Since the beginning of the COVID-19 pandemic, there has been intense debate over SARS-CoV-2’s mode of transmission and appropriate personal protective equipment for health care workers in low-risk settings. The objective of this review is to identify and appraise the available evidence (clinical trials and laboratory studies on masks and respirators, epidemiological studies, and air sampling studies), clarify key concepts and necessary conditions for airborne transmission, and shed light on knowledge gaps in the field.
KEYWORDS: bioaerosols, COVID-19, inhalable aerosols, low-risk settings, respiratory protection, SARS-CoV-2, infection prevention, personal protective equipment, ventilation
SUMMARY
Since the beginning of the COVID-19 pandemic, there has been intense debate over SARS-CoV-2’s mode of transmission and appropriate personal protective equipment for health care workers in low-risk settings. The objective of this review is to identify and appraise the available evidence (clinical trials and laboratory studies on masks and respirators, epidemiological studies, and air sampling studies), clarify key concepts and necessary conditions for airborne transmission, and shed light on knowledge gaps in the field. We find that, except for aerosol-generating procedures, the overall data in support of airborne transmission—taken in its traditional definition (long-distance and respirable aerosols)—are weak, based predominantly on indirect and experimental rather than clinical or epidemiological evidence. Consequently, we propose a revised and broader definition of “airborne,” going beyond the current droplet and aerosol dichotomy and involving short-range inhalable particles, supported by data targeting the nose as the main viral receptor site. This new model better explains clinical observations, especially in the context of close and prolonged contacts between health care workers and patients, and reconciles seemingly contradictory data in the SARS-CoV-2 literature. The model also carries important implications for personal protective equipment and environmental controls, such as ventilation, in health care settings. However, further studies, especially clinical trials, are needed to complete the picture.
INTRODUCTION
The world is facing a devastating new infectious disease, with only preliminary scientific data to guide policy. Disagreement with the World Health Organization’s stance on personal protective equipment (PPE), guideline changes over time (e.g., European CDC, France), and inconsistent data on the effectiveness of medical masks have left health care workers (HCWs) wondering if they are sufficiently protected. The general consensus is that SARS-CoV-2 predominantly transmits through droplets and contact (although precise mechanisms for both modes of transmission are yet to be fully understood), but the airborne debate is still raging. This review attempts to summarize current cumulative data on SARS-CoV-2’s modes of transmission and identify gaps in research while offering preliminary answers to the question on everyone’s mind: is the airborne route significant and should we modify our COVID-19 PPE recommendations for frontline workers in low-risk settings?
This review starts by investigating the differences between droplets and aerosols and goes over prerequisites for clinically significant airborne transmission. It then appraises the evidence in support of the airborne hypothesis: trials and experiments on masks, epidemiological studies, data on SARS-CoV-1, air sampling findings, and aerosol studies. The focus is on low-risk health care settings, in the absence of aerosol-generating procedures (AGPs), with a special look at long-term-care facilities where major outbreaks occurred. National and international guidelines are compared, and alternative hypotheses for SARS-CoV-2’s contagiousness are explored, such as presymptomatic transmission, as well as fomite and fecal routes. Possible mechanisms behind high HCW infection rates are described, and the limits of the precautionary principle are addressed. Finally, a revised model of inhalable particles is proposed to support PPE recommendations and guide future research.
WHAT IS THE DIFFERENCE BETWEEN DROPLET AND AIRBORNE TRANSMISSION?
Determining SARS-CoV-2’s main mode of transmission is essential as it informs clinical guidelines for patient management, prevention practices, and HCW protection. While infectious disease precautions in health care settings are transmission-based (either airborne or droplet), in reality, the distinction is not clear-cut; instead, they are two ends of a spectrum.
In the literature, respiratory droplets are usually defined as larger particles (diameter > 5 μm) sometimes visible to the human eye, produced during spitting, sneezing, and coughing. These droplets are thought to be the main mode of transmission of COVID-19 (1), and they typically travel 1 to 2 m before landing on surrounding surfaces. However, they may be propelled further in the presence of ventilation (2) or forceful ejection (e.g., a violent sneeze) (3) and under certain environmental conditions (e.g., cool and humid) (4). The SARS-CoV-2 virus is also thought to be transmitted by direct contact person to person (e.g., exchange of saliva or a handshake) or by indirect contact through intermediate objects (e.g., sharing of cups, doorknobs). Generally, contact transmissions occur when contaminated hands are brought to the face and touch mucous membranes (eyes, nose, and mouth).
The fate of smaller droplets may be desiccation (evaporation of the liquid) and formation of particles called droplet nuclei, or aerosols, which can contain infectious agents but also secretions, cells, surfactant, and any other product contained in the original droplet. Traditionally, aerosols are defined as particles of <5 μm that can remain airborne for prolonged periods (several minutes or even hours) and travel long distances with air currents (several meters away). With the potential for direct entry into the lungs, they are the primary mode of transmission for tuberculosis, measles, and varicella. In other communicable diseases, such as influenza, aerosols are considered opportunistic and play a role that is of variable importance depending on the context (5).
Conversely, in the field of industrial hygiene, occupational exposure of different body regions to harmful airborne agents is classified into three overlapping categories, according to the median size of penetrating particles (6): 100 μm for nose and mouth (inhalable), 10 μm for trachea and bronchi (thoracic), and 4 μm for alveoli and air exchange regions (respirable). This aerosol classification was recently reviewed and elegantly illustrated by Milton (7). In this model, the concept of aerosol inhalability is defined as the fraction of particles capable of penetrating into the head airways or below, upon inhalation: it excludes larger droplets with ballistic behavior (since inhalation requires suspension in the air) but includes particles that are larger than the traditional 5-μm definition of aerosols. Throughout our review, this more nuanced conceptualization of airborne transmission will be explored, and the larger inhalable aerosols will be contrasted to the smaller respirable aerosols from the classic airborne model.
Finally, some procedures, such as intubation, are known to generate aerosols, while others, such as nebulizer therapy, are associated with an uncertain risk of aerosolization (8). N95s (or similar respiratory protection devices) are unequivocally recommended for HCWs working in high-risk settings with AGPs, although controversy still remains around which interventions constitute an AGP. The design protocol for the N95, and the origin of the name, is based on its efficiency at capturing 95% of the most penetrating size range (0.3 μm) of respirable aerosols (9). By default, respirators are therefore capable of blocking the entire spectrum of airborne particles. Medical masks, on the other hand, are designed to block droplets and do not undergo aerosol-filtering tests; they are therefore not considered to provide respiratory protection against airborne transmission. Given that substantial disagreement persists on the importance of natural aerosol generation by COVID-19 patients, and consequently, the necessary level of respiratory protection in non-AGP contexts, our review will focus on transmission and PPE in low-risk health care settings.
WHAT ARE THE PREREQUISITES FOR SIGNIFICANT AIRBORNE TRANSMISSION?
Natural respiratory activities such as breathing, talking, and coughing can generate a broad range of particle sizes, from submicron aerosols to large droplets (10–14). For the viral aerosols to constitute a clinically significant risk of airborne infection, three conditions are required: viral load (the concentration of infectious particles), infectivity (the ability of a virion to infect a host cell), and tropism (the specificity of a virus for a particular host cell type or tissue).
Since the amount of SARS-CoV-2 virus required to infect a host is unknown, and likely varies from one individual to another (preprint article [15]), it is hard to determine whether typical respiratory activity generates sufficient quantities of infectious aerosols for airborne transmission. In a light-scattering study, Stadnytskyi et al. estimated that 1 min of loud speaking generated at least 1,000 virion-containing droplet nuclei that remain airborne for more than 8 min (16). However, the calculations were based on several theoretical assumptions and data from sputum load was incorrectly applied to saliva, likely overestimating aerosol viral loads. In this model, the probability that a hypothetical speech-generated droplet nucleus of 3 μm contains a SARS-CoV-2 virion is only 0.01%, after aerosolization and desiccation. Furthermore, in a mathematical modeling study on viral aerosol emissions, an individual with a high viral load was estimated to emit only modest amounts of virus with regular breathing (1,248 copies/m3) compared to coughing (7.44 million copies/m3) (17). Accordingly, the authors conclude that the infectious risk posed by a typical COVID-19 patient is low, especially if symptoms are mild, and only a few individuals with high viral load pose a significant risk. These authors suggest that strict respiratory protection may be needed in the case of prolonged exposure to high emitters in poorly ventilated closed environments.
Notwithstanding, evidence of aerosol generation during natural respiratory activity or the presence of viral RNA in the air are not sufficient to prove that the virus remains infectious once airborne. Not all viruses are equally stable in the air, and further aerodynamic and environmental factors may inactivate viruses during aerosolization (18). Therefore, upon detecting SARS-CoV-2 aerosols, infectivity must then be demonstrated. Evaluation of infectivity is usually done with viral cultures: researchers were able to culture rhinovirus (19) and influenza (20) from the fine particles emitted naturally by infected participants, and only recent yet unpublished research has started to achieve the same for SARS-CoV-2. However, it is important to note that culture methods vary between viruses and false-negative results due to the low sensitivity of commonly used SARS-CoV-2 cultures could have possibly underestimated infectivity from air samples until now. For instance, clinical samples (e.g., nasopharyngeal swabs) that yield positive cultures typically have low PCR cycle threshold (CT) values of <25 (Samira Mubareka, University of Toronto, unpublished data), while CT values for environmental samples (including air samples) are often >35.
Finally, since particles penetrate and deposit in different parts of the respiratory tract depending on size, knowledge of target locations for infection (e.g., viral tropism) can hint at typical size range and mode of transmission. SARS-CoV-2’s main entry into host cells is through ACE2 receptors, which seem to be largely expressed in the nose (21, 22). Importantly, the highest and most consistent signs of viral infectivity have been observed for nasal cells, with a gradient along the respiratory tract characterized by a marked reduction in infectivity in the distal bronchioles and alveoli. This may suggest that lower airways are not targets for infection and that transmission via respirable aerosols is not predominant. Interestingly, the typical patchy bilateral pneumonia found in COVID-19 patients is postulated to be caused by oropharyngeal microaspirations rather than direct viral seeding in the lungs, possibly accounting for the increased risk with age and comorbidities (22).
WHAT IS THE EVIDENCE FOR AIRBORNE TRANSMISSION OF SARS-CoV-2?
Different types of studies suggest airborne transmission, but their levels of evidence are variable. In this review, given the focus on health care settings and HCW protection, studies are appraised according to clinical relevance: hard outcomes (e.g., morbidity) are markers of higher levels of evidence, while surrogate outcomes (e.g., pathophysiological mechanisms, modeling, and laboratory results) are considered lower levels of evidence, independent of method or design quality (Table 1).
TABLE 1.
Overview of studies and their level of evidence from a clinical perspective
| Types of studies | Level of evidencea | Clinical limitations |
|---|---|---|
| Trials comparing masks and respirators in health care settings | Moderate | Lack of clinical trials No SARS-CoV-2 trials (extrapolations) High heterogeneity |
| Laboratory studies on masks | Weak | Artificial conditions Nonstandardization of methods Lack of clinical/behavioral factors |
| Epidemiological studies on transmission | Weak to moderate | Observational data Incomplete data Confounding biases |
| SARS-CoV-1 studies | Weak to moderate | Lack of clinical trials High heterogeneity Differences with SARS-CoV-2 |
| Air and no-touch surface sampling | Weak | Variety of methods Confounding biases (e.g., AGPs) Infectivity often not evaluated |
| Laboratory generation of aerosols | Very weak | Artificial conditions Variety of methods |
This hierarchy is based on clinical relevance and outcomes, inspired by GRADE (182).
Trials Comparing Masks and Respirators in Health Care Settings
The term “mask,” as used here, comprises medical masks, surgical masks, procedural masks, fluid-resistant masks, and face masks worn by HCWs. The term “respirator” is used interchangeably with N95, which is the equivalent of FFP2 (European standard filtering facepiece) and KF94 (Korean Filter) respirators.
In the absence of clinical trials on SARS-CoV-2, trials on other viruses with similar infection patterns (i.e., documented droplet and suspected airborne transmission) are the best available alternatives. Recent systematic and narrative reviews comparing the effectiveness of respirators versus masks against common viral respiratory infections (including coronaviruses and influenza viruses such as H1N1) come to similar conclusions: both devices offer comparable protection in health care settings (23–31).
A few reviews (32–34) favor respirators, on the basis of two randomized controlled trials (RCTs) conducted by the same lead authors, MacIntyre et al. (Table 2) (35, 36). Individually and in combination (meta-analysis) (33), these two RCTs report superiority of continuous N95 use over mask use for a single self-reported outcome: clinical respiratory illness (CRI), defined as two or more respiratory symptoms or one respiratory symptom and a systemic symptom. No difference is found for other more rigorous outcomes: influenza-like illness (ILI; defined as fever and one respiratory symptom), laboratory-confirmed viral respiratory infection (LVI), or laboratory-confirmed influenza (LCI). The difference between the self-reported outcome and the laboratory results could be explained by detection bias in the absence of participant blinding and universal testing: higher symptom reporting rates in the medical mask group, rather than true infection, could have skewed CRI results in favor of respirators. Furthermore, selection bias is suspected to have occurred during allocation, given the surprisingly uneven distribution of major confounding variables such as AGPs, age, and handwashing, between the N95 and mask groups.
TABLE 2.
RCTs comparing masks to respirators during HCWs exposure to respiratory virusesa
| Study details | Outcomes | Limitations |
|---|---|---|
|
MacIntyre et al., 2011 (36)
1,441 participants Cluster randomization 23–35% high-risk exposure in N95 group vs 41% in mask group |
Symptom-based PCR swab:
Nonfitted N95s superior to masks for CRI only (not ILI, LVI, and LCI) No difference between fitted N95s and masks |
Serious baseline imbalances Nonfitted outperformed fitted N95 Detection bias Uncertain clinical significance of primary outcome (CRI) |
|
MacIntyre et al., 2013 (35)
1,669 participants Cluster randomization >70% high-risk exposure in both groups |
Symptom-based PCR swab:
Continuous but not intermittent N95s superior to masks for CRI only (not ILI, LVI, and LCI) |
Serious baseline imbalances Detection bias Uncertain clinical significance of primary outcome (CRI) |
|
Loeb et al., 2009 (38)
446 participants Individual randomization High-risk exposure in both groups but % unknown |
Symptom-based PCR swab + serology for all:
No difference for LCIb No difference for ILI, LVI, physician visits, and work-related absenteeismc |
Unknown % high-risk procedures Study ended prematurely |
|
Radonovich et al., 2019 (37)
2,862 participants Cluster randomization 59% high-risk exposure in both groups |
Symptom-based PCR swab + two random swabs and serology for all:
No difference for LCIb No difference for ARI, ILI, LVIc |
Outpatient setting only Underpowered |
ARI, acute respiratory illness; CRI, clinical respiratory illness; ILI, influenza-like illness; LCI, laboratory-confirmed influenza; LVI, laboratory-confirmed viral respiratory infection.
