Abstract
Introduction:
Factor XI (FXI) deficiency is associated with highly variable bleeding, including excessive gynecologic and obstetrical bleeding. Since approximately 20% of FXI-deficient women will experience pregnancy-related bleeding, careful planning and knowledge of appropriate hemostatic management is pivotal for their care.
Areas covered:
In this manuscript, authors present our current understanding of the role of FXI in hemostasis, the nature of the bleeding phenotype caused by its deficiency, and the impact of deficiency on obstetrical care. The authors searched PubMed with the terms, ‘factor XI’, ‘factor XI deficiency’, ‘women’, ‘pregnancy’, and ‘obstetrics’ to identify literature on these topics. Expectations of pregnancy-related complications in women with FXI deficiency, including antepartum, abortion-related, and postpartum bleeding, as well as bleeding associated with regional anesthesia are discussed. Recommendations for the care of these women are considered, including guidance for management of prophylactic care and acute bleeding.
Expert commentary:
FXI deficiency results in a bleeding diathesis in some, but not all, patients, making treatment decisions and clinical management challenging. Currently available laboratory assays are not particularly useful for distinguishing patients with FXI deficiency who are prone to bleeding from those who are not. There is a need for alternative testing strategies to address this limitation.
Keywords: Factor XI, factor XI deficiency, hemostasis, bleeding, pregnancy
1. Introduction
Congenital deficiency of the plasma protein Factor XI (FXI) is associated with a variable propensity to bleed excessively after trauma, particularly when injury involves tissues rich in fibrinolytic activity such as the urinary tract, the nasopharynx, and the mouth [1,2]. While rare in the general population, the disorder is prevalent in certain ethnic groups, particularly Ashkenazi Jews [2,3] and French Basques [4]. FXI deficiency presents several therapeutic dilemmas. While post-traumatic hemorrhage can be significant, many patients with the severe form of the disorder (typically defined as a plasma FXI activity ≤15% of normal) do not experience abnormal bleeding [1,3–5]. Furthermore, FXI replacement therapy is frequently associated with the formation of neutralizing anti-FXI alloantibodies, arguing against wide-spread use of replacement therapy [6]. Approximately 20% of pregnant women with severe FXI deficiency experience excessive bleeding at the time of delivery in the absence of treatment [7]. Here, we discuss our current understanding of the role of FXI in hemostasis, review literature on FXI-deficiency in pregnancy, and make recommendations for management of FXI-deficiency during pregnancy and childbirth.
1.1. Search methodology
The authors initially queried PubMed with the following search terms: factor XI, factor XI deficiency, pregnancy, obstetrics, and women. When used in various combinations, these terms yielded approximately 500 results with significant overlap. The authors reviewed titles and abstracts for relevancy. Clinical trials were reviewed for inclusion and exclusion criteria, and validity of conclusions.
1.2. Factor XI in hemostasis
As a component of the classic intrinsic pathway of coagulation (Figure 1A), FXI is a driver of clot formation in the activated partial thromboplastin time (aPTT) assay [1,8]. In the aPTT, FXI is converted to its active form factor XIa (FXIa) by factor XIIa (FXIIa). FXIa in turn converts factor IX to factor IXa, leading to thrombin generation and clotting (Figure 1A). Specific measurements of FXI activity, given as a percentage of the activity in normal plasma, are based on the aPTT. Nearly all patients with severe FXI deficiency have significantly prolonged aPTTs. However, aPTT and FXI activity values correlate poorly with bleeding propensity in FXI-deficient patients [1,2,5,6]. Indeed, some patients completely lacking FXI (FXI activity <1% of normal, aPTT >100 seconds) have no history of abnormal bleeding despite experiencing significant hemostatic challenges including childbirth and major surgery. Bleeding in FXI-deficient patients is generally milder than in patients with factor IX or factor VIII deficiency (hemophilia B and A, respectively) [1–3], an observation that seems counterintuitive given that the aPTT is usually prolonged to a greater degree in severe FXI deficiency than in severe hemophilia A or B. Furthermore, deficiency of factor XII (FXII), the precursor of FXIIa, is not associated with a bleeding abnormality, despite having a profound effect on the aPTT [9]. These clinical observations indicate that the coagulation model that serves as the basis for the aPTT does not accurately reflect processes involved in hemostasis in vivo.
Figure 1.

(a) Classic cascade/waterfall model of thrombin generation. Coagulation is initiated either through the intrinsic pathway (indicated by black arrows) or the extrinsic pathway (indicated by the light gray circle enclosing factor VIIa and tissue factor [TF]). Coagulation proteases and their precursors are indicated in black type, with a lower case ‘a’ indicating the active form. Cofactors are indicated in white type within dark gray ovals. The intrinsic pathway initates coagulation in the activated partial thromboplastin time (aPTT) assay, while the extrinsic pathway initiates coagulation in the prothrombin time (PT) assay. (b) A model of thrombin generation based on initiation through the factor VIIa/tissue factor (TF) complex. Major reactions involved in thrombin generation and hemostasis in most situations are indicated by white arrows. A feedback loop involving thrombin activation of factor XI to factor XIa, with subsequent activation of factor IX (black arrows) is required for hemostasis in some individuals, particularly when trauma involves tissues with high intrinsic fibrinolytic activity such as those of the urogenital tract.
Current coagulation models are more consistent with clinical phenotypes of individuals with congenital clotting factor deficiencies [8,10,11]. In the model shown in Figure 1B, thrombin generation is initiated at an injury site by a complex formed between the plasma protease factor VIIa and tissue factor, a protein expressed within blood vessel walls and on extravascular tissues. The complex promotes thrombin generation by activating factors X and IX. The reactions indicated by white arrows in Figure 1B are the major reactions required to stem bleeding with most types of injury. In some individuals, FXIa activation of factor IX is required with certain types of trauma to supplement factor IXa generated by factor VIIa/tissue factor [1,8]. The absence of a bleeding disorder in patients lacking FXII indicates an enzyme other than FXIIa activates FXI during hemostasis. In the model in Figure 1B, thrombin is the major FXI activator, although other enzymes may be involved. While this model is consistent with FXI’s ancillary role in hemostasis, assays based on it have not entered clinical practice. The aPTT, and measurements of FXI activity based on the aPTT, remain the standard in evaluating FXI-deficient patients. The discordance between results from these assays and bleeding symptoms presents a major challenge for clinicians tasked with caring for these patients.
2. Factor XI deficiency during pregnancy
2.1. Assessing bleeding risk
Among the congenital coagulation factor deficiencies, the correlation between plasma factor activity and bleeding tendency is weakest for FXI. While some analyses indicate persons with severe deficiency (FXI ≤15% of normal) due bleed more than persons with partial deficiency (FXI 15–45% of normal) with high-risk procedures such as surgery on the urinary tract, oral cavity or nasopharynx [1,3,5], this is not universal [5]. Indeed, some investigators have concluded that it is difficult to distinguish severe and partial deficiency on clinical grounds [12]. The clinical variability undoubtedly impacts estimations of the frequency of the disorder, with many asymptomatic FXI-deficient individuals probably not coming to medical attention. Genetic studies in the Ashkenazi Jewish population indicate a carrier (heterozygous) frequency as high as one in eight, with a homozygous frequency (representing severe deficiency) of one in 450 [13,14]. In the general population, the homozygous frequency has been estimated to be closer to 1 in 1,000,000 (heterozygous frequency 1 in 1000), but supporting data are weak, and this may be an underestimation.
