Abstract
Alveolar type II (ATII) cells synthesize, store, and secrete pulmonary surfactant and restore the epithelium after damage to the alveolar epithelium. Isolation of human ATII cells provides a valuable tool to study their function under normal and pathophysiological conditions. Moreover, maintenance of their differentiated phenotype in vitro allows further study of their function. Here we describe a protocol for efficient ATII cell isolation, characterization, and culture.
Keywords: Lung, Alveolar type II cells, Purification
1. Introduction
The alveolar epithelium is composed of two main cell types, the alveolar type II (ATII) cell and the alveolar type I cell [1]. ATII cells cover about 5% of the alveolar surface area, comprise 15% of peripheral lung cells, and have an apical surface area of about 250 μm2 per cell [2]. They have a cuboidal shape, lamellar bodies, and microvilli. ATII cells synthesize, store, and secrete pulmonary surfactant, which provides the low surface tension in the distal parts of the lung [1]. Moreover, ATII cells also transport sodium and fluid from the apical surface into the interstitium and play an important role in innate immunity. Furthermore, they have a stem cell potential and proliferate to restore the alveolar epithelium after damage to the very sensitive type I cells. ATII cells interact with other cells and have beneficial functions to the whole alveolus [3]. Consequently, the ATII cell expresses a number of receptors and signaling molecules involved in cell–cell and cell–matrix interactions. One of these is epithelial cell adhesion molecule (EpCAM), which can be used to isolate ATII cells, as described below.