Primary outcome.
Secondary outcomes.
The other two RCTs (37, 38) included in the reviews had more robust methodologies and lesser risk of bias (e.g., comparable groups, test results for all participants, and longer follow-up periods). The studies did not find any significant differences between respirators and masks for clinical and laboratory outcomes, in both low and high-risk settings.
A recent systematic review of observational studies suggests that “N95 respirators might be more strongly associated with protection from viral transmission than surgical masks” (39). Regrettably, of 10 studies, not a single one directly compared respirators to masks, and nine of them looked at SARS or MERS rather than SARS-CoV-2. The lone COVID-19 study only compared N95s to no masks and did not include medical masks at all (40). The researchers drew their conclusions by comparing the pooled results for N95 studies with the pooled results for mask studies, obtaining a P value for interaction by mask type that was borderline significant after partial adjustment. However, the difference between the two groups was not statistically significant (overlapping confidence intervals) and the very high heterogeneity (I2 = 88%) could have undermined the validity of the meta-analysis. Also, the presence of AGPs was unknown in 7 of 10 studies: since all the studies were done in a hospital setting where AGPs frequently occur, and N95s are known to be superior in high-risk settings, failure to adjust for AGPs will skew the results in favor of N95s. Finally, all 10 studies were observational and many did not control for important confounding factors, leading the authors themselves to rate the overall certainty for mask data as low.
Since many trials studied airborne viruses (e.g., influenza) and included exposure to AGPs, it may seem surprising that the vast majority of reviews, past and present, did not find respirators to be superior to masks. A possible explanation is that, while not designed to filter very fine particles, the medical mask might nonetheless be effective in blocking the low levels of aerosols produced in most health care contexts. A few case reports seem to support this hypothesis.
For example, in a study of two severely ill COVID-19 patients who were not initially isolated, contact tracing identified 421 HCWs, of whom only 8 tested positive (41). All infected HCWs had close and prolonged contact without wearing the mask or ocular protection and had been present during AGPs. On the other hand, all of the HCWs who used droplet and contact precautions did not get infected, leading the authors to conclude that there was no evidence of airborne transmission. Similarly, two studies reported on 34 and 41 intensive care HCWs exposed to an intubated and mechanically ventilated COVID-19 patient: 50 and 85% wore surgical masks, respectively, and the others wore N95s, yet none were infected according to clinical and laboratory-confirmed results (42, 43). Furthermore, a COVID-19 patient who stayed 35 h in an open cubicle of a general ward, coughed frequently, and received high-flow oxygen at 8 liters/min, did not infect any of the 71 staff members and 49 patients, of which 7 and 10, respectively, had close contacts wearing either N95s or masks (44). Finally, strict contact and droplet precautions, as well as the use of masks rather than respirators, completely prevented nosocomial transmission from three community-infected HCWs to coworkers and patients in an Italian hospital (45).
As for the effectiveness of medical masks as source control (blocking particles emitted by infected individuals), clinical trials are scarce (46–48), and they suggest a reduction of clinical but not laboratory-confirmed viral illnesses. Therefore, we must turn to lower levels of evidence (e.g., laboratory studies) for further guidance.
Laboratory Studies on Masks
The ability of protection devices to control either source emission (e.g., infected individuals) or exposure prevention (e.g., HCWs) has been the subject of several laboratory studies, whose findings are summarized in Table 3. The majority show high filtration capacity for both masks and respirators. The latter, however, are known to provide better protection against fine particles (<5 μm) because of a far superior fit factor. Interestingly, source control with masks may be superior to exposure prevention by either respirators or masks.
TABLE 3.
Major laboratory studies on the filtration efficiency of masks and respiratorsa
| Study details | Design | Findings/conclusions | Strengths (+) and limitations (−) |
|---|---|---|---|
|
Bae et al., 2020 (183)
Source control: Surgical mask Cotton mask |
4 human volunteers SARS-CoV-2 Coughing with and without mask Petri dish sampling (settle plate) Mask surface sampling |
Both mask types ineffective Cotton appears superior to surgical mask Outside layer contamination >> inner contamination |
(−) Implausible findings: superiority of cotton and uncontaminated inner layer (−) Ballistic particles, not aerosols (−) Confounding: cough intensities (−) Underpowered and poorly designed |
|
Kim et al., 2020 (184)
Source control: Surgical mask KF94 N95 |
7 human volunteers SARS-CoV-2 5 coughs with no mask, surgical mask, KF94 and N95 (in this order) Petri dish sampling Mask surface sampling |
Surgical mask:
3 of 7 positive samples Outer and inner layer contamination KF94 and N95: 0 of 7 positive sample No outer layer contamination |
(−) Implausible findings: uncontaminated inner layers of mask and respirators (−) Ballistic particles, not aerosols (−) Confounding: cough intensities and order of device testing (−) Underpowered and poorly designed |
|
Leung et al., 2020 (
185)
Source control: Face mask |
246 human volunteers randomized to mask or no mask Influenza, rhinovirus, coronavirus Breathing and coughing Viral load in droplets and aerosols |
Coronavirus: complete reduction in droplets and aerosols with mask Influenza: partial reduction in droplets but not in aerosols with mask Rhinovirus: no significant reduction with mask |
(+) Similarity to clinical setting (i.e., many infected pts) (+) Viral loads quantified (+) Viral culture for influenza (not the other viruses) (−) No hypotheses provided for differential behavior of viruses (−) No fit factor |
|
Ma et al., 2020 (
186)
Exposure control: Surgical mask N95 Homemade mask (paper and cloth) |
Nebulizer-generated aerosols and bag as aerosol chamber Syringe-simulated human inhalation Avian influenza |
Filtration efficiency N95: 99.98% Medical mask: 97.1% Homemade mask: 95.1% |
(−) Particle sizes not measured but assumed from manufacturer guide (−) Unusual setup for aerosol study (nebulizer, bag, syringe) with unknown risk of bias (−) No fit factor |
|
Patel et al., 2016 (
187)
Source and exposure control: Natural fit and ultrafitted surgical masks N95 with or without Vaseline seal |
2 manikin heads in a chamber, 3 feet apart: Source (simulated coughing) and Receiver (simulated breathing) Nebulizer-generated and radiolabeled aerosols 3 airflow regimes |
Coughing: mask or N95 on Source superior to mask or unsealed N95 on Receiver Breathing: mask on Source superior to mask or N95 on Receiver Fitting/leakage and airflow are important in Source control |
(+) MMAD measured for each setup (+) Various ventilation settings (+) Loose vs tight fit for both devices (−) Vaseline seal does not adequately represent respirator fitting |
|
Milton et al., 2013 (
188)
Source control: Surgical mask |
37 human volunteers Influenza Exhalation Viral load in droplets and aerosols |
Fine particles exhaled contained more viral copies than coarse particles Viral shedding reduced by 2.8-fold (fine) and 25-fold (coarse) when using mask |
(+) Similarity to clinical setting (i.e., many infected pts) (+) Viral loads quantified (+) Viral culture (on subset of fine particle samples) (−) No fit factor |
|
Booth et al., 2013 (
54)
Exposure control: 8 types of surgical masks |
Manikin head (receiver) attached to a breathing simulator Atomiser-generated viral aerosols Influenza Detection of virus in front of and behind mask |
Infectious virus detected behind all masks Reduction of exposure by 1.1- to 55-fold (avg 6-fold), depending on mask Superior performance with integral visor |
(+) Viral culture (+) Variety of mask types (−) Test aerosols different from natural ones (50% < 60 μm and 15% > 100 μm) (−) Unknown size of particles that penetrated mask (−) Fitting and leakage not detailed |
|
Davies et al., 2013 (
189)
Source control: Homemade pleated cloth mask Surgical mask |
Nebulizer-generated microbial aerosols 21 human volunteers coughing (no mask, cloth mask and surgical mask) Bacterial and viral surrogate |
Surgical masks had best filtration efficiency for microbial aerosols and lowered the no. of emitted particles Fit factor: homemade half that of surgical mask |
(+) Fit factor (+) Filtration efficiency measured for particles < and > 4.7 μm (−) Confusion between organism size and aerosol size |
|
Noti et al., 2012 (
53)
Exposure control: Surgical mask N95 |
2 manikin heads attached to a coughing and a breathing simulator Nebulizer-generated aerosols Influenza |
Loosely fitted respirator no better than loosely fitted mask in blocking aerosols (>50–60%) Tightly sealed masks and N95 efficient in blocking aerosols (>90–99%) |
(+) Aerosol sizes measured (+) Fit factor (+) Viral culture (+) Sampling beside mouth and 3 other locations (−) Artificially high fit factor for surgical mask |
|
Wen et al., 2013 (
190)
Exposure control: Medical mask N95 N99 |
A manikin head simulating inhalation Nebulizer-generated aerosols Phage SM702 (viral surrogate) |
>97% filtration for all Low face fit factor for masks (<8) Respirators are superior when considering fit factor |
(+) MMAD = 0.774 μm (+) Fit factor |
|
Diaz and Smaldone 2010 (
191)
Source and exposure control: Surgical mask N95 |
2 manikin heads in a chamber, 3 feet apart: source (simulated exhalation) and receiver Nebulizer-generated and radiolabeled aerosols |
Mask on source effective Mask on receiver not effective unless N95 with Vaseline seal |
(+) MMAD measured for each setup (+) Various ventilation settings (+) Loose versus tight fit (−) Masks on both source and receiver decreased protection (implausible finding) |
|
Johnson et al., 2009 (
192)
Source control: Surgical mask N95 |
9 human volunteers coughing Influenza Petri dish sampling |
Both N95 and surgical equally effective (complete blockage) | (−) Ballistic particles of unknown size, not aerosols (−) No fit factor (−) Poor design with confounding |
MMAD, median mass aerodynamic diameter (indicator of aerosol size); pt(s), patient(s).
Although these studies provide relevant information on the theoretical performances of protection devices, the experimental generation process and particle sizes may not resemble natural respiratory activity. Also, many studies suffer from major limitations and inconsistencies in design: the use of different respiratory viruses with distinct behaviors, the lack of information on the size distribution of particles tested, the use of nonstandardized test particles (e.g., in contrast to standard respirator testing protocols), selection bias for ballistic behavior (petri dish sampling) rather than aerosols (air sampling), and confounding biases (e.g., fit factor and variable cough intensities).
More importantly, many laboratory studies fail to account for crucial clinical and behavioral factors. For example, studies have reported lower adherence to N95 respirators compared to medical masks, due to higher rates of adverse events (35, 36, 49). In one study on the tolerability of respirators in HCWs, the probability of discontinuing respirator use during an 8-h work shift was around 50 to 70%, despite regular 15- or 30-min breaks every 2 h (50). Other studies show that one of the most challenging steps in donning and doffing is N95 use, which can result in a higher risk of contamination (51, 52). In addition, an important, yet overlooked factor is the fitting of the device on the face (or the degree of leakage of particles around the edges). The fit factor varies between mask models and is typically very high for respirators, which is probably its main advantage. However, a poorly fitted respirator could perform no better than a loosely fitting mask (53). Seals used in some laboratory studies are poor surrogates for actual fitting on a HCW. Finally, during exposure to COVID-19 patients, HCWs are instructed to wear ocular protection in addition to masks, and yet very few studies examine the combined effects of overall PPE. Some experiments have shown that masks integrated with visors (54) and face shields individually (55) are protective not only against droplets but also aerosols (but efficiency decreases with exposure time).
Epidemiological Studies on Transmission
The vast majority of epidemiological studies that analyze SARS-CoV-2 outbreak patterns (case identification, contact tracing, epidemiological curves, and basic reproduction number or R0 estimates), undertaken in a variety of contexts, including health care facilities (41–45), homes (56), churches (57), fitness facilities (58), call centers (59), airplanes (60), and company conferences and tour groups (61), are in agreement: contact and droplets were the probable modes of transmission. Rather than long-range propagation and frequent mass outbreaks typical of airborne patterns, the distribution of infected individuals was strongly correlated with close encounters and secondary attack rates were estimated be very low, around 5% (62). Rather than high R0 estimates typical of airborne viral pathogens such as chickenpox (5 to 11) (63) and measles (6 to 27) (64), community reproduction numbers fell between 2 and 4 (65, 66) and were easily lowered by droplet and contact precautions (67). Moreover, the WHO’s large-scale epidemiological analysis of 75,465 COVID-19 patients did not confirm any cases of long-range airborne transmission (68).
In health care settings, the use of medical masks appears to be sufficiently protective of HCWs exposed to COVID-19 patients, as mentioned previously. Several epidemiological reports from hospitals around the world even show little or no nosocomial transmission in the absence of recommended PPE (i.e., no N95s or masks during AGPs or improper mask use during close contact). Combining the findings of six studies, out of a cumulative total of 295 HCWs exposed to COVID-19 patients without proper protection, only 5 HCWs were infected. All five workers either did not wear any mask or used a mask intermittently during an AGP or prolonged exposure (>60 min) (69–74). These low levels of transmission from nonisolated COVID-19 patients to nonequipped HCWs are not suggestive of significant airborne transmission and support the effectiveness of basic IPC measures beyond PPE.
Nonetheless, some epidemiological evidence is compatible with short-range airborne transmission. The Washington choir outbreak is known for linking aerosolization from loud vocalization (i.e., singing) to rapid spread; however, the index case was symptomatic rather than asymptomatic as reported by the media (75), and multiple opportunities for droplet or fomite transmission were revealed in the published investigation (76). In turn, the well-known outbreak at the Guangzhou restaurant has been the subject of controversy: based on epidemiological data, one research team determined that droplets, expelled further than usual by air conditioning, were the probable source of transmission from an index patient to two neighboring tables (2); a second team, based on computer modeling and a tracer gas (a surrogate for exhaled particles), ruled in favor of airborne transmission (preprint article [77]). Moreover, a recently published study analyzed an outbreak involving two groups who rode separate buses to attend a 128-participant worship event (78). While no transmission occurred on bus 1, 23 passengers on bus 2 were infected, some of whom were sitting up to 5 m away from the index case. Seven other participants who did not ride on the buses were infected, all of whom reported close contact with the index case during the outdoor event. Since proximity to source was not correlated with infection risk in the bus, but window and door seats seemed to be protective, the researchers hypothesized that bus 2’s closed environment and air recirculation enabled airborne transmission to occur.
