Abstract
Colorectal cancer (CRC) is one of the most malignant tumors in humans and causes mass mortality. In the age of precise medicine, more and more subtypes of CRC were classified. The caudal-related homeobox transcription factor 2 (CDX2) is an intestine-specific transcription factor which is implicated in differentiation, proliferation, cell-adhesion, and migration. The loss of CDX2 in immunohistochemical stain was reported to be a prognostic factor of colon cancer, but the clinical application remained controversial. Most of the CRCs expressed or over-expressed CDX2. Homeobox genes can display either an oncogenic or a tumor-suppressing activity. CDX2 regulates the developing intestinal epithelium and CRC by different pathways. The complex regulation of CDX2 and its complex targets cause the difficulties of application for CDX2 in the prediction of prognosis. However, CDX2 is a potential biomarker applied in the precise classification of CRC for personalized medicine. This review partially clarifies the role of CDX2 in CRC.
KEYWORDS: Cancer biomarker, Caudal-related homeobox transcription factor 2, Colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is the most common malignant tumor worldwide with increased incidence recently [1]. The CRC is primarily treated by surgical resection and adjuvant treatment, such as chemotherapy and radiation. Although tumor-node-metastasis staging system helps us to predict the prognosis and to decide optimal adjuvant therapy for CRC [2], the outcome is variable within the same cancer stage due to the heterogeneity of the molecular alterations [3]. Carcinogenesis of the colon epithelium is composed of several slowly-accumulated genetic mutations and epigenetic changes, leading to gain-of-function mutations in oncogenes or loss-of-function mutations in tumor-suppressor genes [4].
Several molecular and genetic signatures were used for precise cancer treatment nowadays, including CRC [5]. Due to the heterozygosity of cancers and their behaviors, to identify specific cancer types and to treat them individually are important [6]. Based on their pathogenesis and molecular characteristics, CRCs have been categorized into different subtypes [7].
The Caudal-type homeodomain transcription factors 2 (CDX2) has been reported to be a tumor suppressor and a prognostic factor in CRC [8,9]. CDX2 determines the development of intestine since the morula stage [10]. The expression of CDX2 in adults is restricted to the intestine from the duodenum to the rectum. Therefore, CDX2 can serve as a marker of intestinal origin [11]. Currently, the immunohistochemical (IHC) stain of CDX2 is broadly used to identify the metastatic lesions like intestine-origin [11,12]. Although some meta-analyses have indicated that the IHC stain of CDX2 may be a potential prognostic factor of colon cancer [13,14,15], the clinical application of the CDX2 as a biomarker remains controversial [16]. The reasons arise from the low rate of CDX2 silencing (2%–10%) in CRC [11].
More and more studies have revealed that CDX2 is a potential prognostic factor of CRC. However, the functions of CDX2 are not unveiled clearly in CRC. Salari et al. reported that CDX2 overexpressed in CRC cell lines [Supplemental Materials (212.5KB, pdf) ] is highly linked with cell growth and survival [17]. The regulation mechanism of CDX2 expression is complex and coexisted with many transcription factors. In this review, we went over the published literature and tried to clarify the role of CDX2 in CRC.
CDX2 GENE AND CAUDAL-RELATED HOMEOBOX TRANSCRIPTION FACTOR 2 PROTEIN
The human Cdx2 gene is localized to chromosome 13q12–13. CDX2 protein functions as a transcription factor [18]. It contains homeodomain protein structure, consisting of a 60-amino acid sequence folded into a domain with three-alpha helixes. The helix 2 and helix 3 form a helix-turn-helix structure that binds to the major groove of a specific DNA sequence through hydrogen bonds and hydrophobic interactions. Homeodomain proteins show a preference for interaction with the DNA sequence 5'-TAAT-3' [19]. The homeodomain proteins are tissue specific and regulate different functions. The expression of CDX2 protein distributes from the duodenum to rectum [20,21]. CDX2 is initially presented in the early development, which is essential for the correct intestinal development and differentiation [22]. Aberrant expression of CDX2 leads to pathogenic outcomes, such as gastric or esophageal intestinal metaplasia [23,24] and acute myeloid leukemia [25].