Isolation and purification of ATII cells for culture and functional assays represent a powerful tool by which cellular and molecular mechanisms of respiratory diseases can be studied. This is highlighted by recent reports that investigated the function of human primary ATII cells in the pathogenesis of chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis [4, 5]. Here we describe a protocol for ATII cell isolation from human lung tissue, in which EpCAM MicroBeads are used for positive selection of ATII cells. Our method yields ATII cells with up to 93% viability. Moreover, flow cytometry analysis shows 90% cell purity (Fig. 1). Importantly, the maintenance of the ATII cell phenotype in vitro depends not only on the method of isolation but also on culture conditions [6, 7]. Herein we describe these conditions and demonstrate the use of Western blotting to compare the levels of surfactant proteins: proSP-B, SP-B, proSP-C, and SP-C in both freshly isolated and cultured ATII cells (Fig. 2). Other investigators have used IgG adherence methods [8] or FACS to isolate ATII cells [9]. Notably, protocols for murine ATII cell isolation and culture are similar, although not identical [10]. The reader is referred to Chap. 6 for methods to isolate ATII cells from rodents.
Fig. 1.
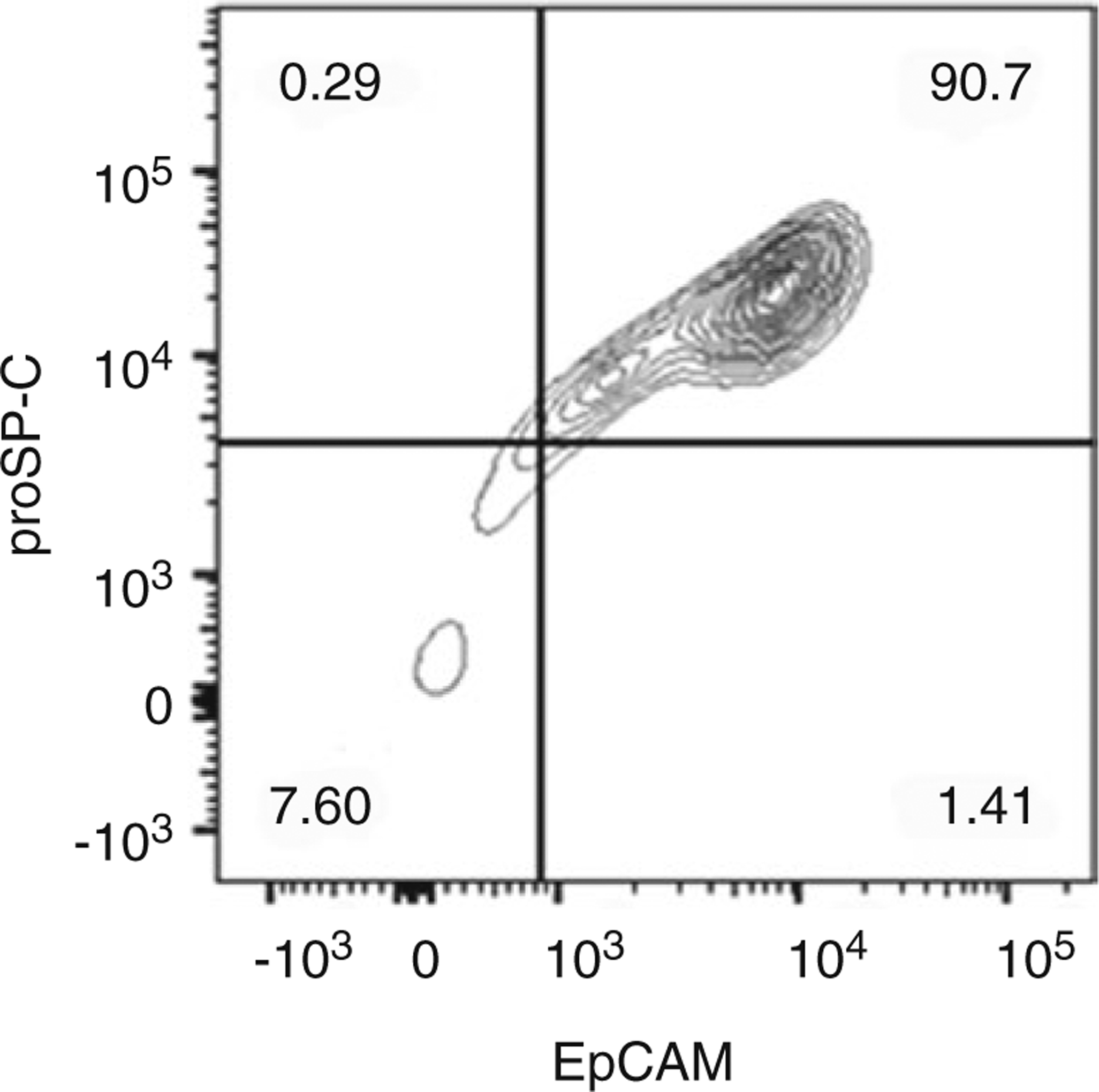
Representative flow cytometry analysis of isolated ATII cells using co-staining for proSP-C and EpCAM
Fig. 2.
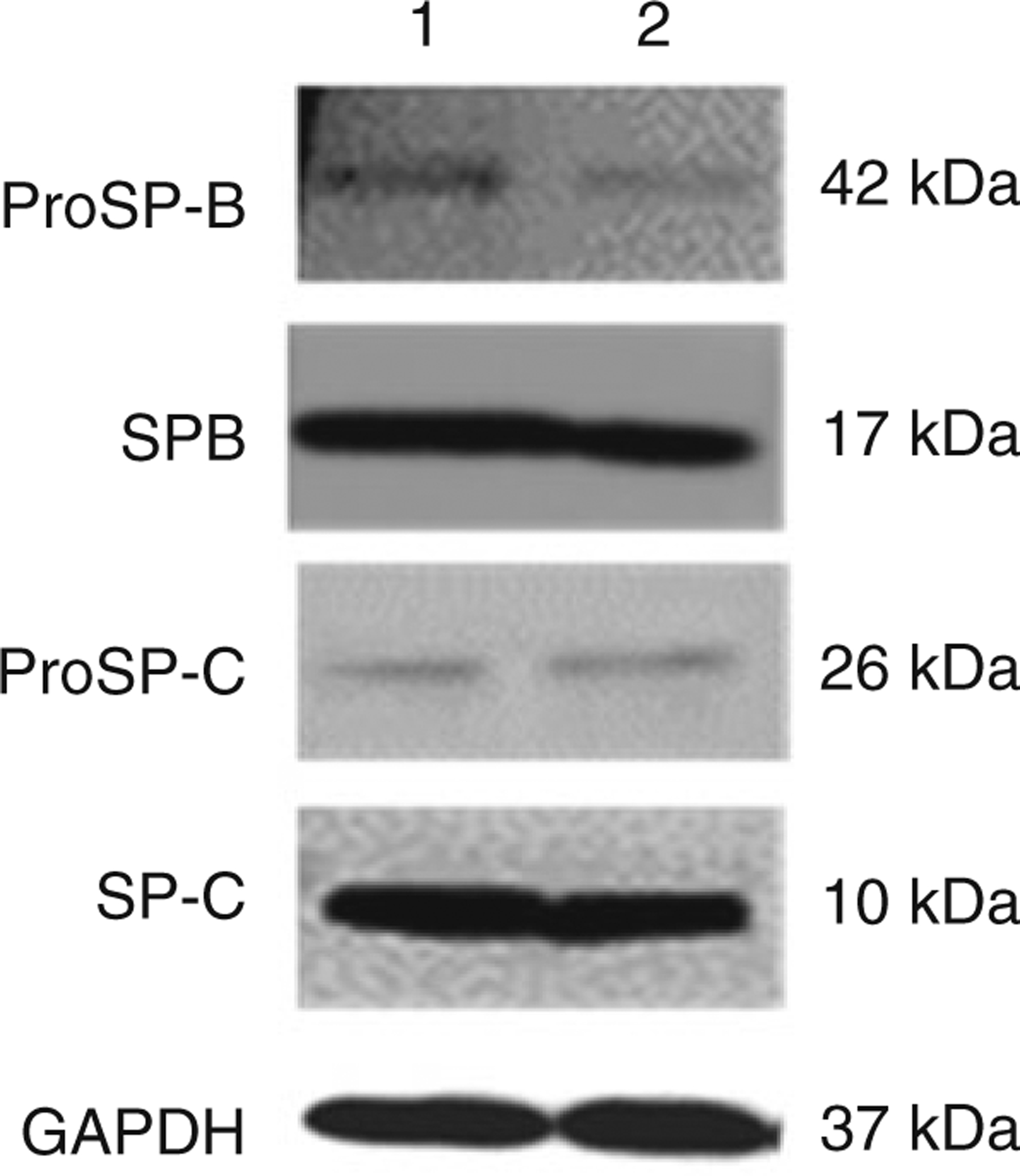