Furthermore, the widely studied Diamond Princess cruise ship outbreak is still up for debate. Based on epidemiological data showing exclusive in-room transmission following imposed quarantine, as well as no correlation between infection patterns and central ventilation system, one research team concluded that close contacts and fomites were the main transmission routes (preprint article [79]). In support of this view, an environmental study failed to detect any virus in air samples despite widespread positive surface sampling; however, passengers had disembarked at the time of sampling (80). Conversely, a modelization study simulating the cruise ship outbreak found that the epidemic models which best predicted the empirical data suggested predominant short-range and long-range airborne transmission (preprint article [81]).
Finally, two studies (82, 83) analyzed the impacts of public health policies on the epidemiological curves of highly impacted regions: the first compared Wuhan, Italy, and New York City (NYC) while the second compared 15 U.S. states. According to the authors, mask-wearing but not social distancing (quarantine, stay-at-home, and lockdown) policies were effective in curtailing COVID-19 outbreaks, suggesting that the main route of transmission is airborne rather than contact and droplets. However, the studies have come under criticism for not accounting for major confounding biases, such as differences between the three regions in terms of timing of lockdown (at >9,000 confirmed cases in Italy and NYC [84, 85] compared to 495 confirmed cases in Wuhan [86]), public health policy (e.g., contact tracing efficiency, testing criteria, and access), and population demographics (87). In addition, using the date of government-mandated mask-wearing as the start point for regression slopes is misleading, since the impacts of any new policy on epidemiological curves are delayed and nonlinear, especially given uneven compliance to mask-wearing, typically around 50% in the United States. (88), but variable between states, compared to over 95% in Asia (89). If we further scrutinize NYC (as well as other states), it appears that the number of daily new cases, hospital admissions, and deaths started to fall before the mask-wearing order (84), thus warranting an alternative explanation for the decline, such as an increasing proportion of immune individuals or the adoption of more aggressive testing. Moreover, researchers could not explain why certain states managed to control their outbreaks without mask-wearing policies and others did not show a decline in new or cumulated cases after facemask adoption.
Beyond the airborne versus droplet debate, there is consensus among epidemiologists: prolonged short-range exposure is the main risk factor. Interestingly, the revised airborne model presented in the Conclusions: Proposed Model (below), involving inhalable aerosols, can accurately explain epidemiological observations as well as the dynamics of several contentious outbreaks.
SARS-CoV-1 Studies
Despite some caveats, SARS-CoV-1 studies may be useful to understand SARS-CoV-2, given that they share around 80% of their genomic sequence (66). A well-studied outbreak at Amoy Gardens in Hong Kong, a high-rise housing estate where >300 tenants were confirmed infected despite little contact between them, was studied by different teams (90, 91). The majority agree on airborne transmission of SARS-CoV-1, originating from the aerosolization of feces and urine through hydraulic action (i.e., toilet flushing) of an index patient who presented with diarrhea and high viral load in excrements. This particular outbreak involved primarily environmental and engineering factors such as unsealed floor drain traps, bathroom fans causing negative pressure, bathroom fixtures contributing to drain overload or backflow, and the specific configuration of the exhaust system, which contributed to drawing aerosolized sewer droplets from the plumbing system back into the bathrooms and spreading them throughout the building (92). The involvement of respiratory aerosols was not hypothesized.
More relevant to health care settings is a Hong Kong hospital outbreak study on medical students exposed to an index SARS patient: proximity with the patient was the main risk factor, but the duration of contact did not appear to be associated with transmission. The researchers conclude that the mode of transmission was probably through droplets and contact, but airborne transmission could not be excluded, especially given the presence of a potential AGP (30-min nebulizer therapy four times a day) (93). Furthermore, in a Canadian study, air samples were collected from 15 SARS patient rooms in low-risk and high-risk settings, as well as four adjacent nursing support areas: 2 of the 40 wet air samples and none of the 28 dry air samples were PCR positive (94). The two positive samples were both from the room of a single recovering SARS patient where AGPs did not appear to be performed. Subsequent viral culture; however, turned out negative.
As for protection devices, a case-control study in five Hong Kong hospitals showed no difference in infection rates between HCWs wearing a mask or a respirator, when exposed to SARS patients (95). Other observational studies (96–98) done in high-risk settings (including AGPs) suggest possible N95 superiority, but the studies either did not adequately compare the two equipment types or did not obtain statistically significant results.
Other lower levels of evidence for SARS-CoV-1 come to similar conclusions regarding PPE. No nosocomial transmission was found in HCWs from eight U.S. hospitals, despite several of them not wearing any masks and 5% of them being exposed to AGPs (99). Furthermore, no nosocomial transmission was found in Vietnamese HCWs exposed for 3 weeks to hospitalized cases, wearing only medical masks (100).
However, given the differences between SARS-CoV-1 and SARS-CoV-2 (e.g., peak viral load, asymptomatic transmission rates, and mortality rates), direct extrapolations from one virus to the other must be made with caution. Similarly to the current pandemic, the significance of airborne transmission for the previous SARS remains uncertain to this day, as the prerequisites (viral load, infectivity, and tropism) are not clearly met. Unfortunately, SARS-CoV-1 seems to suffer from the same lack of rigorous clinical trials as its contemporary cousin.
Air and No-Touch Surface Sampling
Data from air and no-touch surface sampling studies (Tables 4 and 5) conducted in COVID-19 patient rooms and health care facilities are often cited to support airborne transmission. Unfortunately, interstudy comparisons are complicated by the diversity of methodological approaches. For instance, positive air samples correlate with patient features (e.g., viral load and symptom intensity and duration), ventilation parameters, and cleaning procedures, but these elements are not always mentioned or detailed. Moreover, large variations are reported in terms of total volume of air collected (<100 liters to up to 10,000 liters), flow rates (3.5 to 300 liters/min), sampling duration, and technique (gelatin versus polycarbonate filtration, dry cyclonic sampling versus condensation sampling). Furthermore, the sampling of no-touch surfaces, defined as areas typically out of reach of human contact or droplets and therefore assumed to be contaminated by aerosols only, is often poorly described and not always comparable to air samples. Given that each design is associated with its own set of advantages and limitations (e.g., longer duration of air sampling may increase detection probability but decrease infectivity), there is no easy conclusion to be drawn when comparing studies.
TABLE 4.
Positive SARS-CoV-2 air and no-touch surface sampling studies in health care settingsa
| Study setting | Design | Proportion of positive samples | Strengths (+) and limitations (−) |
|---|---|---|---|
| Published studies with negative viral cultures | |||
| Santarpia et al., 2020 (
103)
Nebraska, USA Low- and high-risk |
13 pts in isolation units Air sampling: 50 liters/min for 15 min (750 liters) and personal air sampler (4 liters/min) on HCWs No-touch: air handling grates and window ledges |
Air: 12/19 in rooms, 7/12 in hallway, 4/4 on personal sampler No-touch: 4/5 grates, 16/22 ledges |
(+) Evaluation of long-range (e.g., hallway) and short-range (e.g., personal air sampler) (+) Sample positivity linked to onset of Sx (+) Viral loads measured (−) Unknown particle sizes (−) Sampler positions unknown or suboptimal (e.g., risk of contamination by particles resuspended from the floor) |
| Zhou et al., 2020 (104)
ondon, UK Low- and high-risk (including ICU) |
5 hospitals (including GW, ICU, emergency department) Air sampling: 3 or 4 samplers collecting 1 m3 in pt, staff, and public areas |
Air: 2/31 confirmed positive in cohort ward and acute admission unit; 14/31 suspected positive | (+) Viral loads measured (+) Sample positivity linked to ward type (GW>ICU) and distance from pt (−) Lack of clinical data on pts (−) Air sampling during AGPs (−) Unknown particle sizes (−) Unknown sampling flow rate and duration |
| Binder et al., 2020 (105)
California, USA Assumed low-risk |
20 hospitalized pts Air sampling: 3.5 liters/min for 4 h (840 liters) in in high- and low-risk areas |
Air: 3/195 from 3 different pt rooms (particle sizes, <4 μm and >4 μm) | (+) Aerosol sizes and viral loads measured (−) Lack of clinical data and risk level (−) UV light disinfection (false negative) (−) 50% of pts at day 8 or more of infection |
| Published studies without viral cultures | |||
| Chia et al., 2020 (193)
Singapore Low- and high-risk (including ICU) |
5 pts in AIIRs Air sampling: in 3 GW rooms, 6 samplers at 3.5 liters/min for 4 h (5,040 liters) No-touch: exhaust vents in 4 GW rooms and 1 ICU room |
Air: 2/3 (particle sizes, >4 μm and 1–4 μm) No-touch: 3/5 |
(+) Aerosol sizes and viral loads measured (+) Sample positivity linked to Sx (4/5 pts symptomatic on day of sampling), onset of illness (wk 1) and viral load (+) No AGPs during sampling |
| Liu et al., 2020 (194)
Wuhan, China Low- and high-risk (including ICU) |
Tertiary hospital (severe cases) and make-shift center (mild cases) Air sampling: various sampling duration at 5 liters/min in pt, staff and public areas No-touch: ICU room corner |
Air: 19/33 (particle sizes, 0.25–1.0 μm and >2.5 μm) No-touch: 2/2 |
(+) Aerosol sizes and viral loads measured (+) Sample positivity linked to ventilation (e.g., high loads in mobile toilet) (−) Lack of clinical data on individual pts (−) Floor sampling (risk of contamination by resuspension of settled particles) |
| Ding et al., 2020 (152)
Nanjing, China Unknown risk level |
10 pts in COVID-19 hospital Air sampling: various devices from 10 liters/min for 30 min to 500 liters/min for 20 min; EBC and exhaled air samples No-touch: toilet and roof exhausts |
Air: 1/46 in corridor; 0/2 EBC, 0/2 exhaled air No-touch: 1/1 toilet, 0/5 roof |
(+) Detailed description of pt data and environment (including air flows) (+) Specific data on exhaled breath (+) Viral loads measured (+) Hypothesis on fecal origin of aerosols (−) Unknown particle size |
| Guo et al., 2020 (144)
Wuhan, China Low- and high-risk (including ICU) |
15 ICU pts and 24 GW pts Air sampling: 300 liters/min for 30 min (9,000 liters) near and far from pts (e.g., corridor) No-touch: air outlets |
Air: 14/40 in ICU, 2/16 in GW No-touch: 8/12 in ICU, 1/12 in GW |
(+) Viral loads measured (+) Sample positivity inversely correlated with distance from pt: positive up to 4 m away (−) Unknown particle sizes |
| Ong et al., 2020 (146)
Singapore Assumed low-risk |
3 symptomatic pts in AIIRS Air sampling: 5 liters/min for 4 h and 6 m3/h for 15 min (1,200–1,500 liters) No-touch: air outlet fan |
Air: 0/10 No-touch: 2/3 |
(+) Sample positivity correlated with clinical data and timing of cleaning (+) Viral loads measured (−) Air outlet fan close enough to coughing pt to be contaminated by droplets (195) |
| Ma et al., 2020 (153) Beijing, China Low- and high-risk (including ICU) |
Hospital and quarantine hotel Air sampling: 15 to 400 liters/min for 40 min (600–16,000 liters) Breath sampling from 49 pts: 300–500 μl of EBC No-touch: ventilation duct |
Air: 1/26 in unventilated hotel toilet EBC: 14/52 No-touch: 1/1 |
(+) Specific data on exhaled breath (+) Viral loads measured (+) Sample positivity linked to disease stage (−) Possible saliva contamination of EBC (196) (−) Possible contamination of ventilation duct (located beneath a pt bed) (−) Unknown particle sizes |
| Razzini et al., 2020 (197)
Milan, Italy High-risk (ICU) |
3 pts in a COVID-19 isolation ward Air sampling: 50 liters/min for 40 min (2 m3) in contaminated (pt) areas, semicontaminated and clean (staff) areas |
5 samples total: 100% positive in contaminated areas, 0% in semicontaminated and clean areas | (+) Viral loads measured (−) Unknown particle sizes (−) Lack of clinical data on pts (−) Unspecified no. of samples per area (−) High-risk setting only (2/3 pts intubated) |
| Kenarkoohi et al., 2020 (198)
Iran Low- and high-risk (including ICU) |
10–30 pts in different areas of a COVID-19 hospital Air sampling: 12 liters/min for 3 h (2,160 liters) in ICU, GW, and low-risk areas |
Air: 2/14 (in 2 ICU wards with 10 severely ill pts each) | (+) Viral loads measured (+) Detailed information on environment, pts and interventions (−) PM (particulate matter) sizes measured, but not viral aerosol sizes |
| Mouchtouri et al., 2020 (199)
Greece Low- and high-risk |
Hospital AIIR, long-term care isolation wards, nursing home Air sampling: 50 liters/min for 10 min (500 liters) |
Air: 1/12 (maskless hospitalized pt) No-touch: 1 (nursing home A/C filter) |
(+) Inclusion of long-term care facilities (−) Unknown particle concentration and sizes (−) Unknown total no. of pts and lack of clinical data on pts |
| Unpublished studies with positive viral cultures | |||
| Santarpia et al., 2020 (preprint) (106) Nebraska, USA Unknown risk level |
Air sampling: 6 samplers at 3.5 liters/min for 30 min (105 liters) | Air: 6/6 (particle size, <1 μm) | (+) Aerosol sizes and viral loads measured (+) Viral protein and RNA detection from culture (−) Absence of CT values (−) TCID50 value obtained in culture applied to initial air sample |
| Lednicky et al. (2020) (preprint) (107)
Florida, USA Low-risk |
2 pts in a designated COVID-19 ward Air sampling: 3 h |
Air: 4/4 | (+) Water vapor condensation sampling (+) Matching virus sequence with pt swab (−) Lack of symptom data on pts (−) Unknown flow rate of air sampling (−) Implausible viral loads (−) Unknown particle sizes |
AIIR, airborne infection isolation rooms; ICU, intensive care unit; GW, general ward; TCID50, 50% tissue culture infective dose; pt(s), patient(s); Sx, symptom(s); EBC, exhaled breath concentrate; IPC, infection prevention and control.
TABLE 5.