GENETIC ALTERATIONS OF CAUDAL-RELATED HOMEOBOX TRANSCRIPTION FACTOR 2 IN COLORECTAL CANCER
According to Olsen's review, there were no significant associations of CDX2 with increased risks of CRC in the germline and somatic level [16]. The CDX2 DNA polymorphism was not independently associated with either colon or rectal cancer [26,27]. Some haplotypes were associated with more advanced disease, but not correlated with increased risks of CRC [28]. Although the locus of Cdx2 gene on 13q12.2 was reported to be a target of the amplification, it is possible to be a lineage-survival oncogene deregulated in CRC [17]. The Cdx2 gene is not frequently mutated or lost [29]. The rearrangements are correlated with Cdx2 gene amplification but are not predictive of the CDX2 protein [30]. This finding hinted that the CDX2 protein was not mainly regulated by alterations of Cdx2 gene.
CAUDAL-RELATED HOMEOBOX TRANSCRIPTION FACTOR 2 PLAYS DIFFERENT ROLES IN EMBRYO, INTESTINAL EPITHELIUM, AND COLORECTAL CANCERS
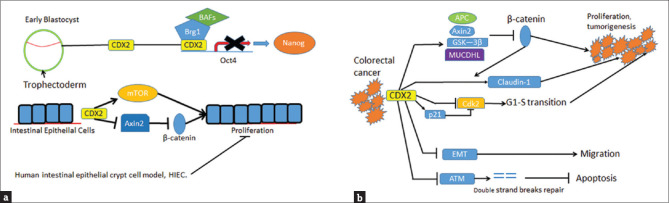
The pluripotent stem cell could be induced to differentiate into hindgut tube formation by CDX2 [31]. Loss of Cdx2 results in failure to downregulate Oct4 and Nanog in outer cells of the blastocyst and subsequent failure of gut development [Figure 1a] [32]. During embryogenesis, the function of CDX2 is to regulate the proliferation-promoting genes.
Figure 1.
Caudal-related homeobox transcription factor 2 plays different roles in embryo, intestinal epithelium and colorectal cancers. (a) In blastocyst, caudal-related homeobox transcription factor 2 determines the gut development by downregulation of Oct 4 and Nanog. In the intestinal epithelial cell, caudal-related homeobox transcription factor 2 could both promote and inhibit proliferation to maintain the renewal of the intestinal epithelium. (b) In the colorectal cancer cells, caudal-related homeobox transcription factor 2 downregulates the Wnt pathway and prevents G1-S cell cycle transition. Overexpression of caudal-related homeobox transcription factor 2 decreases the epithelial mesenchymal transition. Caudal-related homeobox transcription factor 2 protein binds to ATM and prevents DNA double strand break repair in colorectal cancer cells
In the non-cancer intestinal epithelial cell, the CDX2 can modulate the proliferation for maintaining the renewal of intestinal epithelium. In a porcine intestinal epithelial model (porcine jejunum epithelial cell line-J2), the expression of CDX2 leads to proliferation [33]. In the human intestinal epithelial crypt model, over-expression of CDX2 inhibits proliferation [Figure 1a] [34].
We suggested the possible roles of CDX2 in CRC in [Figure 1b]. In cancer cell line (LoVo), over-expression of CDX2 leads to apoptosis, inhibition of proliferation, and epithelial mesenchymal transition (EMT) [35,36]. The CDX2 upregulates the expression of GSK-3β [37], AXIN2, APC [38], and MUCDHL [39] to suppress β-catenin in cells [Supplemental Materials (212.5KB, pdf) ]. Previous literature showed that claudin-1 leads to colon cancer progression (HCT-116, HT-29, SW480, and SW620) [40] and the CDX2 promotes claudin-1 expression and has a modulatory cross-talk with Wnt pathway in colorectal cell lines, HCT-116 and SW480. It could be activated by β-catenin [41].
The homeodomain proteins, including CDX2, directly bind to ATM and MRN complex and suppress DNA repair mechanisms by blocking ATM monomerization through MRN complex in HCT-116 cell line [42]. In cancer cell lines, CDX2 of CRC may serve as a tumor suppressor in DNA damaging environment by interfering DNA damage repair, such as radiation or chemotherapy.
The functions and regulatory mechanisms of CDX2 are different between developing embryo, normal epithelial cell and the CRC.
