal that could lead to a better understanding of the gut’s involvement in BCAA regulation as well as identifying less invasive solutions to obesity and T2D.
Limitations of Study
VSG is the most commonly performed weight loss surgery in the United States (English et al., 2020). Our studies evaluated the role of BCAA levels and BCAA catabolism in sustained weight loss and improved glucose tolerance after VSG, specifically. Therefore, extrapolation to human data and other versions of weight loss surgery should be further evaluated. We focused mainly on sustained weight loss and glucose tolerance as the main two parameters for evaluating the role of BCAA levels and BCAA catabolism in the metabolic improvements after VSG. Our studies did not include a weight-matched control to VSG rodents. Such a control is the only way to determine the role of body weight loss as a factor for the decreasing plasma BCAA levels we see after VSG in mice and rats.
STAR⋆METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Randy J. Seeley (seeleyrj@med.umich.edu).
Materials Availability
Requests for available resources (tissue samples, etc.) and reagents should be directed to and will be fulfilled by the Lead Contact, Randy J. Seeley (seeleyrj@med.umich.edu).
Data and Code Availability
This study did not generate/analyze datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals and Diet
All protocols were approved by the University of Michigan (Ann Arbor, MI) Animal Care and Use Committees and were in accordance to NIH guidelines.
Male mice were single-housed under a 12-hour light/dark cycle with ad libitum access to water and food. Eight-week-old wild-type (C57BL/6) mice were fed 60% HFD (Catalog D16121101; protein (18% kCal) carbohydrate (21% kCal) fat (61% kCal)) or 60% HFD supplemented with BCAA for 8 weeks prior to VSG or Sham surgery (Catalog D16121102; protein (18% kCal) carbohydrate (21% kCal) fat (61% kCal)) from Research Diets, Inc; New Brunswick, NJ. Diet formulations can be found in Table S1. After surgery, the mice were returned to same experimental diets. The diets were formulated and purchased from Research Diets, Inc. (New Brunswick, NJ) and both diets are isocaloric (5.2 kCal/gm). HFD+BCAA have a 4-fold increase in BCAA (leucine, isoleucine and valine) but the same amount of total protein content as HFD control diet. Mice fed the standard chow diet were included as a reference (Envigo Teklad; Catalog 7012).
Pp2cmKO male mice (generous gift by Dr. Yibin Wang, UCLA) (Lu et al., 2009) were single-housed under a 12-hour light/dark cycle with ad libitum access to water and food. Pp2cmKO mice were born in Mendelian ratios and were not distinguishable from their WT littermates. Eight-week-old Pp2cmKO and WT male mice (all littermates) were fed 60% HFD from Research Diets, Inc. (Catalog D12492) for 8 weeks prior to VSG or Sham surgery and returned to HFD after surgery until end of study.
Long Evans male rats were maintained on 45% Tso’s HFD with butter fat (Catalog D03082706 from Research Diets) prior to undergoing Sham or VSG surgery. Fecal matter was collected from the period of 185–191 days post-surgery for energy quantification using bomb-calorimetry (performed by University of Michigan Animal Phenotyping Core).
All animals were euthanized using CO2.
Indirect gas calorimetry and Body composition
Body composition was measured using an EchoMRI (Echo Medical Systems). Indirect gas calorimetry (animal’s oxygen consumption (VO2) and carbon dioxide production (VCO2) to estimate various metabolic parameters, including the respiratory exchange rate (RQ), energy expenditure, substrate utilization, food and liquid intake, and locomotor activity were measured using 24-cage TSE PhenoMaster system (TSE Systems; Germany).
Vertical Sleeve Gastrectomy (VSG) in mice and rats
Mice were maintained on a 60% HFD and Long Evans male rats on a 45% Tso’s HFD for 8 weeks prior to undergoing Sham or VSG surgery, as described previously (Evers et al., 2020; Kim et al., 2019; Patel et al., 2018). See above for diet source and catalog numbers. Animals were anesthetized using isoflurane, and a small laparotomy incision was made in the abdominal wall. The lateral 80% of the stomach along the greater curvature was excised in VSG animals by using an ETS 35-mm staple gun (Ethicon Endo-Surgery). The Sham surgery was performed by the application of gentle pressure on the stomach with blunt forceps for 15 s. The day prior and 3 days following surgery, animals were placed on liquid diet (Osmolite 1.0 Cal, Abbott Nutrition). They were placed back on pre-operative solid diet on day 3 post-surgery. Body weight and food intake as well as overall health were monitored daily for the first 7 days after surgery and once weekly until end of the studies.