Focusing on reproductive tract bleeding, heavy menstrual bleeding is common in women with FXI deficiency [15–17]; however, it is difficult to predict the severity of menstrual bleeding for an individual prior to menarche based on their past history. The presence of heavy menstrual bleeding in this population is not unexpected given the significant hemostatic challenge associated with menstruation. While tissues with high intrinsic fibrinolytic activity appear to be most prone to injury-induced hemorrhage in FXI-deficient patients [13], menstrual and obstetrical bleeding can be significant in FXI-deficient women.
Absence of prior bleeding symptoms in a patient with FXI deficiency does not guarantee normal hemostasis with future hemostatic challenges; however, some investigators feel that a personal evaluation of bleeding phenotype can identify patients who require increased attention. Bolton-Maggs and colleagues classified persons with FXI deficiency (severe or partial) as bleeders or non-bleeders based on responses to a series of questions [15]. A person was considered a bleeder if they experienced at least four specific symptoms: easy bruising, mucous membrane bleeding (e.g. epistaxis, gastrointestinal bleeding, hematuria), heavy menstrual bleeding requiring treatment, post-operative bleeding, post-dental extraction bleeding or bleeding related to childbirth. A scoring system based on these questions was subsequently used in several studies of women with FXI deficiency. There are other bleeding assessment tools, such as the Tosetto bleeding assessment score, that can be useful in identifying persons with a clinically significant bleeding abnormality [18–20]. In addition, heavy menstrual bleeding can be semi-quantitatively assessed using pictorial bleeding assessment charts [21]. While these alternatives have not been rigorously tested in FXI-deficient women, they can be considered when attempting to identify persons with bleeding propensities. As we consider treatment and prevention obstetrical bleeding in FXI-deficient patients, distinguishing between bleeders and non-bleeders will be important.
2.2. Laboratory studies of factor XI during pregnancy
Unlike plasma levels of factor VIII and von Willebrand factor [22,23], FXI levels do not rise significantly during pregnancy. Indeed, FXI activity has been reported to remain stable [24] or decrease [25] during pregnancy in healthy women without FXI deficiency. Data for women with partial or severe FXI deficiency are conflicting. Several studies indicate that FXI activity does not change significantly over the course of pregnancy [26–28], while others reported modest increases or decreases [29,30]. Regardless, the magnitude of changes in FXI levels during pregnancy is unlikely to have an appreciable impact on hemostasis. Furthermore, as discussed, obstetrical bleeding in women with FXI deficiency does not correlate well with plasma factor activity [7,15,16,31,32]. Two groups have commented on obstetrical bleeding and the molecular genotype responsible for FXI deficiency. Salomon et al. did not find a correlation between specific mutations and bleeding tendency in a group of pregnant FXI-deficient women, primarily of Jewish background, in Israel. Specifically, patients homozygous for the type II mutation who have no circulating FXI did not appear to have more severe bleeding than patients with other genotypes [7]. Myers et al. identified four patients from one family with a rare whole gene deletion (obligate). All had bleeding histories and developed pregnancy-associated complications, but other contributors to bleeding were not ruled out [28]. As most FXI-deficient patients have reductions in FXI protein in their plasmas (cross-reactive material negative deficiency), genetic assessment probably does not provide much information on which to base management beyond that provided by the plasma FXI level.
Rotational thromboelastometry (ROTEM) has been evaluated as a monitoring strategy in women with FXI deficiency. Testing in non-pregnant adults demonstrated that ROTEM, like the aPTT, is sensitive to variation in plasma FXI activity, but results do not correlate well with clinical symptoms [33]. In pregnancy, several abnormalities have been reported in FXI-deficient women, including prolonged time to clot formation (analogous to the aPTT) and reduced clot strength. As has been reported for other types of surgical procedures in the general population, algorithms based on thromboelastometry can be used to decrease the overall use of blood products during labor and delivery, and for regional anesthesia [34]. However, at this point, it has not been established that thromboelastometry provides information that can be used to adjust therapy in patients with FXI deficiency.
2.3. Antepartum bleeding and abortions
The general impression is that antepartum bleeding, defined as any bleeding prior to delivery, is similar in women with FXI deficiency and in women without bleeding disorders. Several studies reported rare antepartum bleeding in FXI-deficient women, with clinically significant bleeding occurring only in the presence of additional complications such as preeclampsia or placental abruption (Table 1) [26,30,35]. Other studies focusing on FXI-deficient women simply reported uncomplicated pregnancies in the majority of subjects [28,36]. In one study, antepartum bleeding occurred in 14% of pregnancies in FXI-deficient individuals [29], which is comparable to reported rates for first trimester bleeding in the general population (7–27%) [37], but lower than the incidence in women with von Willebrand disease (33%) [29].
Table 1.
Frequency of pregnancy-related bleeding in women with factor XI deficiency as documented in observational trials.
| Reference | Patients (#SD)/Pregnancies | Antenatal bleeding | Abortion-related bleeding | Postpartum bleeding |
|---|---|---|---|---|
| Kadir et al. [29] | 11 (4 SD)/28 | 4 | 1 of 3 | 4 |
| Myers et al. [28] | 33 (3 SD)/105 | n/a | 2 of 29 | 9 |
| Chi et al. [26] | 30 (2 SD)/61 | 3 | 4 of 14 | 10 (5 primary) |
| Singh et al. [32] | 13 (4 SD)/13 | n/a | n/a | 2 |
| Verghese et al. [30] | 25 (4 SD)/67 | 2 | 0 of 16 | 11 (10 primary) |
| Gerber et al. [35] | 28 (8 SD)/64 | 3 | 2 of 11 | 9 (5 PD/4 SD) |
SD, severe deficiency.
Spontaneous abortion rates in FXI-deficient women (3–24%) [15,26,28–30,35,36] are comparable to those observed in the general population (10–30%) [38,39]. Bolton-Maggs et al. [15] and Myers et al. [28] reported abnormal bleeding after spontaneous abortions in three women with FXI deficiency, all of whom were classified as bleeders. There appears to be an increased risk of excessive bleeding following elective abortion. In a review of obstetrical bleeding in FXI-deficient women, 36% of individuals experienced excessive bleeding following elective abortions, the majority of whom were classified as bleeders based on prior symptoms (Table 1) [40].
2.4. Anesthetic management
Several studies focusing on delivery in woman with partial or severe FXI deficiency have discussed the safety of regional (neuraxial) anesthesia, including epidural anesthesia, spinal anesthesia, and combined spinal/epidural anesthesia, with or without hemostatic prophylaxis. We identified seven studies that described regional anesthesia in 84 women, only 36 of whom received hemostatic prophylaxis (plasma transfusion, FXI concentrate, or antifibrinolytic therapy) [26–30,32,35]. Use of prophylaxis ranged from 0% to 100% across the studies. When reports addressed the issue, it appears that prophylaxis was withheld only in patients without a prior bleeding history. There were no bleeding complications reported (Table 2).
Table 2.
