Abstract
With the exception of a few successes in trials of supportive care, the majority of interventional clinical trials for acute respiratory distress syndrome (ARDS) have not led to new therapies. To improve the likelihood of benefit from clinical trial interventions in ARDS, clinical trial design must be improved. To optimize trial design, many factors need to be considered including the type of therapy to be tested, the type of trial (phase 2 or 3), how patients will be selected, primary and secondary end-points, and strategy for conduct of the trial, including potential newer trial designs such as platform or adaptive trials. Of these, optimization of patient selection is central to the likelihood of success and is particularly relevant in ARDS, which is a heterogeneous clinical syndrome, not a homogeneous disease. Recent advances including improved understanding of pathophysiologic mechanisms and better tools for outcome prediction in ARDS should facilitate both predictive and prognostic enrichment. This commentary focuses on new information and novel methods for prognostic and predictive enrichment that may be useful to optimize patient selection and increase the likelihood of positive clinical trials in ARDS.
Keywords: Acute respiratory distress syndrome, Acute lung injury, Pathophysiology, Clinical trial, Predictive enrichment, Prognostic enrichment
Take-home message
| As we enter the decade of the 2020s, we have the opportunity to design better clinical trials in ARDS that are more likely to demonstrate a beneficial treatment effect. Improved understanding of pathophysiologic mechanisms and better tools for outcome prediction that are now available should facilitate both predictive and prognostic enrichment, hopefully increasing the likelihood of positive trials going forward. |
Introduction
The history of interventional clinical trials for acute respiratory distress syndrome (ARDS) is fraught with many failures and only a few successes in supportive care. The advent of the coronavirus SARS-CoV-2 pandemic in 2019, a new cause of ARDS, has emphasized the need to improve ARDS clinical trial design to maximize the likelihood of positive trial outcomes. To optimize trial design, many factors need to be considered including the type of therapy to be tested, the type of trial (phase 2 or 3), how patients will be selected, primary and secondary end-points, and strategy for conduct of the trial, including potential newer trial designs such as platform or adaptive trials. Of these, optimization of patient selection is central to the likelihood of success and is particularly relevant in ARDS, which is a heterogeneous clinical syndrome, not a homogeneous disease. New information and novel methods for prognostic and predictive enrichment may be useful to optimize patient selection in ARDS trials in 2020 and beyond and will be the focus of this commentary.
Prognostic enrichment involves enriching trial enrollment for patients with a high probability of an actionable outcome of interest, such as mortality, ventilator-free days or days alive and free of organ dysfunction (vasopressors, mechanical ventilation, dialysis). Prognostic enrichment aims to increase the frequency of the outcome of interest, which may increase the power to detect a beneficial treatment effect for a given sample size. In ARDS, efforts at prognostic enrichment have primarily focused on physiologic variables. The arterial to inspired oxygen ratio (PaO2/FiO2) has been used in many trials (see Table 1) to enrich for patients at risk of worse clinical outcomes due to more severe impairment of oxygenation. The best example of successful prognostic enrichment in ARDS is the PROSEVA trial of proning therapy which enriched for patients with moderate-to-severe ARDS by enrolling only those with PaO2/FiO2 less than 150 mmHg and showed a mortality benefit [1]. Earlier trials of proning in ARDS did not enrich for severity and were likely underpowered to detect a survival benefit. Other potential physiologic candidates for prognostic enrichment (see Table 1) include vasopressor-dependent shock and chest imaging criteria that quantify the extent of pulmonary edema (the radiographic assessment of lung edema (RALE) score) [2]. Prognostic biomarkers may also be used for enrichment. Bedside measurement of plasma levels of IL-8, Protein C, and bicarbonate to identify a hyperinflammatory phenotype could potentially identify ARDS patients with higher mortality [3] as does a whole blood gene expression signature in pediatric sepsis [4]. Various ICU risk scores have been tested unsuccessfully because they are not specific for ARDS and other clinical syndromes and because patients at the highest risk may not benefit from therapy [5].
Table 1.