Negative SARS-CoV-2 air sampling studies in health care settingsa
| Study settings | Design | Proportion of positive samples | Strengths (+) and limitations (−) |
|---|---|---|---|
|
Cheng et al., 2020 (
145)
Hong Kong Low- and high-risk (including ICU) |
6 pts in AIIR Air sampling: 50 liters/min for 20 min (1,000 liters), 10 cm from pts’ chin under an umbrella (air shelter) |
Air: 0/6 | (+) Increased proportion of exhaled air sampled under the umbrella (+) Sampling with and without mask-wearing (+) Detailed clinical data on pts |
|
Faridi et al., 2020 (
200)
Iran Mostly high-risk (ICU) |
44 hospitalized pts Air sampling: 1.5 liters/min for 1 h (90 liters) in shared pt rooms |
Air: 0/10 | (+) Detailed information on environment and interventions (−) Lack of clinical data on individual pts (−) Small volume of air sampled |
|
Li et al., 2020 (
201)
Wuhan, China Low- and high-risk (including ICU) |
Designated COVID-19 hospital with 800 severe cases (20 in ICU) Air sampling: 80 liters/min for 30 min (2,400 liters) in 45 areas (low, medium, and high risk) |
Air: 0/135 | (+) Three replicate samples at each location on separate days (−) 4-time-daily air disinfection (false negative) (−) Qualitative reverse transcriptase PCR |
|
Wu et al., 2020 (
147)
Wuhan, China Low- and high-risk (including ICU) |
Designated COVID-19 hospital Sampling in moderate-risk (buffer room for doffing) and high-risk (pt room) areas |
Air: 0/44 | (−) No description of pts (−) Unknown air sampling method (−) Open windows and UV light disinfection (potential false negatives) |
AIIR, airborne infection isolation rooms; ICU, intensive care unit; pt(s), patient(s); Sx, symptom(s).
The majority of published and unpublished studies detected viral RNA in the air and on no-touch surfaces (Table 4), but some did not (Table 5). Unfortunately, few positive studies included viral cultures. The main limitations of these studies were the lack of information on particle sizes and concentrations, unknown or suboptimal air sampler location, unknown time interval between aerosol production and collection (air or surface), and possible false negatives (e.g., negative pressure, open windows, and insufficient sampling volume or duration). For the studies that calculated viral concentrations from the environmental samples, various protocols, target genes (e.g., ORF1ab/RdRp, E, N, and S), and chemistry detection technology, should caution against direct comparisons.
Most studies were carried out in both low- and high-risk areas, and frequently in intensive care units (ICUs) where AGPs commonly occur and ventilation is optimized. Many studies, however, did not specify the general risk level and did not indicate if AGPs were carried out during sampling. Therefore, positive air and no-touch surface samples could not be clearly associated with an emission source (i.e., natural aerosolization versus AGPs) or risk factors (e.g., ventilation rate). This makes the results hard to generalize to most low-risk health care settings, such as long-term-care facilities. Negative results from air sampling studies in home and commercial settings (80, 101), in the definite absence of AGPs, also add to the uncertainty. It is worth noting that when researchers modelized aerosol emission during normal breathing, the observed concentrations of airborne particles were low, frequently under the detection limit for most air sampling approaches (102). This could explain the negative results of many studies (Table 5).
Nonetheless, air and no-touch surface sampling studies support the presence of natural and/or intervention-generated aerosols in COVID-19 health care facilities. However, the infectivity of these aerosols and their significance as a transmission route, beyond the mere detection of viral particles, remain uncertain. Indeed, a better understanding of viral resistance to airborne stress is key to estimating infectious risk. Three published studies (103–105) included viral cultures from air samples, all of which were negative; however, the Santarpia et al. study (103) observed indirect signs of viral replication in two of their samples, including a mild cytopathic effect upon microscopic inspection after 3 to 4 days. On the other hand, in two unpublished studies, Santarpia et al. (106) and Lednicky et al. (107) succeeded in obtaining positive cultures. The former used innovative methods such as detection of viral RNA in supernatant and Western blotting to yield interesting results. However, data scrutiny is impeded by the absence of CT values in the manuscript. In turn, the latter study would benefit from a thorough peer review process given that its methodology is not clearly detailed, and total and culturable viral counts seem implausible, since they are orders of magnitude higher than previously reported in the literature. The use of a condensation-based air sampler could perhaps explain the unusual results.
The fact that few research teams have attempted to culture the virus, and many of those who have did not succeed, could imply that SARS-CoV-2 aerosols are scarce or weakly infectious. However, multiple other factors could be at play. Viral cultures must be done in biosafety level 3 facilities and are therefore not easily accessible to some research teams. Even when culturing is possible, viral shedding dynamics may be unpredictable or intermittent, leading to failed detection within the time frame of air sampling (108). Furthermore, the sampling process of aerosols, in itself, may induce substantial damage to viruses and alter their integrity and, consequently, their infectivity (109). Finally, current culture techniques may not be optimal for the low viral concentration found in air samples. Increased sensitivity could be achieved with a bioassay or alternative methods such as electron microscopy, detection of viral proteins, and RT-qPCR in culture lysis and supernatants (106).
Laboratory Generation of Aerosols
Lastly, studies involving the in vitro generation of SARS-CoV-2 aerosols with Jet Collison nebulizers have been widely cited in support of airborne transmission. Using this method, the well-known van Doremalen et al. letter measured infectious titers per liter of air in a simulated aerosolized environment and showed stability of the SARS-CoV-2 virus in aerosols for up to 3 h, with a half-life of 1.2 h (110). Another similar study made headlines because the aerosols produced were stable for up to 16 h (111).
As with all in vitro models for bioaerosols, while they provide precious information on virus properties in aerosol state, including relative stability (which seems to be high) and comparative viral behavior, it is uncertain whether the mechanically produced SARS-CoV-2 aerosols exhibit the same properties as naturally generated ones. Therefore, such experimental studies are generally considered of low applicability to clinical settings.
ARE LONG-TERM CARE FACILITY OUTBREAKS PROOF OF AIRBORNE TRANSMISSION?
Tragic outbreaks in long-term-care facilities (LTCs) have plagued many countries in Europe (112) and North America (113), with astonishing death tolls. Some facilities report 100% resident infection rates, high HCW infection rates, as well as faulty ventilation systems (114), triggering intense debate over potential airborne transmission.
While aerosols could have contributed in cases involving inadequate ventilation (115), other explanations are also conceivable. Some have justified the devastating statistics by pointing to higher viral loads (116) or longer infection periods (117) in the elderly, two phenomena likely attributable to the weakening of the immune system with age. Notwithstanding, LTCs are fundamentally vulnerable to COVID-19 because of an array of predisposing risk factors (118, 119).
Unlike the general adult population, COVID-infected residents in LTCs are not always capable of communicating their symptoms and frequently have atypical clinical presentations, such as diarrhea, delirium, or falls (120). On the other hand, between 50 and 75% (121, 122) of them are asymptomatic or presymptomatic at the time of their positive test. These geriatric features complicate and delay case detection. The typical patient profile also leads to poor compliance with infection prevention and control (IPC) practices: most residents have neurocognitive disorders and behavioral symptoms, but some also have mental health disorders or intellectual disability, which means isolation, mask-wearing, and hand hygiene are often impossible. Rates of resident noncompliance can reach almost 100% in certain special care units (e.g., wandering ward). Moreover, a majority of residents with severe loss of functional autonomy requiring several hours of proximity care per day (e.g., personal hygiene and bath, urinary and bowel elimination, feeding, and medication administration), means close and sustained contact between HCWs and infected patients (without source control for the most part) and consequently, higher infection risk on both sides (123).
Structural and administrative impediments also come into play. Some LTCs have high bed occupancy rates and tight physical spaces (e.g., shared bedrooms and bathrooms), where distancing becomes a challenge and cross-contamination an inevitability (124). With high population density and limited space, it is very difficult to efficiently segregate patients into zones according to infectious status, leading to mixed units and high infection rates. Moreover, some facilities have defective ventilation systems (115), while others have no mechanical ventilation at all, and must rely on opening windows for air exchange. Most importantly, many already understaffed LTCs were hard hit by pandemic-related absenteeism and had to resort to mobilizing staff between units and facilities or calling on lesser-trained external staff to fill in; this element exaggerated all the other risk factors because it hindered the detection and isolation of suspected cases, the deployment of COVID-19 units with dedicated staff, the optimal application of IPC practices, and the overall quality of care (125).
Unfortunately, despite LTCs being at the epicenter of many regions’ epidemic, data are still lacking. Studies on transmission modes specific to this geriatric subgroup, where various clinical, administrative, and environmental factors intersect, would be very revealing.
ARE THERE DISPARITIES BETWEEN DIFFERENT NATIONAL AND INTERNATIONAL COVID-19 GUIDELINES?
Most authorities agree with the WHO recommendations for droplet and contact precautions with COVID-19 patients. In the United Kingdom (126), Canada (127), France (128), Switzerland (30), Spain (129), Portugal (130), and Australia (131), medical masks are indicated in most situations and respirators are required only in high-risk settings involving AGPs. Recently, the WHO has acknowledged that “short-range aerosol transmission, particularly in specific indoor locations, such as crowded and inadequately ventilated spaces over a prolonged period of time with infected persons cannot be ruled out” but specifies that the significance of COVID-19 airborne transmission has not been convincingly demonstrated and requires further research (1).
While the European Society of Intensive Care Medicine and Society of Critical Care Medicine (132) is also in line with WHO PPE recommendations, the European Centre for Disease Prevention and Control began by recommending respirators at all times, but backtracked in recent updates and now states that both equipment types are appropriate outside of AGPs (133), in agreeance with the Infectious Diseases Society of America (IDSA) (134). On the other hand, the United States (135), South Korea (29), Singapore (136), and China (137) recommend respirators for routine care. The U.S. CDC states that HCWs should wear an N95, but a facemask is a suitable alternative if a respirator is not available.
In summary, most Western countries have adopted similar guidelines in line with WHO recommendations, but comparisons with countries in other parts of the world were not possible due to language barriers.
HOW DO WE EXPLAIN THAT SARS-CoV-2 SPREADS SO EASILY?
Surprising attack rates have been reported. Possible explanations include the high presymptomatic contagion of certain individuals (138), as well as the many asymptomatic or paucisymptomatic cases (139) who seem to have similar viral loads to their symptomatic counterparts (140). Furthermore, unlike SARS-CoV-1 which reached peak viral load (and therefore contagion) at day 7 to 10 from the start of symptoms (141), viral load seems to peak right before the advent of symptoms (108). Given these data, certain researchers estimate that 44% of transmission happens in the presymptomatic phase (108). Finally, nasopharyngeal viral load appears to be much (up to 1,000 times) higher than that of the first SARS (142). We are therefore faced with a very contagious virus that can silently infect a large number of people.
Moreover, another possible mode of transmission that remains to be elucidated is through fomites. Few studies look at SARS-CoV-2 survival on surfaces. A widely cited experiment showed that the virus could subsist between 4 h (on copper) and 72 h (on plastic) (110). However, the study took place under experimental conditions (laboratory surface inoculation, at a stable temperature of 21 to 23°C) which do not represent droplet deposition on surfaces in clinical contexts nor the variations of typical indoor environments. Nonetheless, the potentially prolonged stability of coronaviruses on surfaces (143), as well as the extensive environmental contamination reported by many surface sample studies in health care settings (108, 144–147), needs to be confirmed by future research, including viral cultures for infectivity.
Possible fecal transmission is also worth considering. A significant proportion of patients declare gastrointestinal symptoms before respiratory symptoms, and it is even a predominant form of presentation in some individuals (148). In addition, severe COVID-19 cases appear to have more gastrointestinal symptoms than mild or moderate cases (149). A meta-analysis of over 4,000 patients reported 48% PCR-positive stool samples, of which 70% remained positive even after nasopharyngeal PCR had turned negative (150). Endoscopic studies also found RNA in the esophagi, stomachs, duodena, and recta of patients with severe gastrointestinal symptoms (151). Finally, two studies showed the toilet was among the most contaminated areas in indoor settings (152, 153): interestingly, the patient who’s toilet air sample was positive had a negative exhaled breath sample, warranting the consideration that detectable airborne SARS-CoV-2 could originate from fecal rather than respiratory aerosols. As with air, a limited number of studies have been able to culture infectious viruses from stools (154, 155), supporting infectivity. In theory, fecal transmission could occur through different routes, including contact (e.g., while changing incontinence briefs), short-range aerosolization (i.e., inhalation), or long-range aerosolization due to toilet flushing (156). The latter was well established in the SARS-CoV-1 Amoy Gardens outbreak and was recently considered the main mode of transmission in a SARS-CoV-2 outbreak involving a high-rise building in China, where the nine infected cases lived in three vertically aligned flats connected by drainage pipes in the master bathrooms (157).
HOW DO WE EXPLAIN THE HIGH INFECTION RATE AMONG HCWs, DESPITE ADEQUATE PPE?
HCWs constitute a high-risk population for infection (158). However, the contribution of nosocomial transmission was perhaps overestimated at the beginning of the pandemic, since recent genome-sequencing studies have highlighted the importance of community-acquired infection among HCWs (159). For instance, with epidemiological and genomic data on 50 HCWs and 10 patients at hospitals in the Netherlands, researchers linked these infections with three different clusters, two of which showed local circulation in the community (160). Within each cluster, “identical or near-identical sequences in health care workers at the same hospital, and between patients and health care workers at the same hospital, were found, but no consistent link was noted among health care workers on the same ward or between health care workers and patients on the same ward.” The authors therefore concluded that the patterns observed were consistent with multiple introductions into the hospitals through community-acquired infections. Similarly, studies are pointing to community transmission dynamics and public policies (e.g., universal mask-wearing) as the main drivers of HCWs infection (161–163).
Nonetheless, given that HCWs can both infect patients and get infected from patients, workplace practices deserve a closer look. In the presence of a contagious virus and extensive environmental contamination in health care settings, any breach in protection, as small as it may be, can lead to infection. HCWs who work regularly with COVID-19 patients, especially those in close contact (e.g., patient attendants, nurse aides) can hardly maintain constant and perfect compliance with IPC practices. Besides, risk exposure not only occurs with patients during PPE violations but also with other staff members in shared areas without PPE (e.g., cafeterias and changing rooms).
Unfortunately, few studies looked at PPE compliance during the COVID-19 pandemic: one study reported very poor adherence to mask recommendations due to lack of use (almost 30%) or improper use (164). Before the pandemic, cornerstone practices such as hand hygiene were already poorly applied according to several studies in a variety of hospital departments (including ICUs) across different countries (165–167). A drastic change in a short lapse of time appears improbable, especially in long-term-care facilities where the culture and philosophy are one of “home setting” rather than health care setting. Moreover, HCWs appear to have a false perception of their own compliance with hygiene practices: a MERS-CoV study showed an absence of correlation between staff’s self-assessment and their observed behavior (168). The researchers mention that most HCWs understood the importance of hand hygiene but did not consistently apply it.