Use of regional anesthesia and hemostatic prophylaxis in women with factor XI deficiency.
| Reference | Number of anesthesia events | Number of patients with hemostatic prophylaxis | Factor XI comments |
|---|---|---|---|
| Kadir et al. [29] | 2 | 0 | Documented as mild/moderate factor XI deficiency. |
| Myers et al. [28] | 14 | 0 | Unclear. 14 women received regional anesthesia prior to their diagnosis of FXI deficiency, but specific factor activity was not noted. |
| Chi et al. [26] | 14 | 7 | Both SD patients in this manuscript received prophylaxis (rFVIIa & FXIc then TA, respectively); however, it is not clear what for of anesthesia they received. All others PD. |
| Chi et al. [27] | 5 | 5 | Both SD and PD patients. |
| Singh et al. [32] | 9 | 5 | 1 SD patient, all others PD |
| Verghese et al. [30] | 16 | 16 | All PD (FXI ≥ 20%) |
| Gerber et al. [35] | 24 | 3 | SD patients received prophylaxis. No PD patients received prophylaxis. |
SD, severe deficiency; PD, partial deficiency.
2.5. Postpartum hemorrhage
The incidence of postpartum hemorrhage in the general population is approximately 2% [41]. Primary and secondary postpartum hemorrhage appears to be significantly increased in women with partial and severe FXI deficiency [42]. Kadir et al. reported that primary postpartum hemorrhage, defined as hemorrhage occurring within the first 24 hours after delivery, occurred in 4 of 25 pregnancies (16%), 2 of which involved plasma transfusion for prophylaxis [29]. Secondary postpartum hemorrhage, defined as bleeding occurring between 24 hours after delivery and 6–12 weeks postpartum, occurred in 24% (6 of 25) of FXI-deficient women, five of whom did not receive prophylactic treatment [29]. This report did not distinguish between women who were classified clinically as bleeders or non-bleeders. In studies that focused on distinguishing bleeders from non-bleeders involving 432 pregnancies in 198 women, postpartum bleeding occurred in 13–31% of FXI-deficient women, with the majority of bleeding occurring in individuals classified as bleeders (Table 1) [7,26,28,30,35]. Two studies specifically stated the reasons for postpartum hemorrhage were obstetrically related in 66% [35] and 100% [30] of patients, meaning it was felt that hemostatic defect in patients did not contribute appreciably to bleeding. Hemostatic prophylaxis was not consistently used across, or within these studies, likely contributing to variability in outcomes.
Three studies, one prospective trial and two large retrospective registry trials, collected data on pregnancy-related bleeding in FXI-deficient woman. Chi et al. examined 61 pregnancies in 30 women, and observed that seven (2 with severe deficiency, 5 with partial deficiency) experienced postpartum hemorrhage after 10 pregnancies (five primary and five secondary postpartum hemorrhages) [26]. Four women were classified as bleeders, three of whom received intrapartum prophylaxis with either FXI concentrate or tranexamic acid [26]. Salomon et al. studied 164 pregnancies in 62 women and observed no significant bleeding in women without a bleeding history. There were 36 bleeding episodes, most occurring in women with a history of previous bleeding who did not receive prophylactic therapy [7]. In this study, prophylactic plasma replacement in patients with severe FXI deficiency (five non-bleeders and one bleeder) undergoing cesarean delivery prevented postpartum hemorrhage. Of 12 patients undergoing cesarean delivery who did not receive prophylaxis (8 non-bleeders, 4 bleeders), 2 (16.7%) experienced postpartum hemorrhage [7]. Myers et al. studied 105 pregnancies in 33 women and observed nine episodes of postpartum hemorrhage (8 primary and 1 secondary postpartum hemorrhages), all occurring in women with partial deficiency, eight of whom had a history of abnormal bleeding. This group subsequently calculated the relative risk of obstetrical bleeding associated with FXI deficiency and a prior history of bleeding to be 7.2 (CI 1.99–25.9) [28]. A systematic review by Wiewel-Verschueren et al. identified a postpartum hemorrhage incidence of 19% in FXI-deficient women who did not receive, and 9% in those who did receive, prophylaxis [40].
Variability in bleeding and responses to therapy are substantial in these studies, making it difficult to draw firm conclusions about the incidence of postpartum hemorrhage in FXI-deficient women, risk factors that contribute to bleeding, or the effectiveness of specific types of prophylaxis. From the data available it seems reasonable to conclude that women with a prior bleeding history, particularly obstetrical bleeding, are at higher risk for subsequent bleeding than are patients without such a history. Furthermore, the higher FXI levels in patients with partial deficiency did not reliably provide protection from bleeding compared to patients with severe deficiency.
3. Recommendations
3.1. General recommendations for preparing patients for delivery
Care for pregnant FXI-deficient patients should involve a multidisciplinary approach to obtain input from the obstetrics, hematology, and anesthesia teams. The authors recommend consultation with a hematologist and high-risk maternal-fetal medicine specialist, ideally prior to conception, but at least early in pregnancy to facilitate education of the expectant mother, and to plan for pregnancy-related complications and delivery. The patient may ultimately be referred to a tertiary care center where more specialized resources and expertise are available.
A careful history should be taken that includes a general bleeding history; and a complete review of prior deliveries, surgeries, and invasive procedures, including dental procedures; and responses to prior hemostatic treatment. Providers should consider the use of a validated bleeding assessment tool such as the one described by Bolton-Maggs et al. or a modification of the ISTH bleeding assessment tool [15,18–20]. They appear to provide a useful indicator for hemorrhage risk with delivery in the absence of prophylaxis. In addition, it is important to identify a personal or family history of thrombosis, as use of FXI concentrate has been associated with thrombotic events in some settings [43,44]. The plasma FXI activity should be determined by the third trimester to assist in making hemostatic management decisions in the context of the patient’s bleeding history and responses to prior treatment. Women with FXI-deficiency, particularly those with severe deficiency, who have prior exposure to blood products should be tested for the presence of FXI alloantibody inhibitors that may interfere with factor replacement therapy. The obstetric care plan should be formulated, documented, and communicated early in the third trimester to reduce the likelihood of an unanticipated early admission without a management plan in place. Based on the patient’s past history and FXI activity, a hematologist, in consultation with the obstetrician and anesthesiologist, should determine whether it is appropriate to administer prophylactic treatment, or to withhold treatment unless bleeding occurs.
3.2. Hemostatic management - therapeutic options
Antifibrinolytic therapy is frequently used in persons with and without bleeding disorders to enhance hemostasis. The lysinemimetics tranexamic acid and ε-amino caproic acid interfere with plasminogen binding to fibrin and formation of plasmin, the enzyme primarily responsible for enzymatic degradation of clots. Dosing varies based on the clinical circumstance and administration route. Tranexamic acid is a Category B medication (animal studies fail to demonstrate fetal risk), and is effective for routine treatment of postpartum hemorrhage in patients without bleeding disorders. In the WOMAN trial, an international randomized controlled study, the risks of hemorrhage-related death and laparotomy were reduced when tranexamic acid was given shortly (<3 hours) after postpartum hemorrhage [45]. There was no increase in thrombotic events. A 2015 meta-analysis concluded that tranexamic acid given prophylactically with vaginal or cesarean delivery reduces the risk of postpartum hemorrhage and blood transfusion [46]. Obstetric [47], trauma [48], and orthopedic [49] studies have demonstrated a low risk of thrombosis with tranexamic acid use.