Isotope Studies
Long Evans male rats (Envigo, Indianapolis, IN) were individually housed at arrival and had ad lib access to 45% Tso’s high-fat diet with butter fat (Catalog D03082706 Research Diets) and water. At day 196, food was removed at the start of dark phase (18:00hr, 12:12 light-dark cycle) and animals were fasted overnight. Animals received a mixture of Ensure Plus containing 125mg/ml glucose + 125mg/ml D-glucose-6,6-d2 (Sigma-Aldrich; Catalog 282650) + 12.5mg/ml L-Valine-15N (Sigma-Aldrich; Catalog 490172) at a volume of 8ml/kg and a dosage of 1g/kg glucose, 1g/kg D-glucose-1,6-d2, and 0.1g/kg L-Valine-15N. Circulating glucose levels were measured at 0, 15, 30, 60, 90, and 120 minutes post-gavage from tail blood using a conventional glucometer (Accu-Chek) and an additional 200μl blood sample was collected and centrifuged to collect ~100ul plasma. Plasma samples were stored at −80°C until they were analyzed for circulating isotope levels. Animals were euthanized 120 minutes post-gavage using CO2. Intestines were collected and carefully separated into duodenum, jejunum, and ileum. Contents of each section were collected and stored at −80°C. All samples used for isotope analysis were sent to the metabolomics core at the University of Michigan for isotope analysis.
Metabolic Studies
Body weight was monitored monthly for 8 weeks prior and 17 weeks after Sham/VSG surgery. Intraperitoneal glucose tolerance test (IPGTT) was performed by intraperitoneal (IP) injection of 50% dextrose (2g/kg) in 4–6 hour fasted male mice. Mixed-meal tolerance test (MMTT) was performed via an oral gavage of liquid meal (volume 200 ml Ensure Plus spiked with a 25-mg dextrose) in 4–6 hour fasted male mice. Blood was obtained from the tail vein and blood glucose was measured with Accu-Chek blood glucose meter (Accu-Chek Aviva Plus, Roche Diabetes Care). HOMA2 IR was calculated based on fasting blood glucose and insulin levels (6 hours of fasting).
METHOD DETAILS
Amino Acid and Insulin Measurements
Insulin levels (6-hour fasting) were measured with ELISAs (Crystal Chem) following the manufacturer’s instructions. At week 17 post VSG/Sham surgery in mice (Figures 3 and 7) and 2 weeks post VSG/Sham surgery in rats (Figure 1), food was removed at the start of dark phase (18:00hr, 12:12 light-dark cycle), and mice were fasted overnight. Plasma amino acid level analysis was performed at Michigan Regional Comprehensive Metabolomics Resource Core (University of Michigan, Ann Arbor). Amino acid levels were analyzed in overnight fasted plasma using the Phenomenex EZfast kit. Samples are extracted, semi- purified, derivatized and measured by EI-GCMS using norvaline as an internal standard for normalization. All blood was collected via tail vein in EDTA-coated tubes.
Quantitative Real-Time PCR
RNA was extracted from tissue samples using RNeasy Plus Mini RNA isolation kit (QIAGEN). Gene expression was performed by quantitative real time RT-PCR using Power SYBR Green PCR Mix (Applied Biosystems) using StepOnePlus detection system (Applied Biosystems) with a standard protocol including a melting curve. Relative abundance for each transcript was calculated by a standard curve of cycle thresholds and normalized to Actb. Primers were purchased from Integrated DNA Technologies, Inc (IDT) and sequences can be found in the Key Resources Table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| High capacity cDNA Reverse Transcription kit | Applied Biosystems | Cat# 43-688-14 |
| RNAeasy Plus Mini Kit | QIAGEN | Cat# 74134 |
| Power SYBR Green PCR Master Mix | Applied Biosystems | Cat# 4367659 |
| D-Glucose-6, 6-d2 | Sigma-Aldrich | Cat# 282650 |
| L-Valine-15N | Sigma-Aldrich | Cat# 490172 |
| Critical Commercial Assays | ||
| Ultra Sensitive Mouse Insulin ELISA | Crystal Chem | Cat# 90080 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Pp2cmKO | Yibin Wang (UCLA) | N/A |