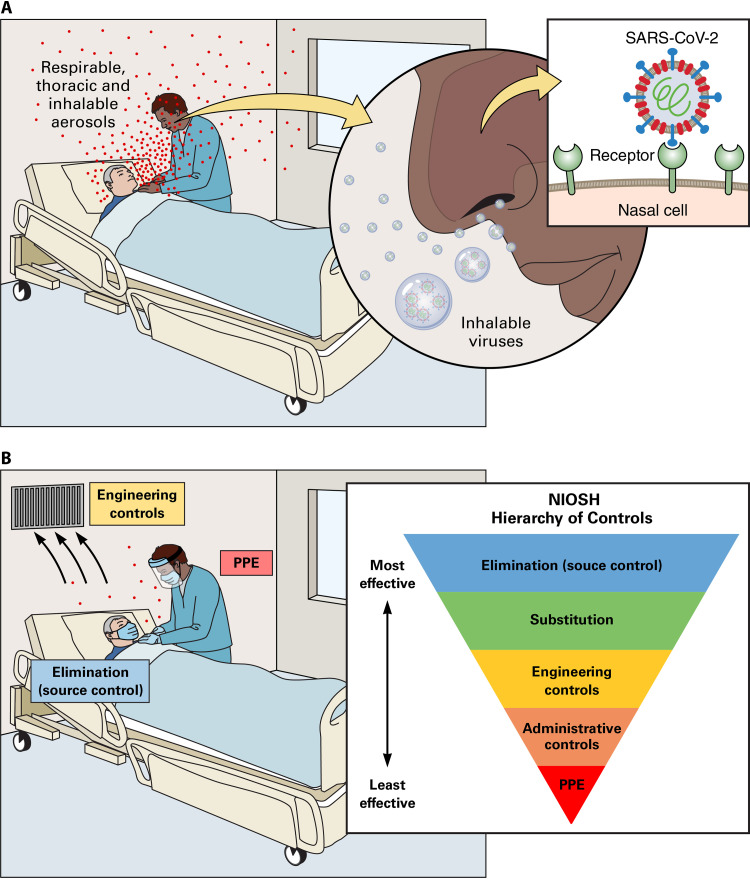
Even so, proper PPE use does not only depend on individual compliance and technique; it is a multidimensional issue with organizational, systemic, and political ramifications (169). More importantly, PPE is neither the only nor the best way to protect HCWs. In fact, when it comes to protection from occupational hazards, PPE is the last and least effective measure in the NIOSH hierarchy of controls (170) (see Fig. 1B). For the current pandemic and future ones, our priority should therefore be elimination strategies (e.g., decreasing bed occupancy rates, source control), engineering controls (e.g., segregated red zones and proper ventilation), and administrative controls (e.g., dedicated staff, adequate training, and strict enforcement of IPC regulations), ending with PPE (29). Unfortunately, we have seen, around the globe, many health care systems fail to meet the structural, human, and material challenges brought on by COVID-19, and some HCWs have paid the price for our collective unpreparedness.
One final potential source for HCW infection could be the combination of risk factors for aerosol accumulation in certain exceptional circumstances, such as an overcrowded and underventilated long-term-care facility (115), or makeshift hospitals such as we have seen built around the world (171). While the vast majority of home and hospital environments are probably safe (172), some care homes are located in old substandard infrastructure which relies on natural ventilation and does not allow for optimization of air exchange. It is plausible that under these specific conditions, normally minimal levels of infectious respirable aerosols could reach a threshold where classic airborne transmission becomes significant. While we wait for future research to confirm this scenario, we must strive to control what we can, eliminating physical, environmental, and administrative risk factors to protect frontline workers (173).
IN THE FACE OF UNCERTAINTY, SHOULD WE APPLY THE PRECAUTIONARY PRINCIPLE?
Drawing the line between precaution and excess is a fundamentally subjective process. Many experts agree that current droplet and contact precautions are adequate in low-risk settings. However, some prefer to exercise precaution by recommending respiratory protection with critically ill patients (e.g., severe desaturation or tachypnea), arguing that these clinical features predict progression to AGPs such as intubation (174). Others consider that the minimum precautionary practice is universal N95 use. Finally, some argue that only drastic measures such as full head hoods and full-body suits, often seen in China, are sufficiently protective.
In the presence of diverging opinions on the definition of so-called precaution, it seems reasonable to use an evidence-based approach to PPE recommendations. The bulk of evidence, until now, indicates that the medical mask is protective in low-risk settings and the respirator is required only for AGPs, although higher levels of evidence in the future may tip the balance the other way. Long-term care facilities, where the risk level may at times be considered high despite the absence of AGPs, deserve special attention from researchers.
Lastly, one could argue that our collective but rather limited energy, time, and resources should be invested in the most impactful areas: proven practices that achieve broad consensus and transmission routes that appear to be predominant. For SARS-CoV-2, long-winded debates on the gray zones and the applicability of the precautionary principle sometimes distract from crucial measures, such as hand hygiene, source control, and optimal ventilation (175), which are uncontroversial and highly effective, yet still unevenly applied in some settings such as long-term-care facilities. We are in favor of a return to core IPC principles, which should dominate the scientific conversation around COVID-19 management.
Beyond the alarming statistics, several success stories around the world prove that much can be achieved quickly and efficiently with basic yet effective practices (45, 176–178), without the need to resort to elaborate theories or equipment.
DISCUSSION
This article is an in-depth literature overview attempting to answer frequently asked questions about droplet and airborne transmission. Although not a systematic review, it goes deeper than current narrative reviews and has important implications for IPC practices, HCW protection, and future research.
However, there are several limitations. The first is the controversial distinction between droplets and aerosols, still commonly used in much of the scientific literature, although deemed arbitrary and inaccurate by many experts. Natural generation of particles belonging to a broad range of size, containing various concentrations of infectious agents, is probably concurrent rather than mutually exclusive, and transmission patterns are likely on a continuum rather than dichotomous. Our proposed model addresses this issue. Going forward, we are in favor of adapting public health policies and PPE recommendations to include a broader industrial hygiene-inspired definition of aerosols, as presented above, in order to lessen confusion and better represent the nuanced and complex reality of SARS-CoV-2 transmission.
The second major limitation is the lack of clinical studies on SARS-CoV-2 transmission and PPE effectiveness, meaning that many conclusions are drawn from lower levels of evidence, extrapolations from other viruses, and laboratory and experimental studies. The available literature, however, is mostly consistent: while airborne transmission exists under certain conditions, there is limited direct evidence of it, especially in low-risk health care settings. Given the very high viral load typical of SARS-CoV-2 infections, it is surprising that, after several months of pandemic, many air samples turn out negative or weakly positive, and subsequent positive cultures remain scarce. This may be attributed to the many logistical and technical limitations associated with air sampling and viral cultures, as mentioned previously, which could underestimate airborne infectivity. We must therefore rely primarily on clinical evidence (trials on masks and epidemiological studies) to study transmission; for now, it suggests that the classic airborne route is not significant. A broader airborne model, involving the short-range inhalation route, could better explain current observations.
Third, only a few national and international guidelines are compared because of the lack of translated documents. A thorough search of guidelines from comparable countries across different continents would allow for an unbiased comparison but is very challenging in practice.
CONCLUSIONS: PROPOSED MODEL
While impatiently waiting for future studies, especially clinical trials, to dispel remaining uncertainties and provide definitive answers to the questions raised here, we would like to propose a revised model for SARS-CoV-2 transmission, involving inhalable aerosols and favorable conditions for airborne transmission (Fig. 1).
FIG 1.
A broader airborne model involving inhalable aerosols for SARS-CoV-2 transmission in low-risk health care settings. (A) Worst-case scenario: no protection on either the sick patient (source) or the health care worker (exposure), emission of particles of various sizes (droplets and aerosols) during natural respiratory activity (breathing, talking, and coughing), entry of infectious inhalable aerosols, and impaction in the nose where viral receptors are abundant and infectivity is greatest. (B) Best-case scenario and NIOSH hierarchy of controls: source control (mask-wearing by the sick patient), engineering control (optimal ventilation), and exposure control (droplet-contact PPE worn by the health care worker) to prevent short-range droplet and inhalable aerosol transmission.
The premises of this model are based on cumulative data and clinical observations. In light of the positive air and no-touch surface samples found in health care facilities, respiratory SARS-CoV-2 aerosols probably occur, but many of their attributes are yet unknown; studies thus far seem to suggest these aerosols are short-range and dilute with distance (102, 103, 144). Similarly, epidemiological studies do not support the existence of long-distance aerosol propagation: the four outbreaks most often cited as evidence of airborne transmission (the Washington choir, the Guangzhou restaurant, the Eastern Chinese bus riders, and the Diamond Princess cruise ship) all involved individuals who were in relatively close contact for a prolonged period of time, in an enclosed space, with the presence of enabling factors (e.g., crowdedness, air currents, and poor ventilation). Indeed, these conditions seem necessary for respiratory airborne transmission to occur. Fecal aerosols, on the other hand, may be more common due to toilet flushing, but further studies are needed to clarify their role and distinguish them from respiratory aerosols.
Moreover, to solve the mystery of particle size, we must first acknowledge that airborne transmission is not exclusive to small aerosols: some larger particles typically classified as droplets may remain airborne, especially if suboptimal airflows contribute to their preservation in suspension and reduce their dilution (179). Thus, inhalable aerosols are the ideal candidate to explain current findings, because they exhibit shorter travel distance and air suspension time than respirable aerosols while having greater potential for infection because of their higher probability of containing virions (16). Furthermore, because inhalable aerosols are larger, they are more likely to deposit proximally in upper airways compared to respirable aerosols (180), which is in line with the robust data suggesting that nasal cells are the main portal for initial infection, with a gradient of infectivity from the proximal (nose) to the distal (lungs) respiratory tract (21, 22).
Therefore, transmission of short-range airborne and inhalable aerosols could explain the seemingly contradictory finding that there are viruses in the air and transmission between individuals without contact, but lack of convincing clinical evidence of classic airborne transmission (i.e., long-distance ranges and superiority of respirators). This size range could exhibit behaviors typical of both droplets and aerosols: higher viral load, airborne behavior, inhalation, and deposition in the nose. Despite relatively shorter suspension time, inhalable aerosols become especially significant in the case of prolonged exposure and close proximity. In addition, they are less likely to follow air streams through leaks in the nonfitted mask, nor make it down to alveolar space, because of larger size, but rather will remain in nasal cells due to natural impaction processes. Consequently, tight seals and superior filtration would not be required in most low-risk settings, as masks (with the help of face shields) could readily block these airborne particles. However, different categories of HCWs may not be exposed to the same level of risk: an attendant who spends an hour feeding, bathing and positioning a patient will be at much higher risk of inhaling aerosols compared to a doctor who questions and examines a patient for 10 min. Finally, ventilation parameters (air exchange rate, flow direction, and airflow patterns) would play a role, since they could contribute to enhancing or reducing airborne suspension and transmission (181).
This model is difficult to assess given the short-range distance and the short airborne stability, as well as the alteration of particle size during most air sampling processes (desiccation and impaction in liquid). However, we believe this novel paradigm, which departs from the outdated aerosol/droplet dichotomy, more accurately portrays the reality of naturally generated viral particles and the nuances in transmission patterns. Broadening the “airborne” definition to inhalable aerosol exposure in the context of proximity care, and considering inhalation as a significant route of entry for the SARS-CoV-2 virus, could open up new paths of exploration.
In summary, traditional droplets (larger particles with ballistic behavior that deposit onto surfaces), as well as our newly defined inhalable aerosols (particles that can be suspended, breathed in, and impacted at the nose, at the location of highest infectivity), could be the predominant modes of transmission of SARS-CoV-2. Classic respirable aerosols, even if present, seem unlikely to be significant in routine health care contexts, possibly due to insufficient quantity, inactivation during aerosolization or shortly after, inability to colonize lower airways, and/or adequate blockage by masks (especially in combination with face shields). In the light of this revised model and the cumulative data analyzed in this review, in line with several major countries’ guidelines, current droplet and contact precautions appear to adequately protect HCWs in most non-AGP settings, with perhaps the exception of proximity caretakers in poorly ventilated settings. Nonetheless, since the health and lives of HCWs depend on our better understanding of SARS-CoV-2, further investigation into all possible routes of transmission—whether airborne, fomite, or fecal—is warranted, especially in settings where they could synergistically contribute to outbreaks (e.g., long-term-care facilities).
ACKNOWLEDGMENTS
We thank Magali-Wen St-Germain for the original design, creation, and development of Fig. 1, as well as Patrick Lane, ScEYEnce Studios, for the final version of Fig. 1. We thank Stéphanie Langevin, Quoc Dinh Nguyen, Luc Trudel, and Jean Barbeau for their contribution in reviewing the original manuscript.
Both authors substantially contributed to the conception, design, analysis, and interpretation of data, as well as reviewing and approving the final version of the manuscript. We agree to be accountable for the contents.
C.D. is holder of Tier-1 Canada Research Chair on Bioaerosols. For this review article, we received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We declare that we do not have commercial or financial relationships that could, in any way, lead to a potential conflict of interest with regard to this publication.
Biographies

Sophie Zhang, M.D., received her medical doctorate from the University of Montreal in 2012. She is a general practitioner who specializes in geriatrics, family medicine, and addiction medicine. She is co-chief of long-term care facilities at CIUSSS Centre-Sud-de-l’Île-de-Montréal and head physician of two care homes, CHSLD Bruchési and CHSLD Jean De La Lande. She is also a clinical educator, teaching residents and students at long-term care facilities, the GMF-U Faubourgs family medicine teaching unit, and the addiction clinic Centre de Recherche et d’Aide aux Narcomanes. This year, she hopes to finish her master’s thesis in Epidemiology at the University of Montreal. During the COVID-19 pandemic, at the head of 15 long-term care facilities, many of which had high attack rates and mortality rates, she helped manage several outbreaks totaling more than 1,000 confirmed COVID-19 cases.

Caroline Duchaine, Ph.D., completed doctoral studies at Université Laval in 1996 and postdoctoral training at University of Montreal and University of Iowa. She holds the Tier-1 Canada Research Chair on Bioaerosols and, since 2000, she is a professor at the Department of Biochemistry, Microbiology, and Bioinformatics at Université Laval and Researcher at the Quebec Heart and Lung Institute (CRIUCPQ). She has been involved in the study of bioaerosols since 1993. Her lab is a leader in aerovirology, microbiota analyses, in vitro bioaerosols, industrial hygiene, and agricultural health and has developed virus models to study viral aerosols, as well as numerous aerosol chamber systems. She has mentored over 110 trainees and led over 105 research projects, funded by national and international agencies. She has published 154 peer-reviewed manuscripts, 25 reports, and 320 abstracts and given 60 invited lectures on bioaerosols. She is currently co-leading projects on COVID-19 bioaerosols, including patient bedside sampling and N95 reuse protocols.
Note Added after Publication
After original publication of this paper, changes were required and have been made in this version of the article. In the last sentence of the second paragraph of the “Epidemiological Studies on Transmission” subsection on page 9, “basic PCI measures beyond PPE” has been changed to “basic IPC measures beyond PPE.” In addition, the author list and page range for reference 99 have been corrected.
Footnotes
[This article was published on 28 October 2020 but required additional changes, now reflected in the Note Added after Publication on p. 23. The changes to the article were made on 7 January 2021.]