Caudal-related homeobox transcription factor 2 regulates the cell cycle in colorectal cancer
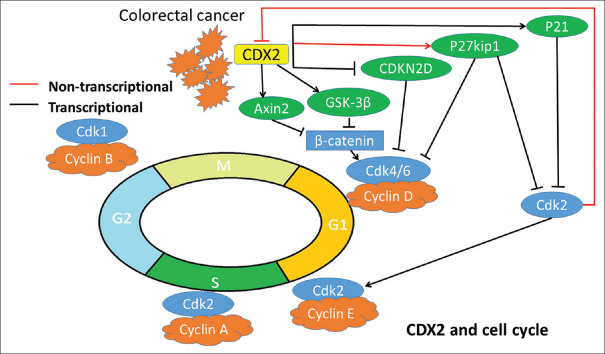
CDX2 regulates the cell cycle in several manners [Figure 2]. Overexpression of CDX2 in HT-29, a colon cancer cell line that displays the low levels of CDX2 [43], leads to the inhibition of cell growth and promotes differentiation [44,45]. Another CDK inhibitor, p21, is a transcriptional target of CDX2 in HT-29 cell line [46]. An animal model study also indicated that knockdown of CDX2 expression promotes G1-S cell cycle transition and tumor formation [8]. The targeted degradation of CDX2 by Cdk2 phosphorylation identifies that CDX2 activity can be regulated in coordination with cancer cell cycle machinery in Caco-2/15 cell line [47]. CDX2 also stabilizes the P27kip1 by nontranscriptional pathway to suppress the proliferation in colon cancer cell lines, DLD-1 and LS174T [48].
Figure 2.
Caudal-related homeobox transcription factor 2 regulates the cell cycle in colorectal cancer. Caudal-related homeobox transcription factor 2 carries out the different mechanisms to control the cell cycle. Black and red lines indicate that caudal-related homeobox transcription factor 2 regulates the cell cycle through the transcriptional and non-transcriptional functions, respectively. Caudal-related homeobox transcription factor 2 can not only inhibit but also promote the G1-S cell cycle transition. Caudal-related homeobox transcription factor 2 downregulates cyclin-dependent kinase inhibitor 2D and in turn inhibits Cdk4/6
The CDX2 also modulates the cell cycle homeostasis by down-regulating the cyclin-dependent kinase (CDK) inhibitor. In cell line study with Caco-2, the CDX2 protein was capable of suppressing the CDK inhibitor 2D by binding its promoter [49], a member of the INK4 family of CDK inhibitors [50].
The nuclear translocation of β-catenin plays the main role in the activation of Wnt pathway [51,52]. The AXIN2 protein promotes the degradation of β-catenin and inhibits the Wnt pathway. In colon cancer cell lines, Caco-2 and SW480, the CDX2 upregulates the expression of AXIN2 during differentiation, thereby inhibiting cell proliferation in colon cancer cell lines [38].
In CRC, the G1-S transition of cell cycle is more likely to be inhibited by CDX2, but there is some machinery to maintain the cell renewal by restricting the inhibitory function.
Caudal-related homeobox transcription factor 2 plays a “dual role” in colorectal cancer
The homeodomain proteins can act either an oncogene or a tumor suppressor. The dual role of CDX2 in cancer progression is dependent on the tissue type. In gastric and esophageal mucosa, CDX2 reexpression indicates to further develop intestinal metaplasia, which is related to bile acid irritation [24,53]. In glioma, the expression of CDX2 indicates more invasiveness of the tumor [54]. The aberrant expression of CDX2 in leukemia indicates proliferation and inferior prognosis [25,55].
An important question that we are interested in is whether the CDX2 is oncogenic or tumor suppressive in CRC. In vitro study with colon cancer cell lines, Caco-2/TC7 and SW480 showed that EMT was inversely correlated with the CDX2 expression [56]. According to an IHC study of the tumor, CDX2 protein expression was significantly lower at the invasive front [57], which indicated that the lower level of CDX2 was associated with more aggressive tumor behavior. In the cell line studies (LoVo, HT-29, and Caco-2), the CDX2 expression leads to less invasiveness and decreased proliferation [36,37,58].
Interestingly, compared with the normal mucosa, the CDX2 mRNA and protein were up-regulated in most tumors [59]. The increased mRNA and the cytoplasmic staining of CDX2 in CRC suggested that CDX2 accumulated in most of colon cancers with controversial function, even tumor suppression, or oncogenesis. We supposed that CDX2 could play a dual role in tumorigenesis [60].