Factor replacement (plasma or an FXI concentrate) is also a mainstay of pre-operative prophylaxis in FXI-deficient patients. Availability of FXI concentrate varies based on location. Hemoleven (LFB Biotechnologies, Les Ulis, France) and Factor XI Concentrate (Bioproducts Laboratory, Elstree, UK) are products containing FXI derived from human plasma [44,50]. In the United States, these products have not been approved by the FDA and are difficult to obtain. Both have been associated with thrombotic events in adults with partial or severe FXI deficiency. This has led to recommendations to reduce the doses administered, as much as possible, and to avoid using the product unless essential [43]. If FXI concentrates are used, concomitant use of an antifibrinolytic should be avoided because of the potential for thrombotic complications [50]. Dosing of FXI concentrate depends on the patient’s baseline FXI activity and the desired target activity, with a 2% increase in plasma FXI level expected for each U/kg of concentrate administered. To reduce thrombotic risk, it is currently recommended that total doses not exceed 30 U/kg [43,50]. In most women a plasma FXI activity of 40% should be adequate for hemostasis during delivery. Initial dosing of FXI concentrate at 15 U/kg is often recommended to minimize the thrombotic risk despite the expectation that this may not achieve a level of 40% in some severely deficient patients. A similar target FXI activity applies to replacement with plasma, although fluid volume considerations may put limits on this therapy in some individuals. Given the relatively long half-life of FXI (45–52 hours), administering plasma at 15 mL/kg every 1–2 days is usually adequate for hemostatic support [51].
3.3. Prophylactic hemostatic management - implementation
Martin-Salces et al. advocated adoption of the UK Haemophilia Centre Doctors’ Organization recommendations that patients with severe FXI deficiency (FXI <15%) receive prophylactic FXI replacement at induction of labor or preoperatively unless there is clear documentation of bleeding challenges without abnormal bleeding. Treatment should be continued for 3–4 days after vaginal delivery and 6–7 days after cesarean delivery [52,53]. Shander et al. also recommended prophylaxis based on FXI activity and a history of bleeding. Specifically, FXI replacement therapy should be administered to those with severe deficiency (FXI <15%) with a history of excessive bleeding or if there is no history of prior hemostatic challenge. For patients with FXI levels in the 15–70% range, they recommend prophylactic tranexamic acid for those with a bleeding history or no prior hemostatic challenge [54]. Generally, for vaginal or cesarean delivery in FXI-deficient patients who have not bled previously with hemostatic challenge, regardless of the severity of the deficiency, recommendations support clinical monitoring without factor replacement, as long as antifibrinolytic therapy such as tranexamic acid is available [52–54].
Women with less severe deficiency (FXI 15–45%) who have negative bleeding histories and who have not bled with prior vaginal or cesarean deliveries in the absence of prophylaxis might be expected to have the lowest risk for bleeding with subsequent deliveries. However, even this specific population’s risk of hemorrhage appears to be greater than that of the general population. Given the relatively high estimated incidence of postpartum hemorrhage among patients with FXI deficiency (19% compared to 2% in the general population), and data indicating the safety of anti-fibrinolytic therapy in the obstetric population, the authors feel it is reasonable to recommend administering a single prophylactic dose of tranexamic acid (1 g IV) at the time of vaginal or cesarean delivery for any FXI-deficient patient, in addition to the routine prophylactic oxytocin administration recommended for all obstetric patients. Continuation of tranexamic acid (1 g IV q8 hours × 72 hours) can be considered for patients at higher risk for primary and/or secondary postpartum hemorrhage. Considering that four in five women with FXI deficiency will deliver without bleeding complications, it is also reasonable to consider routine administration of oxytocin prophylaxis, while only administration of tranexamic acid if bleeding occurs. For this option, tranexamic acid should be prepared and available for rapid administration after bleeding is identified. As tranexamic acid is relatively inexpensive, it can be ordered ahead of time so that it is available at the bedside if needed. In this patient population, partial FXI deficiency and reassuring bleeding history, we advocate avoiding FXI or plasma replacement unless there is significant bleeding.
A drawback to routine prophylactic administration of tranexamic acid is that some patients will subsequently require FXI replacement for severe, life-threatening, or refractory postpartum hemorrhage. As discussed, the combination of FXI concentrate and tranexamic acid may increase the risk for thrombotic complications. Therefore, for patients at high risk of hemorrhage, such as those with severe FXI deficiency (FXI <15%) and a history of bleeding or postpartum hemorrhage, it may be better to treat prophylactically with FXI concentrate (15 U/kg for severe deficiency patients) or plasma (15 mL/kg) in addition to routine oxytocin. Alternatively, one can avoid using FXI concentrate or plasma by using recombinant-activated factor VIIa (rFVIIa) to provide hemostatic management in high-risk patients. rFVIIa in low doses (15–30 μg/kg) has been used to facilitate surgical delivery and regional anesthesia in women with severe or partial FXI deficiency [55,56]. While thrombosis can occur with use of rFVIIa and concurrent use of tranexamic acid, the risk should be relatively low at the doses of rVIIa recommend for FXI-deficient patients.
3.4. Management of postpartum hemorrhage
Although many postpartum bleeds in low-risk populations are unanticipated, there are identifiable risk factors that increase risk for hemorrhage during delivery including multiparity, prolonged oxytocin use, multiple gestation, polyhydramnios, macrosomia, uterine fibroids, intrapartum infection, placental abnormalities, and general anesthesia [57]. The American College of Obstetrics and Gynecology (ACOG) recommends patients in labor or undergoing cesarean section be evaluated on an ongoing basis using risk stratification tools that can identify the majority of patients at-risk for postpartum bleeding [57]. Patients with FXI deficiency would automatically be placed in the highest risk category, but it is important to consider additional risk factors that may evolve over the course of labor or the preoperative period. The obstetrician should, in consultation with the hematologist, consider whether additional risks necessitate alteration of the predetermined treatment plan.
Bleeding should be managed with FXI replacement (FXI concentrate), rFVIIa, and/or antifibrinolytic therapy as previously discussed, taking into account the safety concerns regarding thrombosis in the setting of combination therapy. Identifying the need for transfusion early is essential for these patients to initiate replacement in a timely fashion. In the event of severe postpartum hemorrhage requiring red blood cell blood transfusion, it is critical to replace other blood components with platelet concentrates, plasma, and cryoprecipitate. In the setting of major obstetric hemorrhage requiring transfusion, many hospitals and institutions have established massive transfusion protocols (MTP). Data from trauma and battlefield medicine have informed obstetric practices, although there are little data to support improved outcomes specifically in the obstetric population. There appears to be a benefit to having a hospital-wide MTP that includes fixed ratios of blood products, most commonly 1:1 packed red cells to plasma [58,59]. Approximately 50% of obstetric-specific MTPs also incorporate cryoprecipitate administration [60]. While specific MTPs have not been compared rigorously, ACOG stresses the importance of having an institutional multi-component MTP in place [57]. In patients with FXI deficiency, in addition to MTP protocols and factor replacement, the obstetrician also needs to incorporate standard practice measures to achieve hemostasis, including additional uterotonics, bimanual massage, bladder catheterization, laceration repair, balloon tamponade, or operative interventions such as compression sutures, vascular occlusion sutures, or hysterectomy. The multidisciplinary obstetrics care team on duty should engage in team-based communication with all care team members to anticipate potential medication, equipment, and personnel needs.