REFERENCES
- 1.World Health Organization. 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed 11 July 2020. [Google Scholar]
- 2.Lu J, Gu J, Li K, Xu C, Su W, Lai Z, Zhou D, Yu C, Xu B, Yang Z. 2020. COVID-19 Outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis 26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourouiba L. 2020. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA 323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Qi Y, Luzzatto-Fegiz P, Cui Y, Zhu Y. 2020. COVID-19: effects of environmental conditions on the propagation of respiratory droplets. Nano Lett doi: 10.1021/acs.nanolett.0c03331. [DOI] [PubMed] [Google Scholar]
- 5.Tellier R, Li Y, Cowling BJ, Tang JW. 2019. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Organization for Standardization. 1995. Air quality: Particle size fraction definitions for health-related sampling. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 7.Milton DK. 2020. A Rosetta stone for understanding infectious drops and aerosols. J Pediatr Infect Dis Soc 9:413–415. doi: 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. 2012. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC NIOSH. 1996. NIOSH guide to the selection and use of particulate respirators. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/niosh/docs/96-101/default.html. Accessed 1 July 2020. [Google Scholar]
- 10.Anfinrud P, Stadnytskyi V, Bax CE, Bax A. 2020. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med 382:2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao C, Wan M, Morawska L, Johnson G, Ristovski Z, Hargreaves M, Mengersen K, Corbett S, Li Y, Xie X, Katoshevski D. 2009. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci 40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, Chao CYH, Li Y, Katoshevski D. 2009. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci 40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabian P, Brain J, Houseman EA, Gern J, Milton DK. 2011. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv 24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. 2019. Aerosol emission and superemission during human speech increase with voice loudness. Nature 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes MGM, Corder RM, King JG, Langwig KE, Souto-Maior C, Carneiro J, Goncalves G, Penha-Goncalves C, Ferreira MU, Aguas R. 2020. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv doi: 10.1101/2020.04.27.20081893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadnytskyi V, Bax CE, Bax A, Anfinrud P. 2020. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A 117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riediker M, Tsai DH. 2020. Estimation of viral aerosol emissions from simulated individuals with asymptomatic to moderate coronavirus disease 2019. JAMA Netw Open 3:e2013807. doi: 10.1001/jamanetworkopen.2020.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralton J, Tovey E, McLaws M-L, Rawlinson WD. 2011. The role of particle size in aerosolized pathogen transmission: a review. J Infect 62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stelzer-Braid S, Oliver BG, Blazey AJ, Argent E, Newsome TP, Rawlinson WD, Tovey ER. 2009. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol 81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Grantham M, Pantelic J, de Mesquita PJB, Albert B, Liu F, Ehrman S, Milton DK, EMIT Consortium. 2018. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci U S A 115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network. 2020. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS. 2020. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182:429–446. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. 2020. Medical masks versus N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses 14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qaseem A, Etxeandia-Ikobaltzeta I, Yost J, Miller MC, Abraham GM, Obley AJ, Forciea MA, Jokela JA, Humphrey LL. 2020. Use of N95, surgical, and cloth masks to prevent COVID-19 in health care and community settings: living practice points from the American College of Physicians (version 1). Ann Int Med doi: 10.7326/M20-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou R, Dana T, Jungbauer R, Weeks C, McDonagh MS. 2020. Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings. Ann Int Med doi: 10.7326/M20-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long Y, Hu T, Liu L, Chen R, Guo Q, Yang L, Cheng Y, Huang J, Du L. 2020. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med 13:93–101. doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ippolito M, Vitale F, Accurso G, Iozzo P, Gregoretti C, Giarratano A, Cortegiani A. 2020. Medical masks and respirators for the protection of healthcare workers from SARS-CoV-2 and other viruses. Pulmonology 26:204–212. doi: 10.1016/j.pulmoe.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boškoski I, Gallo C, Wallace MB, Costamagna G. 2020. COVID-19 pandemic and personal protective equipment shortage: protective efficacy comparing masks and scientific methods for respirator reuse. Gastrointest Endosc doi: 10.1016/j.gie.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH. 2020. Personal protective equipment for healthcare workers during the COVID-19 pandemic. Infect Chemother 52:165–182. doi: 10.3947/ic.2020.52.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommerstein R, Fux CA, Vuichard-Gysin D, Abbas M, Marschall J, Balmelli C, Troillet N, Harbarth S, Schlegel M, Widmer A, Swissnoso. 2020. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control 9:100. doi: 10.1186/s13756-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith BP, Agostini G, Mitchell JC. 2020. A scoping review of surgical masks and N95 filtering facepiece respirators: learning from the past to guide the future of dentistry. Saf Sci 131:104920. doi: 10.1016/j.ssci.2020.104920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIntyre CR, Chughtai AA. 2020. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud 108:103629. doi: 10.1016/j.ijnurstu.2020.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iannone P, Castellini G, Coclite D, Napoletano A, Fauci AJ, Iacorossi L, D’Angelo D, Renzi C, La Torre G, Mastroianni CM, Gianola S. 2020. The need of health policy perspective to protect Healthcare Workers during COVID-19 pandemic. A GRADE rapid review on the N95 respirators effectiveness. PLoS One 15:e0234025. doi: 10.1371/journal.pone.0234025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia Godoy LR, Jones AE, Anderson TN, Fisher CL, Seeley KML, Beeson EA, Zane HK, Peterson JW, Sullivan PD. 2020. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health 5:e002553. doi: 10.1136/bmjgh-2020-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacIntyre CR, Wang Q, Seale H, Yang P, Shi W, Gao Z, Rahman B, Zhang Y, Wang X, Newall AT, Heywood A, Dwyer DE. 2013. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med 187:960–966. doi: 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- 36.MacIntyre CR, Wang Q, Cauchemez S, Seale H, Dwyer DE, Yang P, Shi W, Gao Z, Pang X, Zhang Y, Wang X, Duan W, Rahman B, Ferguson N. 2011. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses 5:170–179. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radonovich LJ, Simberkoff MS, Bessesen MT, Brown AC, Cummings DAT, Gaydos CA, Los JG, Krosche AE, Gibert CL, Gorse GJ, Nyquist A-C, Reich NG, Rodriguez-Barradas MC, Price CS, Perl TM, ResPECT investigators. 2019. N95 respirators versus medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA 322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeb M, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, Webby R, Smieja M, Earn DJD, Chong S, Webb A, Walter SD. 2009. Surgical mask versus N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 39.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, on behalf of the COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. 2020. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Pan Z, Cheng Z. 2020. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect 105:104–105. doi: 10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bays DJ, Nguyen MH, Cohen SH, Waldman S, Martin CS, Thompson GR, Sandrock C, Tourtellotte J, Pugashetti JV, Phan C, Nguyen HH, Warner GY, Penn BH. 2020. Investigation of nosocomial SARS-CoV-2 transmission from two patients to health care workers identifies close contact but not airborne transmission events. Infect Control Hosp Epidemiol doi: 10.1017/ice.2020.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik T. 2020. COVID-19 and the efficacy of different types of respiratory protective equipment used by health care providers in a health care setting. Cureus 12:e7621. doi: 10.7759/cureus.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng K, Poon BH, Kiat Puar TH, Shan Quah JL, Loh WJ, Wong YJ, Tan TY, Raghuram J. 2020. COVID-19 and the risk to health care workers: a case report. Ann Intern Med 172:766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong SCY, Kwong RTS, Wu TC, Chan JWM, Chu MY, Lee SY, Wong HY, Lung DC. 2020. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 105:119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durante-Mangoni E, Andini R, Bertolino L, Mele F, Bernardo M, Grimaldi M, Cuomo N, Tiberio C, Falco E, Di Spirito A, Raffone M, Russo M, Atripaldi L, Zampino R. 2020. Low rate of severe acute respiratory syndrome coronavirus 2 spread among health-care personnel using ordinary personal protection equipment in a medium-incidence setting. Clin Microbiol Infect doi: 10.1016/j.cmi.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canini L, Andréoletti L, Ferrari P, D’Angelo R, Blanchon T, Lemaitre M, Filleul L, Ferry JP, Desmaizieres M, Smadja S, Valleron AJ, Carrat F. 2010. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One 5:e13998. doi: 10.1371/journal.pone.0013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barasheed O, Almasri N, Badahdah A-M, Heron L, Taylor J, McPhee K, Ridda I, Haworth E, Dwyer DE, Rashid H, Booy R, Hajj Research Team. 2014. Pilot randomised controlled trial to test effectiveness of facemasks in preventing influenza-like illness transmission among Australian Hajj pilgrims in 2011. Infect Disord Drug Targets 14:110–116. doi: 10.2174/1871526514666141021112855. [DOI] [PubMed] [Google Scholar]
- 48.MacIntyre CR, Zhang Y, Chughtai AA, Seale H, Zhang D, Chu Y, Zhang H, Rahman B, Wang Q. 2016. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open 6:e012330. doi: 10.1136/bmjopen-2016-012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hua W, Zuo Y, Wan R, Xiong L, Tang J, Zou L, Shu X, Li L. 2020. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermat 83:115–121. doi: 10.1111/cod.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radonovich LJ, Cheng J, Shenal BV, Hodgson M, Bender BS. 2009. Respirator tolerance in health care workers. JAMA 301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- 51.Lim SM, Cha WC, Chae MK, Jo IJ. 2015. Contamination during doffing of personal protective equipment by healthcare providers. Clin Exp Emerg Med 2:162–167. doi: 10.15441/ceem.15.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Guio DA, Ricardo-Zapata A, Ospina-Velez J, Gómez-Candamil G, Mora-Martinez S, Rodriguez-Morales AJ. 2020. Cognitive load and performance of health care professionals in donning and doffing PPE before and after a simulation-based educational intervention and its implications during the COVID-19 pandemic for biosafety. Infez Med 28:111–117. [PubMed] [Google Scholar]
- 53.Noti JD, Lindsley WG, Blachere FM, Cao G, Kashon ML, Thewlis RE, McMillen CM, King WP, Szalajda JV, Beezhold DH. 2012. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis 54:1569–1577. doi: 10.1093/cid/cis237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Booth CM, Clayton M, Crook B, Gawn JM. 2013. Effectiveness of surgical masks against influenza bioaerosols. J Hosp Infect 84:22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. 2014. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg 11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M, Ghinai I, Jarashow MC, Lo J, McPherson TD, Rudman S, Scott S, Hall AJ, Fry AM, Rolfes MA. 2020. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January-February 2020. MMWR Morb Mortal Wkly Rep 69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yong SEF, Anderson DE, Wei WE, Pang J, Chia WN, Tan CW, Teoh YL, Rajendram P, Toh MPHS, Poh C, Koh VTJ, Lum J, Suhaimi NAM, Chia PY, Chen MIC, Vasoo S, Ong B, Leo YS, Wang L, Lee VJM. 2020. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis 20:809–815. doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jang S, Han SH, Rhee JY. 2020. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg Infect Dis 26:1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SY, Kim YM, Yi S, Lee S, Na BJ, Kim CB, Kim JI, Kim HS, Kim YB, Park Y, Huh IS, Kim HK, Yoon HJ, Jang H, Kim K, Chang Y, Kim I, Lee H, Gwack J, Kim SS, Kim M, Kweon S, Choe YJ, Park O, Park YJ, Jeong EK. 2020. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis 26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, He H, Cheng W, Liu Y, Sun Z, Chai C, Kong Q, Sun W, Zhang J, Guo S, Shi X, Wang J, Chen E, Chen Z. 2020. Potential transmission of SARS-CoV-2 on a flight from Singapore to Hangzhou, China: an epidemiological investigation. Travel Med Infect Dis doi: 10.1016/j.tmaid.2020.101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pung R, Chiew CJ, Young BE, Chin S, Chen MIC, Clapham HE, Cook AR, Maurer-Stroh S, Toh MPHS, Poh C, Low M, Lum J, Koh VTJ, Mak TM, Cui L, Lin RVTP, Heng D, Leo YS, Lye DC, Lee VJM, Singapore 2019 Novel Coronavirus Outbreak Research Team. 2020. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet 395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klompas M, Baker MA, Rhee C. 2020. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA 324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 63.Farrington CP, Unkel S, Anaya-Izquierdo K. 2013. Estimation of basic reproduction numbers: individual heterogeneity and robustness to perturbation of the contact function. Biostatistics 14:528–540. doi: 10.1093/biostatistics/kxs054. [DOI] [PubMed] [Google Scholar]
- 64.Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. 2017. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis 17:e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 65.He W, Yi GY, Zhu Y. 2020. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. J Med Virol 92:2543–2550. doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. 2020. Coronavirus disease 2019: COVID-19. Clin Microbiol Rev 33:e00028-20. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You C, Deng Y, Hu W, Sun J, Lin Q, Zhou F, Pang CH, Zhang Y, Chen Z, Zhou XH. 2020. Estimation of the time-varying reproduction number of COVID-19 outbreak in China. Int J Hyg Environ Health 228:113555. doi: 10.1016/j.ijheh.2020.113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health Organization. 2020. 02–24. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). World Health Organization, Geneva, Switzerland. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 23 May 2020. [Google Scholar]
- 69.Baker MA, Rhee C, Fiumara K, Bennett-Rizzo C, Tucker R, Williams SA, Wickner P, Beloff J, McGrath C, Poulton A, Klompas M. 2020. COVID-19 infections among HCWs exposed to a patient with a delayed diagnosis of COVID-19. Infect Control Hosp Epidemiol 41:1075–1076. doi: 10.1017/ice.2020.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basso T, Nordbø SA, Sundqvist E, Martinsen TC, Witsø E, Wik TS. 2020. Transmission of infection from non-isolated patients with COVID-19 to health care workers. J Hosp Infect doi: 10.1016/j.jhin.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canova V, Lederer Schläpfer H, Piso RJ, Droll A, Fenner L, Hoffmann T, Hoffmann M. 2020. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss Med Wkly 150:w20257. doi: 10.4414/smw.2020.20257. [DOI] [PubMed] [Google Scholar]
- 72.Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, Sridhar S, Chan JFW, Ho PL, Yuen KY. 2020. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol 41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, Fricchione MJ, Chugh RK, Walblay KA, Ahmed NS, Stoecker WC, Hasan NF, Burdsall DP, Reese HE, Wallace M, Wang C, Moeller D, Korpics J, Novosad SA, Benowitz I, Jacobs MW, Dasari VS, Patel MT, Kauerauf J, Charles EM, Ezike NO, Chu V, Midgley CM, Rolfes MA, Gerber SI, Lu X, Lindstrom S, Verani JR, Layden JE, Illinois COVID-19 Investigation Team. 2020. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, Magill S, Verani JR, Jain S, Acosta M, Epson E. 2020. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep 69:472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Read R. 2020. A choir decided to go ahead with rehearsal: now dozens of members have COVID-19 and two are dead. Los Angeles Times, Los Angeles, CA. https://www.latimes.com/world-nation/story/2020-03-29/coronavirus-choir-outbreak. [Google Scholar]
- 76.Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, Lynn J, Ball A, Narwal S, Russell S, Patrick D, Leibrand H. 2020. High SARS-CoV-2 attack rate following exposure at a choir practice: Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Qian H, Hang J, Chen X, Hong L, Liang P, Li J, Xiao S, Wei J, Liu L, Kang M. 2020. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. medRxiv doi: 10.1101/2020.04.16.20067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Y, Li C, Dong H, Wang Z, Martinez L, Sun Z, Handel A, Chen Z, Chen E, Ebell MH, Wang F, Yi B, Wang H, Wang X, Wang A, Chen B, Qi Y, Liang L, Li Y, Ling F, Chen J, Xu G. 2020. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA Intern Med doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu P, Qian H, Miao T, Yen H-l, Tan H, Cowling BJ, Li YJ. 2020. Transmission routes of Covid-19 virus in the Diamond Princess cruise ship. medRxiv doi: 10.1101/2020.04.09.20059113. [DOI] [Google Scholar]
- 80.Yamagishi T, Ohnishi M, Matsunaga N, Kakimoto K, Kamiya H, Okamoto K, Suzuki M, Gu Y, Sakaguchi M, Tajima T, Takaya S, Ohmagari N, Takeda M, Matsuyama S, Shirato K, Nao N, Hasegawa H, Kageyama T, Takayama I, Saito S, Wada K, Fujita R, Saito H, Okinaka K, Griffith M, Parry AE, Barnetson B, Leonard J, Wakita T. 2020. Environmental sampling for severe acute respiratory syndrome coronavirus 2 during a COVID-19 outbreak on the Diamond Princess cruise ship. J Infect Dis 222:1098–1102. doi: 10.1093/infdis/jiaa437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azimi P, Keshavarz Z, Cedeno Laurent JG, Stephens BR, Allen JG. 2020. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. medRxiv doi: 10.1101/2020.07.13.20153049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang RY, Li YX, Zhang AL, Wang Y, Molina MJ. 2020. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A 117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Zhang R, Zhao J, Molina MJ. 2020. Understanding transmission and intervention for the COVID-19 pandemic in the United States. Sci Total Environ 748:141560. doi: 10.1016/j.scitotenv.2020.141560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.NYC Health. 2020. COVID-19: data summary. NYC Health, New York, NY. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed 24 June 2020. [Google Scholar]
- 85.John Hopkins University Center for Systems Science and Engineering. 2020. COVID-19 dashboard. John Hopkins University, Baltimore, MD. https://coronavirus.jhu.edu/map.html. Accessed 24 June 2020. [Google Scholar]
- 86.Zhuang Z, Cao PH, Zhao S, Lou YJ. 2020. Estimation of local novel coronavirus (COVID-19) cases in Wuhan, China from off-site reported cases and population flow data from different sources. medRxiv doi: 10.1101/2020.03.02.20030080. [DOI] [Google Scholar]
- 87.Haber N, Larremore DB, Goodman SN, Grabowski MK, Wada N, Lessler J. 2020. Formal request for the retraction of Zhang et al., 2020. https://metrics.stanford.edu/PNAS%20retraction%20request%20LoE%20061820. Accessed 1 July 2020.