| Mouse: C57BL/6J | The Jackson Laboratory | Stock # 000664 |
| Rat: Long Evans | Envigo, US | HsdBlu: LE |
| Oligonucleotides | ||
| Actb F-CTA AGG CCA ACC GTG AAA AG | Integrated DNA Technologies | N/A |
| Actb R-ACC AGA GGC ATA CAG GGA CA | Integrated DNA Technologies | N/A |
| Slc7a5 F- CTG TGT GGG TCA CTT GTT AG | Integrated DNA Technologies | N/A |
| Slc7a5 R- TAG GTG CTG TTA GGG TCA T | Integrated DNA Technologies | N/A |
| Slc7a8 F- ACT CAG ATT GTC ACC GTT AAG | Integrated DNA Technologies | N/A |
| Slc7a8 R- GTG GAG AAG AAG GCA AAG AG | Integrated DNA Technologies | N/A |
| BCDKHA F- CCG GAT TGT GAT CTG TTA CTT | Integrated DNA Technologies | N/A |
| BCDKHA R- CAG AAG AAG ATG ATG GGA CAC | Integrated DNA Technologies | N/A |
| BCAT2 F- CAA GCA ACT CCA CAT ACC TAC | Integrated DNA Technologies | N/A |
| BCAT2 R- ACA CCC GAA ACA TCC AAT C | Integrated DNA Technologies | N/A |
| Software and Algorithms | ||
| GraphPad Prism | GraphPad Software | Version 8.2.0 |
| Other | ||
| Regular chow diet (Pp2cmKO and WT) | Envigo Teklad | Cat# 7012 |
| 60% High Fat Diet (Pp2cmKO and WT) | Research Diets | Cat# D12492 |
| 60% High Fat Diet + BCAA | Research Diets | Cat# D16121102 |
| 60% High Fat Diet (control for BCAA diet) | Research Diets | Cat# D16121101 |
| 45% High Fat Diet (rat studies only) | Research Diets | Cat# D03082706 |
| Osmolite 1.0 Cal | Abbott | Cat# 64633 |
| Ensure Plus (for liquid mixed-meal gavage) | Abbott | Cat # 57263 |
| Accu-Chek Aviva Meter | Accu-Chek, Roche Diabetes Care | https://www.accu-chek.com |
| Nuclear Magnetic Resonance | EchoMRI LLC, USA | EchoMRI-900 TM |
| TSE PhenoMaster System | TSE Systems, Germany | https://www.tse-systems.com |
QUANTIFICATION AND STATISTICAL ANALYSIS
The statistical analysis for comparisons between 2 groups was performed by unpaired (2-tailed) Student’s t test. One-way ANOVA with post hoc Tukey’s multiple comparisons test was used for comparisons among 3 groups. Two-way ANOVA with post hoc Tukey’s multiple comparisons test was used for comparisons among 4 groups. P values < 0.05 were considered significant (GraphPad Prism 8.2.0).
Supplementary Material
Highlights.
VSG surgery causes reduced levels of circulating branched-chain amino acids (BCAAs)
An increase in dietary BCAAs does not reduce benefits of VSG on weight and glucose
Deletion of Pp2cm blocks VSG’s lowering of BCAAs but does not reduce benefits
BCAAs are markers of VSG benefits but are not a driver of weight loss and glucose levels
ACKNOWLEDGMENTS
The authors thank the surgeons for conducting mouse VSG (Alfor Lewis, Andriy Myronovych, Mouhamadoul Toure, and Diana Farris) and Kelli Rule, Jack Magrisso, and Stace Kernodle for the technical assistance. We thank Dr. Michael Pellizzon from Research Diets, Inc. for help with diet formulation. We thank Michigan Regional Comprehensive Metabolomics Resource Core (DK097153), University of Michigan Animal Phenotyping Core (1U2CDK110678-01), and University of Michigan In-Vivo Animal Core (IVAC) (University of Michigan, Ann Arbor). This work was supported by NIH grants DK108740 and DK071212 (to N.B.K.), HL140116 (to Y.W.), DK107282 (to D.A.S.), DK020572 (to Michigan Diabetes Research Center), and DK089503 (to Michigan Nutrition and Obesity Research Center). N.B.K. was also funded by Michigan Institute for Clinical and Health Research (UL1TR002240).
DECLARATION OF INTERESTS
R.J.S. has received research support from Zafgen, Novo Nordisk, Kallyope, Astra Zeneca, Kintai, and Ionis. R.J.S. has served as a paid consultant for Novo Nordisk, Sanofi, Kallyope, Scohia, Kintai, and Ionis. R.J.S. has equity or options from Zafgen, Calibrate, and Rewind. S.S.E. is a paid employee of Gubra (Denmark).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108239.