3.5. Anesthesia care
General anesthesia carries higher maternal risk of failed intubation, aspiration, and postpartum hemorrhage in addition to the impact on maternal birth experience and bonding, making regional anesthesia the method of choice among the population at low risk of bleeding. Regional anesthesia has been historically contraindicated in patients with FXI deficiency; however, there are data to support its safe use in this patient population. While spinal hematomas may produce severe sequelae, the risk for this complication appears to be low. Intravenous analgesia early in labor and inhalational nitrous oxide, where available, can be excellent low-risk options. Because there is no general consensus on the risk of regional anesthesia for patients with FXI deficiency, and no established parameters for decision-making, anesthesiologists should weigh the risks and benefits for each individual, engaging in shared decision-making with an adequately counseled patient. Tranexamic acid can be considered for prophylaxis at the time of regional anesthesia placement, reserving FXI concentrate for more severe deficiency or patients with known bleeding history.
3.6. Neonatal management
Risk of intracranial hemorrhage in infants with severe and partial FXI deficiency is likely similar to that of infants without a bleeding disorder. However, the FXI status of the neonate will in most cases be unknown at the time of delivery and conservative management should be considered. Based on recommendations for delivery of neonates at risk of having severe hemophilia (factor VIII or IX deficiencies), certain traumatic procedures should be avoided in the intrapartum period and until the neonate’s factor activity is known. Operative vaginal delivery with forceps or vacuum assistance should be avoided, as well as invasive monitors such as fetal scalp electrodes. Vitamin K and Hepatitis B immunization can be administered by intramuscular injection; however, pressure should be held for 15 minutes after injection to reduce the chances of bleeding. Additional intramuscular injections, heel sticks, and invasive procedures, including circumcision for male neonates, should be delayed until FXI activity is known to allow for increased monitoring or hemostatic support if needed. Of note, normal FXI activity in neonates is substantially lower than in adults [61,62], and formal diagnosis of FXI deficiency may need to be delayed until the infant is 6 months old (corrected for gestational age).
4. Expert opinion
Women with FXI deficiency are at increased risk of bleeding with child-birth. In this review, we suggest a relatively conservative approach that favors careful multidisciplinary planning combined with standard-of-care treatment in the setting of a negative bleeding history, with more aggressive management reserved for patients who have bled in the past or who have not experienced a hemostatic challenge sufficient to determine if they are prone to bleeding (Figure 2 and Table 3). We feel that our current understanding of the pathophysiology of FXI deficiency justifies this flexible approach, although there is room for improvement.
Figure 2.
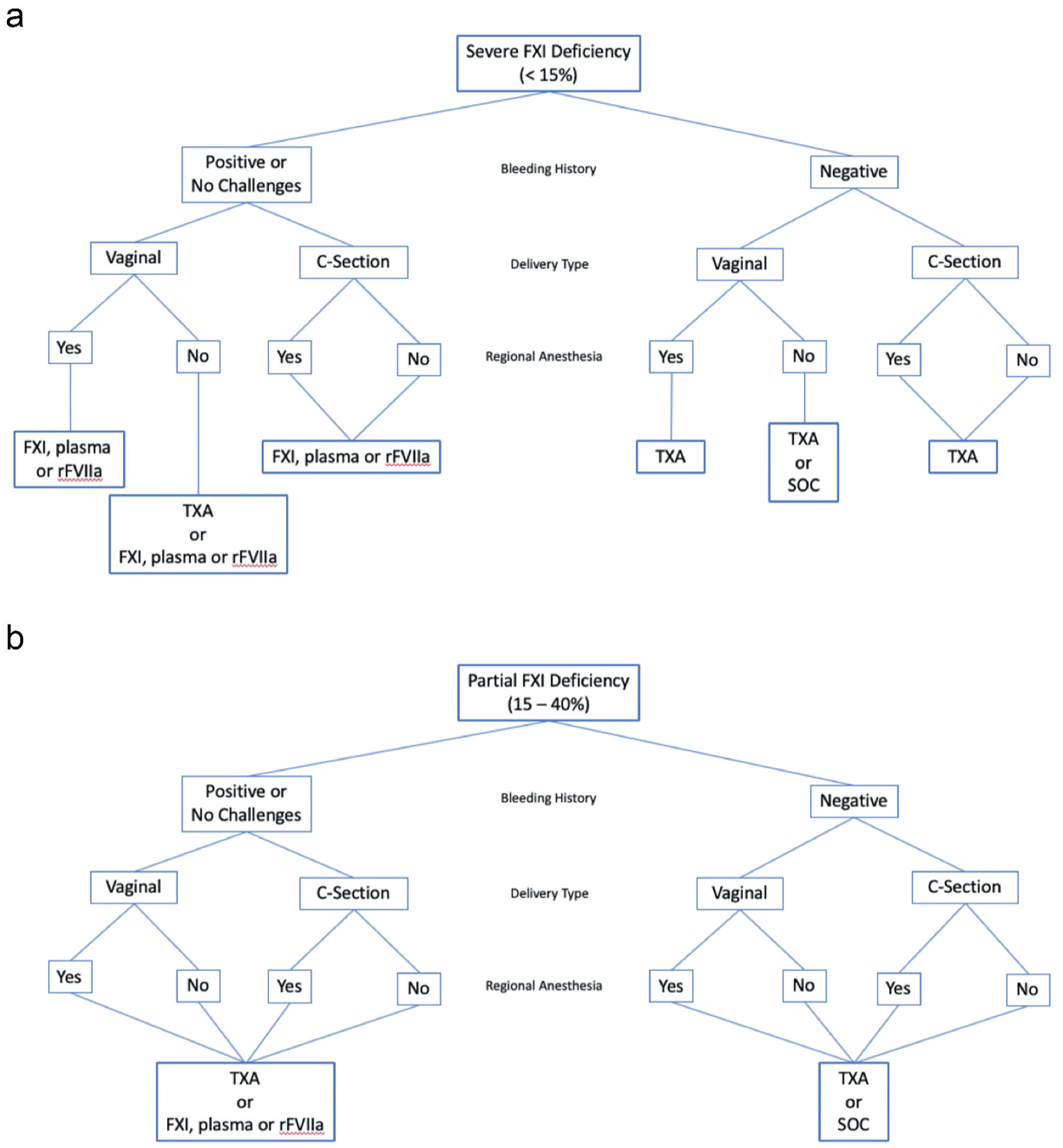
Treatment recommendations for delivery in women with FXI deficiency based on bleeding history, delivery type, and presence or absence of regional anesthesia. (a) Patients with severe deficiency and (b) patients with partial deficiency.
Table 3.
Summary of recommendations for obstetrical care of women with factor XI deficiency.
| Multidisciplinary Care Team |
|
| Laboratory Data |
|
| Intrapartum and Delivery Care |
|
| Anesthesia Care |
|
| Neonatal Care |
|
Treating FXI-deficient patients presents some unique challenges for clinicians. The deficiency state clearly compromises hemostasis sufficiently in some individuals to render surgery and childbirth dangerous without intervention. At the same time, many patients do not appear to require protein. The laboratory tests we routinely use to assess hemostasis in disorders such as hemophilia A and B are less reliable in FXI deficiency. This is probably partly due to the poor correlation between FXI plasma levels and bleeding propensity, and partly because FXI is activated in the aPTT by a mechanism that has little relevance for hemostasis. These points of uncertainty could be rendered moot if we had a readily available, easily administered, safe source of FXI for replacement that could be administered to all FXI-deficient patients. At this point, such therapy is not available.