- 88.Huffington Post. 2020. HuffPost: Facemasks, May 14–16 2020: 1,000 US adult citizens. Huffington Post, New York, NY. https://big.assets.huffingtonpost.com/athena/files/2020/05/20/5ec58353c5b63a90e1d578fe.pdf. Accessed 24 June 2020. [Google Scholar]
- 89.Cheng VCC, Wong SC, Chuang VWM, So SYC, Chen JHK, Sridhar S, To KKW, Chan JFW, Hung IFN, Ho PL, Yuen KY. 2020. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect 81:107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Health Organization. 2003. 05–17. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). World Health Organization, Geneva, Switzerland. https://www.who.int/csr/sars/en/WHOconsensus.pdf. Accessed 23 May 2020. [Google Scholar]
- 91.Yu ITS, Li Y, Wong TW, Tam W, Chan AT, Lee JHW, Leung DYC, Ho T. 2004. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 92.McKinney KR, Gong YY, Lewis TG. 2006. Environmental transmission of SARS at Amoy Gardens. J Environ Health 68:26–30. [PubMed] [Google Scholar]
- 93.Wong TW, Lee CK, Tam W, Lau JTF, Yu TS, Lui SF, Chan PKS, Li Y, Bresee JS, Sung JJY, Parashar UD, Outbreak Study Group. 2004. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis 10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, Li Y, Spence M, Paton S, Henry B, Mederski B, White D, Low DE, McGeer A, Simor A, Vearncombe M, Downey J, Jamieson FB, Tang P, Plummer F. 2005. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis 191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seto WH, Tsang D, Yung RWH, Ching TY, Ng TK, Ho M, Ho LM, Peiris JSM. 2003. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T, Levie J, McQueen J, Smith S, Moss L, Smith A, Green K, Walter SD. 2004. SARS among critical care nurses, Toronto. Emerg Infect Dis 10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. 2006. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth 53:122–129. doi: 10.1007/BF03021815. [DOI] [PubMed] [Google Scholar]
- 98.Scales DC, Green K, Chan AK, Poutanen SM, Foster D, Nowak K, Raboud JM, Saskin R, Lapinsky SE, Stewart TE. 2003. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis 9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park BJ, Peck AJ, Kuehnert MJ, Newbern C, Smelser C, Comer JA, Jernigan D, McDonald LC. 2004. Lack of SARS transmission among healthcare workers, United States. Emerg Infect Dis 10:244–248. doi: 10.3201/eid1002.030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ha LD, Bloom SA, Hien NQ, Maloney SA, Mai LQ, Leitmeyer KC, Anh BH, Reynolds MG, Montgomery JM, Comer JA, Horby P, Plant AJ. 2004. Lack of SARS transmission among public hospital workers, Vietnam. Emerg Infect Dis 10:265–268. doi: 10.3201/eid1002.030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Döhla M, Wilbring G, Schulte B, Kümmerer BM, Diegmann C, Sib E, Richter E, Haag A, Engelhart S, Eis-Hübinger AM, Exner M, Streeck H, Schmithausen RM. 2020. SARS-CoV-2 in environmental samples of quarantined households. medRxiv doi: 10.1101/2020.05.28.20114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones RM. 2020. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. J Occup Environ Hyg 17:408–415. doi: 10.1080/15459624.2020.1784427. [DOI] [PubMed] [Google Scholar]
- 103.Santarpia JL, Rivera DN, Herrera VL, Morwitzer MJ, Creager HM, Santarpia GW, Crown KK, Brett-Major DM, Schnaubelt ER, Broadhurst MJ, Lawler JV, Reid SP, Lowe JJ. 2020. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep 10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou J, Otter JA, Price JR, Cimpeanu C, Garcia DM, Kinross J, Boshier PR, Mason S, Bolt F, Holmes AH, Barclay WS. 2020. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis doi: 10.1093/cid/ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Binder RA, Alarja NA, Robie ER, Kochek KE, Xiu L, Rocha-Melogno L, Abdelgadir A, Goli SV, Farrell AS, Coleman KK, Turner AL, Lautredou CC, Lednicky JA, Lee MJ, Polage CR, Simmons RA, Deshusses MA, Anderson BD, Gray GC. 2020. Environmental and aerosolized SARS-CoV-2 among hospitalized COVID-19 patients. J Infect Dis doi: 10.1093/infdis/jiaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santarpia JL, Herrera VL, Rivera DN, Ratnesar-Shumate S, Reid SP, Denton PW, Martens JWS, Fang Y, Conoan N, Callahan MV, Lawler JV, Brett-Major DM, Lowe JJ. 2020. The infectious nature of patient-generated SARS-CoV-2 aerosol. medRxiv doi: 10.1101/2020.07.13.20041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lednicky JA, Lauzardo M, Fan ZH, Jutla AS, Tilly TB, Gangwar M, Usmani M, Shankar SN, Mohamed K, Eiguren-Fernandez A, Stephenson CJ, Alam MM, Elbadry MA, Loeb JC, Subramaniam K, Waltzek TB, Cherabuddi K, Morris JG, Wu CY. 2020. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. medRxiv doi: 10.1101/2020.08.03.20167395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. 2020. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 109.Verreault D, Moineau S, Duchaine C. 2008. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev 72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. 2020. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fears AC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, Mirchandani D, Plante JA, Aguilar PV, Fernandez D, Nalca A, Totura A, Dyer D, Kearney B, Lackemeyer M, Bohannon JK, Johnson R, Garry RF, Reed DS, Roy CJ. 2020. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis 26:2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Orecchio-Egresitz H. 2020. As many as half of Europe’s COVID-19 deaths were people in long-term care facilities. Business Insider, Berlin, Germany. https://www.businessinsider.com/half-europes-covid-19-deaths-in-long-term-care-facilities-2020-4. [Google Scholar]
- 113.Stockman F, Richtel M, Ivory D, Smith M. 2020. “They’re death pits”: virus claims at least 7,000 lives in U.S. nursing homes. The New York Times, New York, NY. https://www.nytimes.com/2020/04/17/us/coronavirus-nursing-homes.html. [Google Scholar]
- 114.Gerbert T, Krishnan S. 2020. Investigators look into catastrophic outbreak that infected all residents of TMR seniors’ home, killing 70. CBC News, New York, NY. https://www.cbc.ca/news/canada/montreal/vigi-long-term-care-home-all-residents-infected-1.5569178. [Google Scholar]
- 115.de Man P, Paltansing S, Ong DSY, Vaessen N, van Nielen G, Koeleman JGM. 2020. Outbreak of COVID-19 in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis doi: 10.1093/cid/ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, Lung DC, Yip CCY, Cai JP, Chan JMC, Chik TSH, Lau DPL, Choi CYC, Chen LL, Chan WM, Chan KH, Ip JD, Ng ACK, Poon RWS, Luo CT, Cheng VCC, Chan JFW, Hung IFN, Chen Z, Chen H, Yuen KY. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. 2020. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang XS, Chabot J, Dousseau A. 2020. Guide COVID-19 pour les médecins en CHSLD. https://docs.google.com/document/d/1aqY84tYB0ZRyZkFVBr0a7VlNaeY7KenDJD9wJPI13LE/edit?usp=sharing. Accessed 1 July 2020.
- 119.Barnett ML, Grabowski DC. 2020. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum 1:e200369. doi: 10.1001/jamahealthforum.2020.0369. [DOI] [PubMed] [Google Scholar]
- 120.Godaert L, Proye E, Demoustier-Tampere D, Coulibaly PS, Hequet F, Dramé M. 2020. Clinical characteristics of older patients: the experience of a geriatric short-stay unit dedicated to patients with COVID-19 in France. J Infect 81:e93–e94. doi: 10.1016/j.jinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Chisty Z, Bell JM, Methner M, Harney J, Jacobs JR, Carlson CM, McLaughlin HP, Stone N, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Russell D, Hiatt B, Gant J, Duchin JS, Clark TA, Honein MA, Reddy SC, Jernigan JA, Public Health—Seattle & King County, CDC COVID-19 Investigation Team. 2020. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoxha A, Wyndham-Thomas C, Klamer F, Dubourg D, Vermeulen M, Hammami N, Cornelissen L. 2020. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Louie JK, Scott HM, DuBois A, Sturtz N, Lu W, Stoltey J, Masinde G, Cohen S, Sachdev D, Philip S, Bobba N, Aragon T. 2020. Lessons from mass-testing for COVID-19 in long-term care facilities for the elderly in San Francisco. Clin Infect Dis doi: 10.1093/cid/ciaa1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stall NM, Jones A, Brown KA, Rochon PA, Costa AP. 2020. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. Can Med Assoc J doi: 10.1503/cmaj.201197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Estabrooks CA, Straus S, Flood CM, Keefe J, Armstrong P, Donner G, Boscart V, Ducharme F, Silvius J, Wolfson M. 2020. Restoring trust: COVID-19 and the future of long-term care. Royal Society of Canada, Ottawa, Canada. https://rsc-src.ca/en/restoring-trust-covid-19-and-future-long-term-care. [Google Scholar]
- 126.NHS. 2020. Recommended PPE for healthcare workers by secondary care inpatient clinical setting, NHS and independent sector. National Health Service, London, United Kingdom. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881361/Table_1._Recommended_PPE_for_healthcare_workers_by_secondary_care_clinical_context.pdf. Accessed 11 July 2020. [Google Scholar]
- 127.Public Health Agency of Canada. 2020. 06–29. Coronavirus disease (COVID-19): for health professionals. Public Health Agency of Canada, Ottawa, Ontario, Canada. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals.html. Accessed 11 July 2020. [Google Scholar]
- 128.Lepelletier D, Grandbastien B, Romano-Bertrand S, Aho S, Chidiac C, Géhanno JF, Chauvin F, French Society for Hospital Hygiene and the High Council for Public Health. 2020. What face mask for what use in the context of COVID-19 pandemic? The French guidelines. J Hosp Infect 105:414–418. doi: 10.1016/j.jhin.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dirección General de Salud Pública Calidad e Innovación. 2020. 06–17. Prevención y control de la infección en el manejo de pacientes con COVID-19. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Documento_Control_Infeccion.pdf. Accessed 11 July 2020.