Caudal-related homeobox transcription factor 2 Interacts with the tumor microenvironment
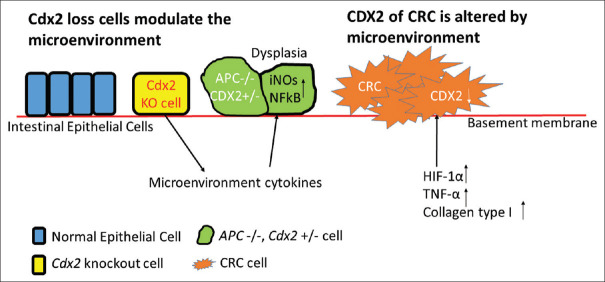
In previous review, to completely knock out the CDX2 expression of CRC cell lines is lethal [16]. CDX2-loss cells might act as the conclusive minority to promote carcinogenesis by non-cell-autonomous manner [61]. The Cdx2 metaplastic knockout cells were not the main part of tumorigenic but altering the microenvironment. The Cdx2-defect cells promote the tumorigenesis of adjacent Cdx2-intact tumor-prone cells by activation of NF-κB, induction of inducible nitric oxide synthase, and loss of function of Apc [Figure 3] [61].
Figure 3.
Caudal-related homeobox transcription factor 2-loss cells and the microenvironment strongly influence each other. The caudal-related homeobox transcription factor 2 knock-out cells induce tumorigenesis of the caudal-related homeobox transcription factor 2 heterozygous cells via inducible nitric oxide synthase and NF-κB signalings. Caudal-related homeobox transcription factor 2 actively makes the microenvironment to be more tumorigenic. The caudal-related homeobox transcription factor 2 expression of colorectal cancer cells can be passively suppressed by the hypoxia-inducible factor-1α, tumor necrosis factor-1α and type I collagen, which are released by the microenvironment
In CRC cell lines, SW480 and LS174T, the CDX2 expression was altered by hypoxia. Hypoxia-inducible factor-1α overexpression led to the lower expression of CDX2 [62]. CDX2 expression was also reported to be inversely correlated with tumor necrosis factor-1α (TNF-α) at the invasive front and in tumor buddings of rectal cancer specimens and in Caco-2 cell line [63]. This finding also hinted that CDX2 probably associated with the alteration of TNF-α. Type I collagen distributes in stromal and it decreases 55% of Cdx2 mRNA expression in a colon cancer cell lines (DLD1, Caco-2, and LS174T) [64] [Figure 3].
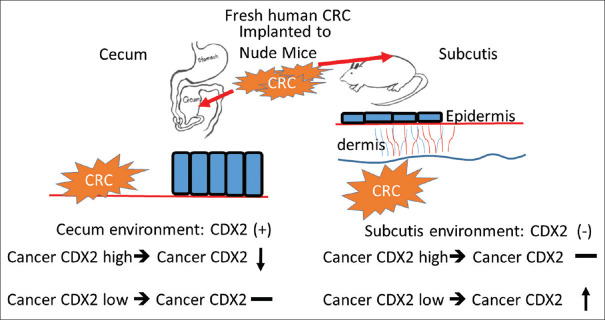
An experiment was designed to prove the CDX2 expression was altered by its environment. The researchers injected the surgically resected fresh human colon cancer cells into the subcutis and the cecal wall of nude-mice. The CDX2 showing variant expression patterns according to the different implantation sites. In the nonintestinal environment, the implanted colon cancer cells increased homogeneous expression of CDX2, but the CDX2 expression was decreased in the cecum [65]. According to previous studies, the expression of CDX2 in CRC and microenvironment could strongly affect each other [Figure 4].
Figure 4.
Caudal-related homeobox transcription factor 2 expression of colorectal cancer depends on its microenvironment. The fresh resected colorectal cancer displays different caudal-related homeobox transcription factor 2 expression in cecum and subcutis, indicating that the caudal-related homeobox transcription factor 2 expression of colorectal cancer cells is regulated by the microenvironment
Different microenvironments indicate to the complex upstream and downstream regulatory machineries of CDX2. In tumor cell lines, tumor xenografts and even patient-derived xenografts are different from those in patients. There are still many gaps from laboratories to the clinical applications of CDX2.