Surfactant protein levels in ATII cells. ProSP-B, SP-B, proSP-C, and SP-C expression was detected by immunoblotting. Lanes: 1, freshly isolated cells; 2, cells cultured for 6 days
2. Materials
2.1. Supplies and Equipment
Surgical scissors.
Surgical suture.
Forceps.
Perfusion tube.
60 mL syringe.
20 μm and 100 μm nylon mesh.
40 μm cell strainer.
Cheesecloth.
Graduated cylinder.
Containers.
Hydrometer.
LS columns (Miltenyi Biotec Inc.).
QuadroMACS Separator (Miltenyi Biotec Inc.).
Hemocytometer.
Shaking water bath.
Vacuum aspirator.
Neutator.
Refrigerated centrifuge.
2.2. Reagents
HEPES/saline buffer: 17.532 g NaCl; 2.383 g HEPES, 2 L final volume, pH 7.4.
HEPES/saline/EDTA buffer: 17.532 g NaCl; 2.383 g HEPES; 3.722 g EDTA, 2 L final volume, pH 7.4.
BSS-A buffer: 31.8 g NaCl; 1.6 g KCl; 0.2 g NaH2PO4; 1.28 g Na2HPO4; 10.12 g HEPES; 4 g dextrose, 10 μg/mL gentamycin, 4 L final volume, pH 7.4. Store at 4 °C.
BSS-B buffer: 31.04 g NaCl; 1.544 g KCl; 0.2 g NaH2PO4; 1.236 g Na2HPO4; 9.86 g HEPES; 4 g dextrose; 1.116 g CaCl2·2H20; 1.276 g MgSO4·7H20, 10 μg/mL gentamycin, pH 7.4, 4 L final volume. Store at 4 °C.
Column buffer in PBS: 10 g BSA; 2 mM EDTA, 2 L final volume, pH 7.4; degas and store at 4 °C.
4 mg/mL DNase I in BSS-B buffer.
Krebs HEPES buffer: 0.25 M NaCl, 2.65 mM sodium phosphate buffer (pH 7.4), 5.31 mM KCl, 10 mM HEPES.
OptiPrep to make light and heavy gradients. Light gradient: 45 mL OptiPrep, 8 mL FBS, 1 mL phenol red; adjust with Krebs HEPES buffer to relative density 1.038–1.045, 400 mL final volume, and store at −20 °C. Heavy gradient: 110 mL OptiPrep, 8 mL FBS; adjust with Krebs HEPES buffer to relative density 1.088–1.092, 400 mL final volume, and store at −20 °C.