Replacement therapy with factor concentrate has not been adopted as widely for treating FXI-deficient patients as it has for treating hemophilia A or B. FXI concentrates, while effective, are used cautiously because of early experience with thrombosis after relatively high doses, particularly in patients with other thrombotic risk factors [50,63,64]. This is obviously a concern when managing peripartum patients. The culprit was likely FXIa contamination, and current FXI concentrates contain heparin and one or more inhibitors (antithrombin and/or C1-inhibitor) to neutralize the protease. FXIa, indeed, appears to be pro-thrombotic at relatively low concentrations. Clusters of thrombotic episodes were linked to intravenous infusion of certain preparations of human immunoglobulin contaminated with FXIa [65–67]. As a consequence of the rarity of the disorder and, to some extent, remaining concern over thrombosis, FXI concentrates are not widely available, leaving plasma infusion as the main source of replacement in many parts of the world.
Another concern with replacement therapy (concentrate or plasma) is formation of neutralizing antibodies. A significant number of patients with severe FXI-deficiency, particularly Jewish patients homozygous for the type II nonsense mutation, completely lack FXI. These individuals are at high risk (30%) for developing alloantibodies after as little as a single exposure to FXI [68,69]. In these patients, it makes sense to limit exposure to FXI as much as possible, reserving replacement therapy for situations where alternatives are likely to be ineffective.
Recombinant factor VIIa has been used been successfully in FXI-deficient patients with FXI-neutralizing alloantibodies as prophylaxis during invasive procedures, including surgery on vulnerable tissues such as the urinary tract and oropharynx, and major surgeries where risk of bleeding is high such as abdominal aortic aneurysm repair [55,56,70,71]. The efficacy of factor VIIa in these patients makes sense considering the role of FXI depicted in Figure 1B. Here FXIa activates factor IX to supplement the factor IX activated by the factor VIIa/tissue factor complex. Administration of factor VIIa would enhance thrombin generation through factor VIIa/tissue factor, reducing or obviating the need for FXIa. Given the issues with FXI replacement, some practitioners now opt to use factor VIIa as front-line therapy for prophylaxis in FXI-deficient patients who do not have alloantibodies [72]. While the potential for factor VIIa-induced thrombosis is a concern in pregnant women, the doses of drug used in FXI-deficient patients are low, and would probably not enhance thrombotic risk appreciably [73].
Additional considerations for future treatment may also include non-factor replacement therapies. Inhibitory and factor mimic proteins have recently exploded in the context of therapy for persons with hemophilia [74]. These new mechanisms for creating hemostasis have targeted improved hemostasis without reliance on traditional replacement of factor proteins. These options may be able to provide a means of safely enhancing hemostasis without dependence on FXI and its prothrombotic side effects.
Ultimately, it would be advantageous to have a laboratory test that can distinguish FXI-deficient patients who are prone to bleeding from those who are not. So-called global hemostatic assays, such as thromboelastometry, have not clarified the clinical picture. While these assays demonstrate abnormalities in this patient population, there has not been clear evidence that they provide the clinical insight that is needed for treatment decisions [33,34]. Research-based laboratory assays, however, have demonstrated some promise in this area. Thrombin generation assays have been studied in this population and have the potential to differentiate bleeding from non-bleeding persons [33,75]; however, additional research into this testing modality and its clinical utility is still needed at this time. In addition, clinical implementation of thrombin generation assays has significant challenges to overcome [76]. Zucker and colleagues noted that the fibrin structure and sensitivity to fibrinolysis of plasma clots from FXI-deficient patients differed depending on their bleeding history [77]. The methods used in this study would be difficult to introduce into most hospital clinical laboratories; however, some progress has been made in this direction. Gidley et al. studied clot formation and sensitivity to fibrinolysis in plasmas from FXI-deficient patients using microtiter plate assays [78]. They proposed a model that combined results from the aPTT assay with rates of clot formation and dissolution to distinguish bleeders from non-bleeders. This type of assay, once validated, could be a valuable tool for tailoring therapy in FXI-deficient patients. Novel methods of assessing plasma coagulation will be developed that better reflect the role of FXI in hemostasis. Until these assays are available clinically, the care of patients with FXI deficiency will continue to be centered on presumptions based on personal bleeding history and the types of hemostatic challenges anticipated.
Article highlights.
Factor XI deficiency causes a variable bleeding phenotype.
During pregnancy, women with factor XI deficiency demonstrate significant variability in their propensity to bleed, and their responses to prophylactic and therapeutic treatment.
Identification of factor XI-deficient patients at increased risk of bleeding during obstetrical events is challenging; however, a personal history of bleeding is likely a relevant factor.
Therapeutic management of pregnant women with factor XI deficiency should be multidisciplinary and anticipatory.
There is a need for tests that are better predictors of bleeding in factor XI-deficient patients to replace the tests currently available.
Footnotes
Declaration of interest
D Gailani has served as a consultant for Bioproducts Laboratory and wishes to acknowledge grant support from the National Heart, Lung and Blood Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wheeler AP, Gailani D. Why factor XI deficiency is a clinical concern. Expert Rev Hematol. 2016;9(7):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James P, Salomon O, Mikovic D, et al. Rare bleeding disorders - bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemophilia. 2014. May;20(Suppl 4):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asakai R, Chung DW, Davie EW, et al. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991. July 18;325(3):153–158. [DOI] [PubMed] [Google Scholar]
- 4.Zivelin A, Bauduer F, Ducout L, et al. Factor XI deficiency in French Basques is caused predominantly by an ancestral Cys38Arg mutation in the factor XI gene. Blood. 2002. April 1;99(7):2448–2454. [DOI] [PubMed] [Google Scholar]
- 5.Bolton-Maggs PH. Factor XI deficiency–resolving the enigma? Hematology Am Soc Hematol Educ Program. 2009(1);97–105. [DOI] [PubMed] [Google Scholar]
- 6.Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006. September;12(5):490–493. [DOI] [PubMed] [Google Scholar]
- 7.Salomon O, Steinberg DM, Tamarin I, et al. Plasma replacement therapy during labor is not mandatory for women with severe factor XI deficiency. Blood Coagul Fibrinolysis. 2005. January;16 (1):37–41. [DOI] [PubMed] [Google Scholar]; •• This review of obstetrical bleeding in women with factor XI deficiency provided support of management without prophylactic plasma or factor XI replacement, demonstrating the variable needs of this complex population.