Caudal-related homeobox transcription factor 2 in more specific subgroups of colorectal cancer
The subgrouping of CRC was utilized for more precise individualized treatment. In a previous report, the CRC was classified into six subtypes (CIT) [7]. In the CIT C4 group, which was more like serrated and stem-cell like CRC, low expression of CDX2 was significantly related to poor disease-free survival [61]. Another study also indicated that CDX2 was an independent prognostic factor of serrated pathway in CRC [43]. Combined applications of CDX2 and consensus molecular subtypes (CMSs) were reported to be prognostic in Stage II and Stage III resected colon cancer. In CMS4 group, which were marked with prominent transforming growth factor–β activation, stromal invasion and angiogenesis, CDX2 loss indicated poor prognosis [66,67]. A literature reported that CDX2 loss was not related to the poor outcome in CRC, but might be associated with the poor prognosis among patients with a family history of CRC [13]. The applications of CDX2 might be potential for predicting tumor behavior [68], especially in specific subgroups.
CONCLUSION
Due to its complex functions in CRC and the interactions with tumor microenvironment, CDX2 expression is not suitable to be a single biomarker for prognosis. Under the consideration of the tumor environment and the dual-agent character, the clinical application of CDX2 in CRC is controversial. However, CDX2 remains potential to be a marker for personalized medicine in future, especially in the serrated pathway or CRC with stem-cell character. Further studies are necessary to identify the function of CDX2 and the application of CDX2 expression in CRC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplementary material available online
Cancer cell lines cited in the text
REFERENCES
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. gastroenterology. 2008;134:1296–310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozoe T, Kohno M, Iguchi T, Maeda T, Ezaki T. Five-point scoring system based on clinicopathological data: A convenient criterion to determine prognosis of patients with colorectal carcinoma. Oncol Lett. 2013;5:978–82. doi: 10.3892/ol.2013.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carethers JM. DNA testing and molecular screening for colon cancer. Clin Gastroenterol Hepatol. 2014;12:377–81. doi: 10.1016/j.cgh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011;29:17–24. doi: 10.1200/JCO.2010.30.1077. [DOI] [PubMed] [Google Scholar]
- 7.Marisa L, De Reyniès A, Duval A, Selves J, Gaub MP, Vescovo L, et al. Gene expression classification of colon cancer into molecular subtypes: Characterization, validation, and prognostic value. PLOS Med. 2013;10:e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawengsaksophak K, James R, Hammond VE, Köntgen F, Beck F. Homeosis and intestinal tumours in cdx2 mutant mice. Nature. 1997;386:84–7. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 9.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, et al. cdx2 as a prognostic biomarker in stage ii and stage iii colon cancer. N Engl J Med. 2016;374:211–22. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Sengupta S, Magnani L, Wilson CA, Henry RW, Knott JG. Brg1 is required for cdx2-mediated repression of oct4 expression in mouse blastocysts. PLOS One. 2010;5:e10622. doi: 10.1371/journal.pone.0010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werling RW, Yaziji H, Bacchi CE, Gown AM. cdx2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–10. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Saad RS, Cho P, Silverman JF, Liu Y. Usefulness of cdx2 in separating mucinous bronchioloalveolar adenocarcinoma of the lung from metastatic mucinous colorectal adenocarcinoma. Am J Clin Pathol. 2004;122:421–7. doi: 10.1309/UMF7-15KR-G2V1-98YD. [DOI] [PubMed] [Google Scholar]
- 13.Baba Y, Nosho K, Shima K, Freed E, Irahara N, Philips J, et al. Relationship of cdx2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665–73. doi: 10.1158/1078-0432.CCR-09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomasello G, Barni S, Turati L, Ghidini M, Pezzica E, Passalacqua R, et al. Association of cdx2 expression with survival in early colorectal cancer: A systematic review and meta-analysis. Clin Colorectal Cancer. 2018;17:97–103. doi: 10.1016/j.clcc.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Zhang H, Cao Q, Zhu W. The prognostic value of cdx2 in colorectal cancer: A meta-analysis. Int J Clin Exp Med. 2016;9:15955–60. [Google Scholar]
- 16.Olsen J, Espersen ML, Jess P, Kirkeby LT, Troelsen JT. The clinical perspectives of cdx2 expression in colorectal cancer: A qualitative systematic review. Surg Oncol. 2014;23:167–76. doi: 10.1016/j.suronc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Salari K, Spulak ME, Cuff J, Forster AD, Giacomini CP, Huang S, et al. cdx2 is an amplified lineage-survival oncogene in colorectal cancer. Proc Natl Acad Sci U S A. 2012;109:e3196–205. doi: 10.1073/pnas.1206004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.German MS, Wang J, Fernald AA, Espinosa R, 3rd, Le Beau MM, Bell GI. Localization of the genes encoding two transcription factors, lmx1 and cdx3, regulating insulin gene expression to human chromosomes 1 and 13. Genomics. 1994;24:403–4. doi: 10.1006/geno.1994.1639. [DOI] [PubMed] [Google Scholar]
- 19.Damante G, Pellizzari L, Esposito G, Fogolari F, Viglino P, Fabbro D, et al. A molecular code dictates sequence-specific DNA recognition by homeodomains. Embo J. 1996;15:4992–5000. [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 21.Human Protein Atlas. [Last accessed on 2020 Mar 10]. Available from: http://wwwproteinatlasorg .