RTC mix: 10% 10× MEM and 90% of 2.5 mg/mL RTC, and adjust pH using 0.32 M NaOH to get pink color.
Pharm Lyse.
Elastase (Worthington Biochemical Corp.).
Epithelial cell adhesion molecule (EpCAM) MicroBeads (Miltenyi Biotec Inc.).
FcR Blocking Reagent (Miltenyi Biotec Inc.).
Matrigel Matrix.
10× MEM.
Fetal bovine serum.
Fetal calf serum.
Dimethyl sulfoxide (DMSO).
Rat tail collagen (RTC).
DMEM.
Charcoal-stripped FBS (CS-FBS).
Keratinocyte growth factor (KGF).
Isobutylmethylxanthine (IBMX).
8-Bromo-cyclic AMP (cAMP).
Dexamethasone.
Streptomycin.
Penicillin.
Amphotericin B.
Glutamine.
Gentamycin.
3. Methods
3.1. Lung Perfusion and Lavage
Select the lobe to be processed. The right middle lobe is the easiest to process but the lingula can also be used.
Identify the bronchus and corresponding pulmonary artery that supplies the lobe. Place a small tube in the pulmonary artery, and use a 60 mL syringe to inject HEPES/saline buffer to flush blood from the lobe’s blood vessels. Repeat until blood no longer flushes out.
Put the cannula in the lobar bronchus. Tie off with surgical suture to secure the cannula in place.
Lavage the lobe by filling it completely with HEPES/saline/EDTA four times to remove alveolar macrophages and debris from the airspaces. The volume depends on the size of the lobe and is usually between 100 and 250 mL.
Lavage the lobe with BSS-A buffer (37 °C) twice as described above. This will rinse EDTA from the lungs and remove additional alveolar macrophages.
Lavage the lobe with BSS-B buffer (37 °C) twice to neutralize any remaining EDTA. Drain this last lavage very well.
Instill BSS-B buffer with 12.9 U/mL elastase (37 °C) into the lobe. The volume depends on the size of the lobe and is usually between 200 and 250 mL.
Clamp the cannula that was used to lavage the lobe so that none of the elastase leaks out.
Place the lobe in a container and incubate in the water bath for 40 min at 37 °C (see Note 1).
Remove the lung from the water bath. Take out the cannula and trim away any surrounding bronchial tissue. Cut the lung into small pieces.
Homogenize lung pieces in 150 mL BSS-B buffer with 5 mL DNase I using a food processor (five bursts of 2–3 s).
Filter cell suspension through cheesecloth into a container with 90 mL FCS, and shake in a water bath for 5 min at 37 °C.
Sequentially filter the cell suspension through cheesecloth, 100 μm and 20 μm nylon mesh.
Centrifuge the suspension at 200 × g for 10 min at 4 °C; aspirate and discard the supernatant (see Note 2).
Prepare six conical tubes (50 mL) with OptiPrep medium by pipetting a 10 mL light density gradient and underlying 10 mL heavy density gradient.
Resuspend the cell pellet in 150 mL BSS-B buffer, and load 25 mL onto OptiPrep medium (see Note 3).
Centrifuge at 200 × g for 20 min at 4 °C.
Aspirate the liquid above cells located at the interface and discard.
Collect cells into separate tubes.
Wash cells by adding 45 mL BSS-B buffer and centrifuge at 200 × g for 10 min.
Resuspend cell pellet with column buffer, and count total number of cells using a hemocytometer.
3.2. Optional Collection of Alveolar Macrophages from Lavage Fluid
Combine lavage fluid obtained from Steps 4 and 5 in Subheading 3.1.
Filter lavage fluid through a 100 μm filter.
Centrifuge at 200 × g for 10 min at 4 °C.
Resuspend cell pellet in 1× Pharm Lyse buffer to lyse red blood cells.
Incubate cells for 10 min in the dark at room temperature.
Centrifuge the cells at 200 × g for 10 min at 4 °C.
Resuspend the cell pellets in DMEM with 10% FBS; centrifuge at 200 × g for 10 min.
Freeze cells in a liquid nitrogen tank in freezing media (90% FBS and 10% DMSO).
3.3. ATII Cell Purification Using EpCAM MicroBeads
Prepare conical tubes based on the cell count obtained from Step 21 in Subheading 3.1. Use 100 × 106–150 × 106 cells per tube.