- 130.Peres D, Boléo-Tomé JP, Santos G. 2020. Respiratory and facial protection: current perspectives in the context of the COVID-19 pandemic. Acta Med Port 33:amp.14108. doi: 10.20344/amp.14108. [DOI] [PubMed] [Google Scholar]
- 131.Communicable Diseases Network Australia. 2020. National guidelines for public health units. Communicable Diseases Network Australia, Melbourne, Australia. https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-novel-coronavirus.htm. Accessed 11 July 2020. [Google Scholar]
- 132.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. 2020. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.ECDC. 2020. Infection prevention and control and preparedness for COVID-19 in healthcare settings, 4th update. ECDC, Solna, Sweden. https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings. Accessed 11 July 2020. [Google Scholar]
- 134.Lynch JB, Davitkov P, Anderson DJ, Bhimraj A, Cheng VC, Guzman-Cottrill J, Dhindsa J, Duggal A, Jain MK, Lee GM, Liang SY, McGeer A, Lavergne V, Murad MH, Mustafa RA, Morgan RL, Falck-Ytter Y, Sultan S. 2020. Infectious Diseases Society of America guidelines on infection prevention for health care personnel caring for patients with suspected or known COVID-19. Clin Infect Dis doi: 10.1093/cid/ciaa1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.CDC. 2020. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 11 July 2020. [Google Scholar]
- 136.Ong SWX, Coleman KK, Chia PY, Thoon KC, Pada S, Venkatachalam I, Fisher D, Tan YK, Tan BH, Ng OT, Ang BSP, Leo YS, Wong MSY, Marimuthu K. 2020. Transmission modes of severe acute respiratory syndrome coronavirus 2 and implications on infection control: a review. Singapore Med J doi: 10.11622/smedj.2020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Islam MS, Rahman KM, Sun Y, Qureshi MO, Abdi I, Chughtai AA, Seale H. 2020. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: a global analysis. Infect Control Hosp Epidemiol doi: 10.1017/ice.2020.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH, for the Taiwan COVID-19 Outbreak Investigation Team. 2020. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 180:1156. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.INESSS. 2020. COVID-19 et détection moléculaire du SARS-CoV-2 chez les individus asymptomatiques. INESSS, Quebec City, Canada. https://www.inesss.qc.ca/fileadmin/doc/INESSS/COVID-19/COVID-19_INESSS_detection_moleculaire_individus_asymptomatiques.pdf. Accessed 23 May 2020. [Google Scholar]
- 140.Zhou X, Li Y, Li T, Zhang W. 2020. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect 26:957–959. doi: 10.1016/j.cmi.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, Law KI, Tang BSF, Hon TYW, Chan CS, Chan KH, Ng JSC, Zheng BJ, Ng WL, Lai RWM, Guan Y, Yuen KY. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 143.Kampf G, Todt D, Pfaender S, Steinmann E. 2020. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, Cui Y, Fu RB, Dong YZ, Chi XY, Zhang MY, Liu K, Cao C, Liu B, Zhang K, Gao YW, Lu B, Chen W. 2020. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng VCC, Wong S-C, Chan VWM, So SYC, Chen JHK, Yip CCY, Chan KH, Chu H, Chung TWH, Sridhar S, To KKW, Chan JFW, Hung IFN, Ho PL, Yuen KY. 2020. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol doi: 10.1017/ice.2020.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. 2020. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu S, Wang Y, Jin X, Tian J, Liu J, Mao Y. 2020. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am J Infect Control doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tian Y, Rong L, Nian W, He Y. 2020. Review article: gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther 51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. 2020. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheung KS, Hung IF, Chan PP, Lung K, Tso E, Liu R, Ng Y, Chu MY, Chung TW, Tam AR, Yip CC, Leung KH, Yim-Fong Fung A, Zhang RR, Lin Y, Cheng HM, Zhang AJ, To KK, Chan KH, Yuen KY, Leung WK. 2020. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. 2020. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. BMJ Gut 69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 152.Ding Z, Qian H, Xu B, Huang Y, Miao T, Yen HL, Xiao S, Cui L, Wu X, Shao W, Song Y, Sha L, Zhou L, Xu Y, Zhu B, Li Y. 2020. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci Total Environ 753:141710. doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma J, Qi X, Chen H, Li X, Zhang Z, Wang H, Sun L, Zhang L, Guo J, Morawska L, Grinshpun SA, Biswas P, Flagan RC, Yao M. 2020. COVID-19 patients in earlier stages exhaled millions of SARS-CoV-2 per hour. Clin Infect Dis doi: 10.1093/cid/ciaa1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y, Chen C, Zhu S, Shu C, Wang D, Song J, Song Y, Zhen W, Feng Z, Wu G, Xu J, Xu W, National Health Commission Key Laboratory for Medical Virology, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China. 2020. Notes from the field: isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19). CCDC Wkly 2:123–124. doi: 10.46234/ccdcw2020.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. 2020. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li YY, Wang JX, Chen X. 2020. Can a toilet promote virus transmission? From a fluid dynamics perspective. Phys Fluids (1994) 32:e065107. doi: 10.1063/5.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kang M, Wei J, Yuan J, Guo J, Zhang Y, Hang J, Qu Y, Qian H, Zhuang Y, Chen X, Peng X, Shi T, Wang J, Wu J, Song T, He J, Li Y, Zhong N. 2020. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann Intern Med doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo CH, Kwon S, Song M, Mucci LA, Stampfer MJ, Willett WC, Eliassen AH, Hart JE, Chavarro JE, Rich-Edwards JW, Davies R, Capdevila J, Lee KA, Lochlainn MN, Varsavsky T, Sudre CH, Cardoso MJ, Wolf J, Spector TD, Ourselin S, Steves CJ, Chan AT, Coronavirus Pandemic Epidemiology Consortium. 2020. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Safdar N, Moreno GK, Braun KM, Friedrich TC, O’Connor DH. 2020. Using virus sequencing to determine source of SARS-CoV-2 transmission for healthcare worker. Emerg Infect Dis 26:2489–2491. doi: 10.3201/eid2610.202322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sikkema RS, Pas SD, Nieuwenhuijse DF, O’Toole Á, Verweij J, van der Linden A, Chestakova I, Schapendonk C, Pronk M, Lexmond P, Bestebroer T, Overmars RJ, van Nieuwkoop S, van den Bijllaardt W, Bentvelsen RG, van Rijen MML, Buiting AGM, van Oudheusden AJG, Diederen BM, Bergmans AMC, van der Eijk A, Molenkamp R, Rambaut A, Timen A, Kluytmans J, Oude Munnink BB, Kluytmans van den Bergh MFQ, Koopmans MPG. 2020. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hunter E, Price DA, Murphy E, van der Loeff IS, Baker KF, Lendrem D, Lendrem C, Schmid ML, Pareja-Cebrian L, Welch A, Payne BAI, Duncan CJA. 2020. First experience of COVID-19 screening of health-care workers in England. Lancet 395:e77–e78. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng C, Hafezi-Bakhtiari N, Cooper V, Davidson H, Habibi M, Riley P, Breathnach A. 2020. Characteristics and transmission dynamics of COVID-19 in healthcare workers at a London teaching hospital. J Hosp Infect 106:325–329. doi: 10.1016/j.jhin.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Seidelman J, Lewis S, Advani S, Akinboyo I, Epling C, Case M, Said K, Yancey W, Stiegel M, Schwartz A, Stout J, Sexton D, Smith B. 2020. Universal masking is an effective strategy to flatten the SARS-2-CoV healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol doi: 10.1017/ice.2020.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Supehia S, Singh V, Sharma T, Khapre M, Gupta PK. 2020. Rational use of face mask in a tertiary care hospital setting during COVID-19 pandemic: an observational study. Indian J Public Health 64:S225–S227. doi: 10.4103/ijph.IJPH_493_20. [DOI] [PubMed] [Google Scholar]
- 165.Powell-Jackson T, King JJC, Makungu C, Spieker N, Woodd S, Risha P, Goodman C. 2020. Infection prevention and control compliance in Tanzanian outpatient facilities: a cross-sectional study with implications for the control of COVID-19. Lancet Glob Health 8:e780–e789. doi: 10.1016/S2214-109X(20)30222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Musu M, Lai A, Mereu NM, Galletta M, Campagna M, Tidore M, Piazza MF, Spada L, Massidda MV, Colombo S, Mura P, Coppola RC. 2017. Assessing hand hygiene compliance among healthcare workers in six intensive care units. J Prev Med Hyg 58:e231–e237. [PMC free article] [PubMed] [Google Scholar]
- 167.Erasmus V, Daha TJ, Brug H, Richardus JH, Behrendt MD, Vos MC, van Beeck EF. 2010. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol 31:283–294. doi: 10.1086/650451. [DOI] [PubMed] [Google Scholar]
- 168.Alshammari M, Reynolds KA, Verhougstraete M, O’Rourke MK. 2018. Comparison of perceived and observed hand hygiene compliance in healthcare workers in MERS-CoV endemic regions. Healthcare (Basel) 6:122. doi: 10.3390/healthcare6040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Institute of Medicine (US) Committee on Personal Protective Equipment for Healthcare Personnel to Prevent Transmission of Pandemic Influenza and Other Viral Respiratory Infections. 2011. Using PPE: individual and organizational issues. In Larson EL, Liverman CT (ed), Preventing transmission of pandemic influenza and other viral respiratory diseases: personal protective equipment for healthcare personnel: update 2010. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 170.CDC NIOSH. 2015. Hierarchy of controls. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/niosh/topics/hierarchy/default.html. Accessed 3 June 2020. [Google Scholar]
- 171.Jayaweera M, Perera H, Gunawardana B, Manatunge J. 2020. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res 188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haut Conseil de la Santé Publique. 2020. Réduction du risque de transmission du coronavirus SARS-CoV-2 par la ventilation et gestion des effluents des patients. Haut Conseil de la Santé Publique, Paris, France. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=783. Accessed 30 May 2020. [Google Scholar]
- 173.Morawska L, Tang JW, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, Cao J, Dancer S, Floto A, Franchimon F, Haworth C, Hogeling J, Isaxon C, Jimenez JL, Kurnitski J, Li Y, Loomans M, Marks G, Marr LC, Mazzarella L, Krikor Melikov A, Miller S, Milton DK, Nazaroff W, Nielsen PV, Noakes C, Peccia J, Querol X, Sekhar C, Seppänen O, Tanabe S, Tellier R, Wai Tham K, Wargocki P, Wierzbicka A, Yao M. 2020. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.INSPQ. 2020. COVID-19: Mesures de prévention et contrôle des infections pour les milieux de soins aigus: recommandations intérimaires. INSPQ, Quebec City, Canada. https://www.inspq.qc.ca/sites/default/files/covid/2906-mesures-prevention-milieux-soins-aigus-covid19.pdf. Accessed 29 August 2020. [Google Scholar]
- 175.Morawska L, Milton DK. 2020. It is time to address airborne transmission of COVID-19. Clin Infect Dis doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harris K, Burke A. 2020. The long-term care crisis: how B.C. controlled COVID-19 while Ontario, Quebec face disaster. CBC News, New York, NY. https://www.cbc.ca/news/politics/long-term-care-crisis-covid19-pandemic-1.5589097. [Google Scholar]
- 177.Seak CJ, Liu YT, Ng CJ. 2020. Rapid responses in the emergency department of Linkou Chang Gung Memorial Hospital, Taiwan effectively prevent spread of COVID-19 among healthcare workers of emergency department during outbreak: lessons learnt from SARS. Biomed J doi: 10.1016/j.bj.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gan WH, Lim JW, Koh D. 2020. Preventing intra-hospital infection and transmission of coronavirus disease 2019 in health-care workers. Saf Health Work 11:241–243. doi: 10.1016/j.shaw.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fernstrom A, Goldblatt M. 2013. Aerobiology and its role in the transmission of infectious diseases. J Pathog 2013:493960. doi: 10.1155/2013/493960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roy CJ, Milton DK. 2004. Airborne transmission of communicable infection: the elusive pathway. N Engl J Med 350:1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 181.Qian H, Zheng X. 2018. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J Thorac Dis 10:S2295–S2304. doi: 10.21037/jtd.2018.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schünemann H, Brożek J, Guyatt G, Oxman A. 2013. GRADE handbook. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 3 June 2020.
- 183.Bae S, Kim MC, Kim JY, Cha HH, Lim JS, Jung J, Kim MJ, Oh DK, Lee MK, Choi SH, Sung M, Hong SB, Chung JW, Kim SH. 2020. Effectiveness of surgical and cotton masks in blocking SARS–CoV-2: a controlled comparison in 4 patients. Ann Intern Med doi: 10.7326/M20-1342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Kim MC, Bae S, Kim JY, Park SY, Lim JS, Sung M, Kim SH. 2020. Effectiveness of surgical, KF94, and N95 respirator masks in blocking SARS-CoV-2: a controlled comparison in 7 patients. Infect Dis (Lond) doi: 10.1080/23744235.2020.1810858. [DOI] [PubMed] [Google Scholar]
- 185.Leung NHL, Chu DKW, Shiu EYC, Chan K-H, McDevitt JJ, Hau BJP, Yen H-L, Li Y, Ip DKM, Peiris JSM, Seto W-H, Leung GM, Milton DK, Cowling BJ. 2020. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma QX, Shan H, Zhang HL, Li GM, Yang RM, Chen JM. 2020. Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol 92:1567–1571. doi: 10.1002/jmv.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patel RB, Skaria SD, Mansour MM, Smaldone GC. 2016. Respiratory source control using a surgical mask: an in vitro study. J Occup Environ Hyg 13:569–576. doi: 10.1080/15459624.2015.1043050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. 2013. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 9:e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. 2013. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep 7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wen ZB, Yu L, Yang WH, Hu LF, Li N, Wang J, Li JS, Lu JC, Dong XK, Yin Z, Zhang K. 2013. Assessment the protection performance of different level personal respiratory protection masks against viral aerosol. Aerobiologia 29:365–372. doi: 10.1007/s10453-012-9286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Diaz KT, Smaldone GC. 2010. Quantifying exposure risk: surgical masks and respirators. Am J Infect Control 38:501–508. doi: 10.1016/j.ajic.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 192.Johnson DF, Druce JD, Birch C, Grayson ML. 2009. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 193.Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Lim XF, Lim AS, Sutjipto S, Lee PH, Son TT, Young BE, Milton DK, Gray GC, Schuster S, Barkham T, De PP, Vasoo S, Chan M, Ang BSP, Tan BH, Leo YS, Ng OT, Wong MSY, Marimuthu K, for the Singapore 2019 Novel Coronavirus Outbreak Research Team. 2020. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun 11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, Liu X, Xu K, Ho KF, Kan H, Fu Q, Lan K. 2020. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 195.Lewis D. 2020. Is the coronavirus airborne? Experts can’t agree. Nature 580:175–175. https://www.nature.com/articles/d41586-020-00974-w#ref-CR6. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- 196.Hunt J. 2007. Exhaled breath condensate: an overview. Immunol Allergy Clin North Am 27:587–596. doi: 10.1016/j.iac.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Razzini K, Castrica M, Menchetti L, Maggi L, Negroni L, Orfeo NV, Pizzoccheri A, Stocco M, Muttini S, Balzaretti CM. 2020. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci Total Environ 742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kenarkoohi A, Noorimotlagh Z, Falahi S, Amarloei A, Mirzaee SA, Pakzad I, Bastani E. 2020. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci Total Environ 748. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mouchtouri VA, Koureas M, Kyritsi M, Vontas A, Kourentis L, Sapounas S, Rigakos G, Petinaki E, Tsiodras S, Hadjichristodoulou C. 2020. Environmental contamination of SARS-CoV-2 on surfaces, air-conditioner and ventilation systems. Int J Hyg Environ Health 230:113599. doi: 10.1016/j.ijheh.2020.113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M, Momeniha F, Mokamel A, Hassanvand MS, MokhtariAzad T. 2020. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ 725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li YH, Fan YZ, Jiang L, Wang HB. 2020. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol Infect 148:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]