- 22.Silberg DG, Swain GP, Suh ER, Traber PG. cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–71. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 23.Barros R, Da Costa LT, Pinto-De-Sousa J, Duluc I, Freund JN, David L, et al. cdx2 autoregulation in human intestinal metaplasia of the stomach: Impact on the stability of the phenotype. Gut. 2011;60:290–8. doi: 10.1136/gut.2010.222323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazumori H, Ishihara S, Rumi MA, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene cdx2 expression in oesophageal keratinocytes in barrett's epithelium. Gut. 2006;55:16–25. doi: 10.1136/gut.2005.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholl C, Bansal D, Döhner K, Eiwen K, Huntly BJ, Lee BH, et al. The homeobox gene cdx2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117:1037–48. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slattery ML, Herrick J, Wolff RK, Caan BJ, Potter JD, Sweeney C. cdx2 vdr polymorphism and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2752–5. doi: 10.1158/1055-9965.EPI-07-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozek Ls, Lipkin SM, Fearon ER, Hanash S, Giordano TJ, Greenson JK, et al. Cdx2 polymorphisms, rna expression, and risk of colorectal cancer. Cancer Res. 2005;65:5488–92. doi: 10.1158/0008-5472.CAN-04-3645. [DOI] [PubMed] [Google Scholar]
- 28.Sivagnanasundaram S, Islam I, Talbot I, Drummond F, Walters JR, Edwards YH. The homeobox gene cdx2 in colorectal carcinoma: A genetic analysis. Br J Cancer. 2001;84:218–25. doi: 10.1054/bjoc.2000.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi OK, Akiyama Y, Yuasa Y. Genomic structure and alterations of homeobox gene cdx2 in colorectal carcinomas. Br J Cancer. 1999;79:440–4. doi: 10.1038/sj.bjc.6690068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subtil C, Guérin E, Schneider A, Chenard MP, Martin E, Domon-Dell C, et al. Frequent rearrangements and amplification of the cdx2 homeobox gene in human sporadic colorectal cancers with chromosomal instability. Cancer Lett. 2007;247:197–203. doi: 10.1016/j.canlet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro . Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chickarmane V, Peterson C. A computational model for understanding stem cell, trophectoderm and endoderm lineage determination. PLOS One. 2008;3:e3478. doi: 10.1371/journal.pone.0003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan HB, Zhai ZY, Li XG, Gao CQ, Yan HC, Chen ZS, et al. cdx2 stimulates the proliferation of porcine intestinal epithelial cells by activating the mtorc1 and wnt/β-catenin signaling pathways. Int J Mol Sci. 2017;18:e2447. doi: 10.3390/ijms18112447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escaffit F, Paré F, Gauthier R, Rivard N, Boudreau F, Beaulieu JF. cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem Biophys Res Commun. 2006;342:66–72. doi: 10.1016/j.bbrc.2006.01.128. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J, He S, Qi J, Wang X, Yu J, Wu Y, et al. Targeted cdx2 expression inhibits aggressive phenotypes of colon cancer cells in vitro and in vivo. Int J Oncol. 2017;51:478–88. doi: 10.3892/ijo.2017.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng JB, Sun XJ, Qi J, Li SS, Wang W, Ren HL, et al. Effects of homeodomain protein cdx2 expression on the proliferation and migration of lovo colon cancer cells. Pathol Oncol Res. 2011;17:743–51. doi: 10.1007/s12253-011-9380-0. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C, et al. cdx2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing wnt/β-catenin signaling via transactivation of gsk-3β and axin2 expression. Cell Death Dis. 2019;10:26. doi: 10.1038/s41419-018-1263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen Ak, Coskun M, Bzorek M, Kristensen MH, Danielsen ET, Jørgensen S, et al. Regulation of apc and axin2 expression by intestinal tumor suppressor cdx2 in colon cancer cells. Carcinogenesis. 2013;34:1361–9. doi: 10.1093/carcin/bgt037. [DOI] [PubMed] [Google Scholar]