- 8.Mohammed BM, Matafonov A, Ivanov I, et al. An update on factor XI structure and function. Thromb Res. 2018;161:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016. January;14(1):28–39. [DOI] [PubMed] [Google Scholar]
- 10.Broze GJ Jr., Girard TJ, Novotny WF. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry. 1990. August 21;29 (33):7539–7546. [DOI] [PubMed] [Google Scholar]
- 11.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018. April;38(4):709–725. [DOI] [PubMed] [Google Scholar]
- 12.Bolton-Maggs PH. Bleeding problems in factor XI deficient women. Haemophilia. 1999. May;5(3):155–159. [DOI] [PubMed] [Google Scholar]
- 13.Seligsohn U Factor XI deficiency. Thromb Haemost. 1993. July 1;70 (1):68–71. [PubMed] [Google Scholar]
- 14.Seligsohn U High gene frequency of factor XI (PTA) deficiency in Ashkenazi Jews. Blood. 1978. June;51(6):1223–1228. [PubMed] [Google Scholar]
- 15.Bolton-Maggs PH, Patterson DA, Wensley RT, et al. Definition of the bleeding tendency in factor XI-deficient kindreds-a clinical and laboratory study. Thromb Haemost. 1995. February;73 (2):194–202. [PubMed] [Google Scholar]; •• This pivotal manuscript demonstrated the importance of a bleeding history in persons with factor XI deficiency to determine their bleeding tendency and risk.
- 16.Leiba H, Ramot B, Many A. Heredity and coagulation studies in ten families with factor XI (plasma thromboplastin antecedent) deficiency. Br J Haematol. 1965;November;11(6):654–665. [DOI] [PubMed] [Google Scholar]
- 17.Rapaport SI, Proctor RR, Patch MJ, et al. The mode of inheritance of PTA deficiency: evidence for the existence of major PTA deficiency and minor PTA deficiency. Blood. 1961;18:149–165. [PubMed] [Google Scholar]
- 18.Tosetto A, Castaman G, Plug I, et al. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. J Thromb Haemost. 2011. June;9(6):1143–1148. [DOI] [PubMed] [Google Scholar]
- 19.Federici AB, Bucciarelli P, Castaman G, et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014. June 26;123 (26):4037–4044. [DOI] [PubMed] [Google Scholar]
- 20.Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012. November;10(11):2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnay JL, O’Brien S, Gerlinger C, et al. Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: a systematic literature review. BMC Womens Health. 2020. February 10;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drury-Stewart DN, Lannert KW, Chung DW, et al. Complex changes in von Willebrand factor-associated parameters are acquired during uncomplicated pregnancy. PLoS One. 2014;9 (11):e112935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaman G, James PD. Pregnancy and delivery in women with von Willebrand disease. Eur J Haematol. 2019. August;103(2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerova L, Chabada J, Drobny J, et al. Longitudinal evaluation of markers of hemostasis in pregnancy. Bratisl Lek Listy. 2014;115 (3):140–144. [DOI] [PubMed] [Google Scholar]
- 25.Phillips LL, Rosano L, Skrodelis V. Changes in factor XI (plasma thromboplastin antecedent) levels during pregnancy. Am J Obstet Gynecol. 1973. August 15;116(8):1114–1116. [DOI] [PubMed] [Google Scholar]
- 26.Chi C, Kulkarni A, Lee CA, et al. The obstetric experience of women with factor XI deficiency. Acta Obstet Gynecol Scand. 2009;88 (10):1095–1100. [DOI] [PubMed] [Google Scholar]; • This retrospective review provided data regarding the unpredicable nature of obstetrical bleeding in women with factor XI deficiency, and thus the need for careful planning and hemostatic management.
- 27.Chi C, Lee CA, England A, et al. Obstetric analgesia and anaesthesia in women with inherited bleeding disorders. Thromb Haemost. 2009. June;101(6):1104–1111. [PubMed] [Google Scholar]; • This retrospective review provided critcial data regarding the safety of regional anesthesia in women with factor XI deficiency.
- 28.Myers B, Pavord S, Kean L, et al. Pregnancy outcome in Factor XI deficiency: incidence of miscarriage, antenatal and postnatal haemorrhage in 33 women with Factor XI deficiency. BJOG. 2007. May;114(5):643–646. [DOI] [PubMed] [Google Scholar]
- 29.Kadir RA, Lee CA, Sabin CA, et al. Pregnancy in women with von Willebrand’s disease or factor XI deficiency. Br J Obstet Gynaecol. 1998. March;105(3):314–321. [DOI] [PubMed] [Google Scholar]
- 30.Verghese L, Tingi E, Thachil J, et al. Management of parturients with factor XI deficiency-10year case series and review of literature. Eur J Obstet Gynecol Reprod Biol. 2017. August;215:85–92. [DOI] [PubMed] [Google Scholar]; • A 10-year review of obstetrical bleeding in women with factor XI deficiency, emphasizing the imporance of individual treatment decisions based on bleeding history and factor activity.
- 31.Ragni MV, Sinha D, Seaman F, et al. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood. 1985. March;65(3):719–724. [PubMed] [Google Scholar]
- 32.Singh A, Harnett MJ, Connors JM, et al. Factor XI deficiency and obstetrical anesthesia. Anesth Analg. 2009. Jun;108(6):1882–1885. [DOI] [PubMed] [Google Scholar]
- 33.Livnat T, Shenkman B, Martinowitz U, et al. The impact of thrombin generation and rotation thromboelastometry on assessment of severity of factor XI deficiency. Thromb Res. 2015. August;136 (2):465–473. [DOI] [PubMed] [Google Scholar]
- 34.Davies J, Harper A, Kadir RA. The role of rotational thromboelastometry in assessment of haemostasis during pregnancy in women with factor XI deficiency. Haemophilia. 2016. March;22 (2):276–284. [DOI] [PubMed] [Google Scholar]
- 35.Gerber GF, Klute KA, Chapin J, et al. Peri- and postpartum management of patients with factor XI deficiency. Clin Appl Thromb Hemost. 2019;25:1076029619880262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed S, Russo LA, Siddiqui AK, et al. Prolonged activated partial thromboplastin time in pregnancy: a brief report. Am J Med Sci. 2004. March;327(3):123–126. [DOI] [PubMed] [Google Scholar]
- 37.Hasan R, Baird DD, Herring AH, et al. Patterns and predictors of vaginal bleeding in the first trimester of pregnancy. Ann Epidemiol. 2010. Jul;20(7):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinaman MJ, Clegg ED, Brown CC, et al. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996. March;65(3):503–509. [PubMed] [Google Scholar]
- 39.Committee on Practice Bulletins - Gynecology. ACOG practice bulletin no. 200: early pregnancy loss. Obstetrics Gynecol. 2018;132(5): e197–e207. [DOI] [PubMed] [Google Scholar]
- 40.Wiewel-Verschueren S, Meijer K. Gynaecological and obstetrical bleeding in women with factor XI deficiency - a systematic review: response to rebuttal. Haemophilia. 2016. September;22(5):e436–e437. [DOI] [PubMed] [Google Scholar]
- 41.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010. April;202(4):353e1–353e6. [DOI] [PubMed] [Google Scholar]
- 42.Kadir R, Chi C, Bolton-Maggs P. Pregnancy and rare bleeding disorders. Haemophilia. 2009. September;15(5):990–1005. [DOI] [PubMed] [Google Scholar]