REFERENCES
- Afshin A, Reitsma MB, and Murray CJL (2017). Health Effects of Over-weight and Obesity in 195 Countries. N. Engl. J. Med. 377, 1496–1497. [DOI] [PubMed] [Google Scholar]
- Arany Z, and Neinast M (2018). Branched Chain Amino Acids in Metabolic Disease . Curr. Diab. Rep. 18, 76. [DOI] [PubMed] [Google Scholar]
- Bevington A, Brown J, Butler H, Govindji S, M-Khalid K, Sheridan K, and Walls J (2002). Impaired system A amino acid transport mimics the catabolic effects of acid in L6 cells. Eur. J. Clin. Invest. 32, 590–602. [DOI] [PubMed] [Google Scholar]
- Blouet C, Ono H, and Schwartz GJ (2008). Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 8, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S (2008). Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88, 249–286. [DOI] [PubMed] [Google Scholar]
- Cavanaugh AR, Schwartz GJ, and Blouet C (2015). High-fat feeding impairs nutrient sensing and gut brain integration in the caudomedial nucleus of the solitary tract in mice. PLoS One 10, e0118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, et al. (2013). The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144, 50–52.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, and Seeley RJ (2006). Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930. [DOI] [PubMed] [Google Scholar]
- National Research Council. (1995). Nutrient Requirements of Laboratory Animals, Fourth Revised Edition (National Research Council; ). [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballèvre O, and Beaufrère B (2001). The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab 280, E340–E348. [DOI] [PubMed] [Google Scholar]
- Dave MH, Schulz N, Zecevic M, Wagner CA, and Verrey F (2004). Expression of heteromeric amino acid transporters along the murine intestine. J. Physiol. 558, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LE, Yu W, Goodpaster BH, DeLany JP, Widen E, Lemos T, Strain GW, Pomp A, Courcoulas AP, Lin S, et al. (2018). Fat-Free Mass and Skeletal Muscle Mass Five Years After Bariatric Surgery. Obesity (Silver Spring) 26, 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English WJ, DeMaria EJ, Hutter MM, Kothari SN, Mattar SG, Brethauer SA, and Morton JM (2020). American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis 16, 457–463. [DOI] [PubMed] [Google Scholar]
- Evers SS, Lewis AG, Tong C, Shao Y, Alvarez R, Ridelman E, Grant B, and Seeley RJ (2020). The Unconventional Role for Gastric Volume in the Response to Bariatric Surgery for Both Weight Loss and Glucose Lowering. Ann. Surg. 271, 1102–1109.. [DOI] [PubMed] [Google Scholar]
- Ferraris RP, and Diamond JM (1989). Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu. Rev. Physiol. 51, 125–141. [DOI] [PubMed] [Google Scholar]
- Friedrich AE, Damms-Machado A, Meile T, Scheuing N, Stingel K, Basrai M, Küper MA, Kramer KM, Königsrainer A, and Bischoff SC (2013). Laparoscopic sleeve gastrectomy compared to a multidisciplinary weight loss program for obesity—effects on body composition and protein status. Obes. Surg. 23, 1957–1965. [DOI] [PubMed] [Google Scholar]
- Guasch-Ferrè M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, and Hu FB (2016). Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 39, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba Y, Fujimura T, Oyama K, Kinoshita J, Miyashita T, Fushida S, Harada S, and Ohta T (2019). Effect of Oral Branched-Chain Amino Acids and Glutamine Supplementation on Skeletal Muscle Atrophy After Total Gastrectomy in Rat Model. J. Surg. Res. 243, 281–288. [DOI] [PubMed] [Google Scholar]
- Hanvold SE, Vinknes KJ, Bastani NE, Turner C, Løken EB, Mala T, Refsum H, and Aas AM (2018). Plasma amino acids, adiposity, and weight change after gastric bypass surgery: are amino acids associated with weight regain? Eur. J. Nutr 57, 2629–2637. [DOI] [PubMed] [Google Scholar]
- Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, and Kraus WE (2009). Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32, 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenngott HG, Nickel F, Wise PA, Wagner F, Billeter AT, Nattenmüller J, Nabers D, Maier-Hein K, Kauczor HU, Fischer L, and Müller-Stich BP (2019). Weight Loss and Changes in Adipose Tissue and Skeletal Muscle Volume after Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study with 12-Month Follow-Up. Obes. Surg. 29, 4018–4028. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hutch CR, Wood L, Magrisso IJ, Seeley RJ, and Sandoval DA (2019). Glycemic effect of pancreatic preproglucagon in mouse sleeve gastrectomy. JCI Insight 4, e129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, et al. (2011). Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 3, 80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, and Bueter M (2014). The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp. Physiol. 99, 1128–1132. [DOI] [PubMed] [Google Scholar]
- Lips MA, Van Klinken JB, van Harmelen V, Dharuri HK, t Hoen PA, Laros JF, van Ommen GJ, Janssen IM, Van Ramshorst B., Van Wagensveld BA, et al. (2014). Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 37, 3150–3156. [DOI] [PubMed] [Google Scholar]
- Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, Schmidt AF, Imamura F, Stewart ID, Perry JR, et al. (2016). Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med 13, e1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, and Wang Y (2009). Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest. 119, 1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, and Adams SH (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, Newgard CB, and Klein S (2013). Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 62, 2757–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jørgensen ME, Grarup N, Pedersen O, Kilpeläinen TO, and Hansen T (2017). Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 60, 873–878. [DOI] [PubMed] [Google Scholar]
- Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ, et al. (2013). Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62, 4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moizè V, Andreu A, Rodríguez L, Flores L, Ibarzabal A, Lacy A, Jimènez A, and Vidal J (2013). Protein intake and lean tissue mass retention following bariatric surgery. Clin. Nutr. 32, 550–555. [DOI] [PubMed] [Google Scholar]
- Neinast M, Murashige D, and Arany Z (2019a). Branched Chain Amino Acids. Annu. Rev. Physiol. 81, 139–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG, et al. (2019b). Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 29, 417–429.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB (2017). Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 25, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. (2009). A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakiet A, Wilczynski M, Rostkowska O, Korczynska J, Jablonska P, Kaska L, Proczko-Stepaniak M, Sobczak E, Stepnowski P, Magkos F, et al. (2020). The Effect of One Anastomosis Gastric Bypass on Branched-Chain Fatty Acid and Branched-Chain Amino Acid Metabolism in Subjects with Morbid Obesity . Obes. Surg. 30, 304–312. [DOI] [PubMed] [Google Scholar]
- Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, Wagenknecht LE, Newgard CB, and Bowden DW (2015). Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 100, E463–E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ND, Okut H, Hsu FC, Ng MCY, Chen YI, Goodarzi MO, Taylor KD, Norris JM, Lorenzo C, Rotter JI, et al. (2018). Metabolomics Identifies Distinctive Metabolite Signatures for Measures of Glucose Homeostasis: The Insulin Resistance Atherosclerosis Family Study (IRAS-FS). J. Clin. Endocrinol. Metab. 103, 1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Yusta B, Matthews D, Charron MJ, Seeley RJ, and Drucker DJ (2018). GLP-2 receptor signaling controls circulating bile acid levels but not glucose homeostasis in Gcgr−/− mice and is dispensable for the metabolic benefits ensuing after vertical sleeve gastrectomy. Mol. Metab. 16, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pèrez HE, Sandoval DA, Kohli R, Bäckhed F, and Seeley RJ (2014). FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Debarba LK, Werneck-de-Castro JP, Ali Awada A, Baker TA, and Bernal-Mizrachi E (2019). Sexual dimorphism in hypothalamic inflammation in the offspring of dams exposed to a diet rich in high fat and branched-chain amino acids. Am. J. Physiol. Endocrinol. Metab. 317, E526–E534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, and Kashyap SR; STAMPEDE Investigators (2017). Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 376, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B, et al. (2012). Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, and Hutson SM (2007a). Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 6, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Van Horn C, Reid T, Hutson SM, Cooney RN, and Lynch CJ (2007b). Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab 293, E1552–E1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, et al. (2010). Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhäusl W, Marette A, and Roden M (2005). Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54, 2674–2684. [DOI] [PubMed] [Google Scholar]
- Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, and Gerszten RE; Diabetes Prevention Program Research Group (2016). Metabolite Profiles of Diabetes Incidence and Intervention Response in the Diabetes Prevention Program. Diabetes 65, 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, and Holst J (2015). L-type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 5, 1281–1294. [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. (2011). Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu Y, Lian K, Shentu X, Fang J, Shao J, Chen M, Wang Y, Zhou M, and Sun H (2019). BCAA Catabolic Defect Alters Glucose Metabolism in Lean Mice. Front. Physiol. 10, 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, and Newgard CB (2019). Branched-chain amino acids in disease. Science 363, 582–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, Grenier-Larouche T, An J, Lapworth AL, Astapova I, et al. (2018). The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 27, 1281–1293.e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayatunga NN, Sams VG, Dawson JA, Mancini ML, Mancini GJ, and Moustaid-Moussa N (2018). Roux-en-Y gastric bypass surgery alter serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab. Res. Rev. 34, e3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Pèrez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pèrez-Tilve D, and Seeley RJ (2013a). Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62, 2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Pèrez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, and Seeley RJ (2013b). The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. 37, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, Viikari JS, Raitakari OT, and Ala-Korpela M (2013). Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, Chen M, Wynn RM, Wang J, Wang J, et al. (2019). Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 68, 1730–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets or code.