- 39.Hinkel I, Duluc I, Martin E, Guenot D, Freund JN, Gross I. cdx2 controls expression of the protocadherin mucdhl, an inhibitor of growth and β-catenin activity in colon cancer cells. Gastroenterology. 2012;142:875–85 e3. doi: 10.1053/j.gastro.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–76. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhat AA, Sharma A, Pope J, Krishnan M, Washington MK, Singh AB, et al. Caudal homeobox protein cdx-2 cooperates with wnt pathway to regulate claudin-1 expression in colon cancer cells. PLOS One. 2012;7:e37174. doi: 10.1371/journal.pone.0037174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson TE, Lee JH, Myler LR, Zhou Y, Mosley TJ, Yang SH, et al. Homeodomain proteins directly regulate ATM kinase activity. Cell Rep. 2018;24:1471–83. doi: 10.1016/j.celrep.2018.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graule J, Uth K, Fischer E, Centeno I, Galván JA, Eichmann M, et al. cdx2 in colorectal cancer is an independent prognostic factor and regulated by promoter methylation and histone deacetylation in tumors of the serrated pathway. Clin Epigenetics. 2018;10:120. doi: 10.1186/s13148-018-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallo GV, Soubeyran P, Lissitzky JC, André F, Farnarier C, Marvaldi J, et al. Expression of the cdx1 and cdx2 homeotic genes leads to reduced malignancy in colon cancer-derived cells. J Biol Chem. 1998;273:14030–6. doi: 10.1074/jbc.273.22.14030. [DOI] [PubMed] [Google Scholar]
- 45.Soubeyran P, Mallo GV, Moucadel V, Dagorn JC, Iovanna JL. Overexpression of cdx1 and cdx2 homeogenes enhances expression of the hla-i in ht-29 cells. Mol Cell Biol Res Commun. 2000;3:271–6. doi: 10.1006/mcbr.2000.0226. [DOI] [PubMed] [Google Scholar]
- 46.Bai YQ, Miyake S, Iwai T, Yuasa Y. cdx2, a homeobox transcription factor, upregulates transcription of the p21/waf1/cip1 gene. Oncogene. 2003;22:7942–9. doi: 10.1038/sj.onc.1206634. [DOI] [PubMed] [Google Scholar]
- 47.Boulanger J, Vézina A, Mongrain S, Boudreau F, Perreault N, Auclair BA, et al. cdk2-dependent phosphorylation of homeobox transcription factor cdx2 regulates its nuclear translocation and proteasome-mediated degradation in human intestinal epithelial cells. J Biol Chem. 2005;280:18095–107. doi: 10.1074/jbc.M502184200. [DOI] [PubMed] [Google Scholar]
- 48.Aoki K, Kakizaki F, Sakashita H, Manabe T, Aoki M, Taketo MM. Suppression of colonic polyposis by homeoprotein cdx2 through its nontranscriptional function that stabilizes p27kip1. Cancer Res. 2011;71:593–602. doi: 10.1158/0008-5472.CAN-10-2842. [DOI] [PubMed] [Google Scholar]
- 49.Boyd M, Hansen M, Jensen TG, Perearnau A, Olsen AK, Bram LL, et al. Genome-wide analysis of cdx2 binding in intestinal epithelial cells (caco-2) J Biol Chem. 2010;285:25115–25. doi: 10.1074/jbc.M109.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okuda T, Hirai H, Valentine VA, Shurtleff SA, Kidd VJ, Lahti JM, et al. Molecular cloning, expression pattern, and chromosomal localization of human cdkn2d/ink4d, an inhibitor of cyclin d-dependent kinases. Genomics. 1995;29:623–30. doi: 10.1006/geno.1995.9957. [DOI] [PubMed] [Google Scholar]