Centrifuge cells at 200 × g for 5 min at 4 °C.
Resuspend cell pellet with 900 μL column buffer per tube. Add 300 μL FcR Blocking Reagent and 300 μL EpCAM MicroBeads per conical tube.
Place conical tubes with cells on the neutator for 30 min at 4 °C.
Add 10 mL column buffer to each tube to wash cells.
Centrifuge the cells at 200 × g for 10 min at 4 °C. Aspirate and discard supernatant.
Place LS columns in the QuadroMACS Separator, and rinse with 3 mL column buffer (see Note 4).
Resuspend cell pellet in 3 mL column buffer; filter cells through 40 μm cell strainer and add to the column. When this volume runs through the column completely, wash the column three times with 3 mL column buffer.
Elute ATII cells by adding 5 mL column buffer and then plunging with the provided plunger. Count EpCAM-positive ATII cells using a hemocytometer (see Note 5). The unlabeled fraction contains other cell types.
3.4. ATII Cell Culture
Use a mixture of 80% RTC mix and 20% Matrigel Matrix to coat inserts for 12-well plates. Incubate the plates at 37 °C for 10 min.
Plate 1 × 106 ATII cells on inserts in DMEM with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B, and 10 μg/mL gentamycin (see Note 6).
After 2 days add DMEM with 1% CS-FBS, 10 ng/mL KGF, and antibiotics as described in Step 2 to the basolateral side, and culture ATII cells under air/liquid interface conditions (see Note 7).
After 2 additional days, add DMEM with 1% CS-FBS, 10 ng/mL KGF, 0.1 mM IBMX, 0.1 mM cAMP, 10−8 M dexamethasone, and antibiotics to the basolateral side.
After 2 days start experiments.
4. Notes
We typically place the lobe in a plastic bag for the incubation step. This provides an easy way to collect any buffer that leaks out of the lobe. After 20 min you may re-instill the lobe with elastase that leaked out.
Use Pharm Lyse buffer as described in Subheading 3.2 if you see many red blood cells.
Add 1–2 mL DNase I if you see cell clumps.
Avoid air bubbles which may block the column.
Final ATII cell viability and yield depend on lung condition, how much time passed from lung recovery to start processing, and the speed of lung processing.
ATII cell plating efficiency may vary among lung donors.
ATII cells are plated on a filter insert coated with the mixture or RTC and Matrigel Matrix. After cell attachment for 2 days, the medium is placed outside the insert to culture cells under air/liquid interface conditions. The reader is referred to Chap. 8 for details.
Acknowledgments
This work was supported by NIH R01 HL118171 (BK) and FAMRI grant CIA130046 (BK). We thank Chih-Ru Lin for helping with experiments.
References
- 1.Mason RJ (2006) Biology of alveolar type ii cells. Respirology 11(Suppl):S12–S15 [DOI] [PubMed] [Google Scholar]
- 2.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD (1992) Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol 6(2):235–243 [DOI] [PubMed] [Google Scholar]
- 3.Fehrenbach H (2001) Alveolar epithelial type ii cell: defender of the alveolus revisited. Respir Res 2(1):33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D et al. (2016) Hyaluronan and TLR4 promote surfactant-protein-c-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med 22(11):1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skronska-Wasek W, Mutze K, Baarsma HA, Bracke KR, Alsafadi HN, Lehmann M, Costa R, Stornaiuolo M, Novellino E, Brusselle GG et al. (2017) Reduced frizzled receptor 4 expression prevents wnt/beta-catenin-driven alveolar lung repair in copd. Am J Respir Crit Care Med 196:172–185 [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ (2007) Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 36(6):661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahmed K, Messier EM, Zhou W, Tuder RM, Freed CR, Chu HW, Kelsen SG, Bowler RP, Mason RJ, Kosmider B (2016) Dj-1 modulates NRF2-mediated protection in human primary alveolar type ii cells in smokers. Am J Respir Cell Mol Biol 55:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O’Neal WK, Boucher RC (2010) Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285(45):34939–34949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino N, Kubo H, Ota C, Suzuki T, Suzuki S, Yamada M, Takahashi T, He M, Suzuki T, Kondo T et al. (2012) A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol 46(4):422–430 [DOI] [PubMed] [Google Scholar]
- 10.Messier EM, Mason RJ, Kosmider B (2012) Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res 38(7):363–373 [DOI] [PubMed] [Google Scholar]