- 43.Bolton-Maggs P, Goudemand J, Hermans C, et al. FXI concentrate use and risk of thrombosis. Haemophilia. 2014. July;20(4):e349–e351. [DOI] [PubMed] [Google Scholar]
- 44.Ling G, Kagdi H, Subel B, et al. Safety and efficacy of factor XI (FXI) concentrate use in patients with FXI deficiency: a single-centre experience of 19 years. Haemophilia. 2016. May;22 (3):411–418. [DOI] [PubMed] [Google Scholar]
- 45.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017. May 27;389(10084):2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015. June;16(6):CD007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindoff C, Rybo G, Astedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thromb Haemost. 1993. August 2;70(2):238–240. [PubMed] [Google Scholar]
- 48.CRASH-2 Trial Collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010. July 3;376(9734):23–32. [DOI] [PubMed] [Google Scholar]
- 49.Madsen RV, Nielsen CS, Kallemose T, et al. Low risk of thrombo-embolic events after routine administration of tranexamic acid in hip and knee arthroplasty. J Arthroplasty. 2017. Apr;32 (4):1298–1303. [DOI] [PubMed] [Google Scholar]
- 50.Bauduer F, de Raucourt E, Boyer-Neumann C, et al. Factor XI replacement for inherited factor XI deficiency in routine clinical practice: results of the HEMOLEVEN prospective 3-year postmarketing study. Haemophilia. 2015. July;21(4):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gailani D, Wheeler AP, Neff AT. Rare coagulation factor deficiencies In: editor, Hoffman R Hematology basic principles and practice. 7th ed. Philadelphia, PA: Elsevier; 2018:2034–2050. [Google Scholar]
- 52.Martin-Salces M, Jimenez-Yuste V, Alvarez MT, et al. Review: factor XI deficiency: review and management in pregnant women. Clin Appl Thromb Hemost. 2010. Apr;16(2):209–213. [DOI] [PubMed] [Google Scholar]
- 53.Lee CA, Chi C, Pavord SR, et al. The obstetric and gynaecological management of women with inherited bleeding disorders-review with guidelines produced by a taskforce of UK Haemophilia Centre Doctors’ Organization. Haemophilia. 2006. July;12(4):301–336. [DOI] [PubMed] [Google Scholar]
- 54.Shander A, Friedman T, Palleschi G, et al. The evolving dilemma of factor XI in pregnancy: suggestions for management. Anesth Analg. 2018. June;126(6):2032–2037. [DOI] [PubMed] [Google Scholar]
- 55.Setty S, Reddell A, England A, et al. The role of recombinant factor VIIa for obstetric block in women with severe factor XI deficiency. Haemophilia. 2011. November;17(6):906–909. [DOI] [PubMed] [Google Scholar]
- 56.Riddell A, Abdul-Kadir R, Pollard D, et al. Monitoring low dose recombinant factor VIIa therapy in patients with severe factor XI deficiency undergoing surgery. Thromb Haemost. 2011. September;106 (3):521–527. [DOI] [PubMed] [Google Scholar]
- 57.Committee on Practice Bulletins - Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstetrics Gynecol. 2017;130(4): e168–e186. [DOI] [PubMed] [Google Scholar]
- 58.Treml AB, Gorlin JB, Dutton RP, et al. Massive transfusion protocols: a survey of academic medical centers in the United States. Anesth Analg. 2017. January;124(1):277–281. [DOI] [PubMed] [Google Scholar]
- 59.Thomasson RR, Yazer MH, Gorham JD, et al. International assessment of massive transfusion protocol contents and indications for activation. Transfusion. 2019. May;59(5):1637–1643. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka H, Matsunaga S, Yamashita T, et al. A systematic review of massive transfusion protocol in obstetrics. Taiwan J Obstet Gynecol. 2017. December;56(6):715–718. [DOI] [PubMed] [Google Scholar]
- 61.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the healthy premature infant. Blood. 1988. November;72(5):1651–1657. [PubMed] [Google Scholar]
- 62.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987. July;70 (1):165–172. [PubMed] [Google Scholar]
- 63.Pike GN, Bolton-Maggs PH. Factor XI-related thrombosis and the role of concentrate treatment in factor XI deficiency. Haemophilia. 2015. July;21(4):477–480. [DOI] [PubMed] [Google Scholar]
- 64.Batty P, Honke A, Bowles L, et al. Ongoing risk of thrombosis with factor XI concentrate: 5 years experience in two centres. Haemophilia. 2015. July;21(4):490–495. [DOI] [PubMed] [Google Scholar]
- 65.Wolberg AS, Kon RH, Monroe DM, et al. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am J Hematol. 2000. September;65(1):30–34. [DOI] [PubMed] [Google Scholar]
- 66.Menis M, Sridhar G, Selvam N, et al. Hyperimmune globulins and same-day thrombotic adverse events as recorded in a large health-care database during 2008–2011. Am J Hematol. 2013. December;88 (12):1035–1040. [DOI] [PubMed] [Google Scholar]
- 67.Germishuizen WA, Gyure DC, Stubbings D, et al. Quantifying the thrombogenic potential of human plasma-derived immunoglobulin products. Biologicals. 2014. September;42(5):260–270. [DOI] [PubMed] [Google Scholar]
- 68.Salomon O, Zivelin A, Livnat T, et al. Prevalence, causes, and characterization of factor XI inhibitors in patients with inherited factor XI deficiency. Blood. 2003. June 15;101(12):4783–4788. [DOI] [PubMed] [Google Scholar]
- 69.Gailani D Factor XI inhibitors. Blood. 2003;101(12):4649. [DOI] [PubMed] [Google Scholar]
- 70.Livnat T, Tamarin I, Mor Y, et al. Recombinant activated factor VII and tranexamic acid are haemostatically effective during major surgery in factor XI-deficient patients with inhibitor antibodies. Thromb Haemost. 2009. September;102(3):487–492. [DOI] [PubMed] [Google Scholar]
- 71.O’Connell NM, Riddell AF, Pascoe G, et al. Recombinant factor VIIa to prevent surgical bleeding in factor XI deficiency. Haemophilia. 2008. July;14(4):775–781. [DOI] [PubMed] [Google Scholar]
- 72.Salomon O, Budnik I, Avishai E, et al. Single low dose of rFVIIa combined with antifibrinolytic agent is a simple and safe treatment for factor XI-deficient patients undergoing surgery. Thromb Haemost. 2019. December;119(12):1927–1932. [DOI] [PubMed] [Google Scholar]
- 73.Levi M, Levy JH, Andersen HF, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010. November 4;363(19):1791–1800. [DOI] [PubMed] [Google Scholar]
- 74.Hartmann J, Croteau SE. 2017 Clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2016. December;91(12):1252–1260. [DOI] [PubMed] [Google Scholar]
- 75.Rugeri L, Quelin F, Chatard B, et al. Thrombin generation in patients with factor XI deficiency and clinical bleeding risk. Haemophilia. 2010. September 1;16(5):771–777. [DOI] [PubMed] [Google Scholar]
- 76.Adams M Assessment of thrombin generation: useful or hype? Semin Thromb Hemost. 2009. Feb;35(1):104–110. [DOI] [PubMed] [Google Scholar]
- 77.Zucker M, Seligsohn U, Salomon O, et al. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014. July;12 (7):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gidley GN, Holle LA, Burthem J, et al. Abnormal plasma clot formation and fibrinolysis reveal bleeding tendency in patients with partial factor XI deficiency. Blood Adv. 2018. May 22;2(10):1076–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]