- 51.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (apc) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995;92:3046–50. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tejeda-Muñoz N, Robles-Flores M. Glycogen synthase kinase 3 in wnt signaling pathway and cancer. Iubmb Life. 2015;67:914–22. doi: 10.1002/iub.1454. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, et al. Bile acids induce cdx2 expression through the farnesoidxreceptor in gastric epithelial cells. J Clin Biochem Nutr. 2010;46:81–6. doi: 10.3164/jcbn.09-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F, Ruan X, Ma J, Liu X, Zheng J, Liu Y, et al. dgcr8/zfat-as1 promotes cdx2 transcription in a prc2 complex-dependent manner to facilitate the malignant biological behavior of glioma cells. Mol Ther. 2020;28:613–30. doi: 10.1016/j.ymthe.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoene S, Rawat VP, Heilmeier B, Hoster E, Metzeler KH, Herold T, et al. The homeobox gene cdx2 is aberrantly expressed and associated with an inferior prognosis in patients with acute lymphoblastic leukemia. Leukemia. 2009;23:649–55. doi: 10.1038/leu.2008.355. [DOI] [PubMed] [Google Scholar]
- 56.Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, et al. The intestine-specific homeobox gene cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene. 2008;27:107–15. doi: 10.1038/sj.onc.1210601. [DOI] [PubMed] [Google Scholar]
- 57.Karamitopoulou E, Zlobec I, Panayiotides I, Patsouris ES, Peros G, Rallis G, et al. Systematic analysis of proteins from different signaling pathways in the tumor center and the invasive front of colorectal cancer. Hum Pathol. 2011;42:1888–96. doi: 10.1016/j.humpath.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Hryniuk A, Grainger S, Savory JG, Lohnes D. Cdx1 and cdx2 function as tumor suppressors. J Biol Chem. 2014;289:33343–54. doi: 10.1074/jbc.M114.583823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witek ME, Nielsen K, Walters R, Hyslop T, Palazzo J, Schulz S, et al. The putative tumor suppressor cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549–56. doi: 10.1158/1078-0432.CCR-05-1624. [DOI] [PubMed] [Google Scholar]
- 60.Shen L, Shi Q, Wang W. Double agents: Genes with both oncogenic and tumor-suppressor functions. Oncogenesis. 2018;7:25. doi: 10.1038/s41389-018-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balbinot C, Armant O, Elarouci N, Marisa L, Martin E, de Clara E, et al. The cdx2 homeobox gene suppresses intestinal tumorigenesis through non-cell-autonomous mechanisms. J Exp Med. 2018;215:911–26. doi: 10.1084/jem.20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng J, Sun X, Wang W, Lu S. Hypoxia-inducible factor-1alpha modulates the down-regulation of the homeodomain protein cdx2 in colorectal cancer. Oncol Rep. 2010;24:97–104. [PubMed] [Google Scholar]
- 63.Coskun M, Olsen AK, Bzorek M, Holck S, Engel UH, Nielsen OH, et al. Involvement of cdx2 in the cross talk between tnf-α and wnt signaling pathway in the colon cancer cell line caco-2. Carcinogenesis. 2014;35:1185–92. doi: 10.1093/carcin/bgu037. [DOI] [PubMed] [Google Scholar]
- 64.Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, et al. Down-regulation of the homeodomain factor cdx2 in colorectal cancer by collagen type I: An active role for the tumor environment in malignant tumor progression. Cancer Res. 2004;64:6973–7. doi: 10.1158/0008-5472.CAN-04-1132. [DOI] [PubMed] [Google Scholar]
- 65.Benahmed F, Gross I, Guenot D, Jehan F, Martin E, Domon-Dell C, et al. The microenvironment controls cdx2 homeobox gene expression in colorectal cancer cells. Am J Pathol. 2007;170:733–44. doi: 10.2353/ajpath.2007.060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pilati C, Taieb J, Balogoun R, Marisa L, de Reyniès A, Laurent-Puig P. cdx2 prognostic value in stage II/III resected colon cancer is related to CMS classification. Ann Oncol. 2017;28:1032–5. doi: 10.1093/annonc/mdx066. [DOI] [PubMed] [Google Scholar]
- 68.Singh J. Pattern of expression of CDX2 in colorectal cancer and its role in prognosis. J Clin Oncol. 2019;37(15 Suppl):e15115–e. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cancer cell lines cited in the text