Summary of potential strategies for prognostic and predictive enrichment in ARDS clinical trials
| Prognostic factor | Metric for enrichment | Outcome targeted by enrichment strategy | Used in published ARDS trials? |
|---|---|---|---|
| Strategies for prognostic enrichment | |||
| Severity of hypoxemia | PaO2/FiO2 | Death and/or prolonged mechanical ventilation | Yes |
| Presence of shock | Need for vasopressors | Death | No |
| Severity of pulmonary edema | RALE score | Prolonged mechanical ventilation | No |
| Biomarkers of poor prognosis | Model incorporating IL-8, Protein C, bicarbonate | Death and/or prolonged mechanical ventilation | No |
| Predictive factor | Metric for enrichment | Mechanism targeted by enrichment strategy | |
|---|---|---|---|
| Strategies for predictive enrichment | |||
| Higher likelihood of fibroproliferative ARDS | BAL PCP III | Anti-fibroproliferative effects of corticosteroids | No, one trial is enrolling |
| Higher likelihood of oxidative injury from cell-free hemoglobin | Plasma cell-free hemoglobin | Hemoprotein-reductant effects of acetaminophen | Used in a pilot sepsis trial |
| Early lung injury more likely to respond | Enrollment prior to invasive ventilation | Anti-inflammatory effects of inhaled budesonide and formoterol | No, one trial is enrolling |
| Focal vs. diffuse ARDS | Chest CT distribution of infiltrates | Personalized ventilator strategy | Yes |
| Hyperinflammatory ARDS | Latent class analysis of clinical and biomarker features | Anti-inflammatory effects of simvastatin | No |
| Impaired vascular integrity | Plasma adrenomedullin | Vascular protective effects of adrecizumab | No, one trial is enrolling |
| Higher likelihood of ventilator-induced lung injury | Increased dead space fraction and lower compliance of the respiratory system | Identify group with highest predicted drop in driving pressure with extracorporeal CO2 removal | No |
Predictive enrichment involves the enrollment of patients who are more likely to respond to a given treatment based on the mechanism of benefit and thus is more specific than prognostic enrichment. Predictive enrichment has transformed cancer treatment trials, wherein analysis of genetic mutations in an individual’s tumor is used to predictively enrich for enrollment in trials that mechanistically target these mutations. In severe asthma, identification of a hypereosinophilic/type 2-like phenotype has led to successful trials that enrich for this phenotype. In ARDS, a number of potential strategies for predictive enrichment have been proposed, with many being specific to a single therapy (Table 1); as of yet, there are few examples of completed predictively enriched trials. One example is a currently enrolling trial of systemic corticosteroids for moderate-to-severe ARDS that enriches enrollment for patients with elevation of bronchoalveolar lavage procollagen peptide III, an early biomarker of activation of profibrotic pathways in the lung (NCT#03371498). Another form of predictive enrichment is being used in the ARREST trial of inhaled budesonide and formoterol for severe pneumonia from COVID-19 or other causes, where the target population is enriched for early acute lung injury within 12 h of hospitalization and prior to intubation. The rationale behind this temporal enrichment is the hypothesis that early acute lung injury is more likely to respond to inhaled corticosteroids and beta-agonists than more established ARDS [6]. Another strategy is to assess less enriched trials for patterns of heterogeneity of treatment effect as has been recently proposed by Goligher and colleagues [7]. Using data from a trial of extracorporeal CO2 removal in moderate-to-severe ARDS to simulate future trials, they found that restricting enrollment to patients with a larger predicted decrease in driving pressure based on the alveolar dead space fraction and static respiratory compliance might increase the predicted mortality benefit, and reduce predicted sample size and screening size requirements [8]. As another example, the hyperinflammatory phenotype of ARDS that has been consistently identified among ARDS patients enrolled in clinical trials was associated with reduced mortality with simvastatin treatment in retrospective analysis of trial data, an effect that was not seen in the hypo-inflammatory phenotype nor in the trial as a whole [9]. A future trial of simvastatin that enriches for the hyperinflammatory phenotype might be more likely to show a treatment benefit.
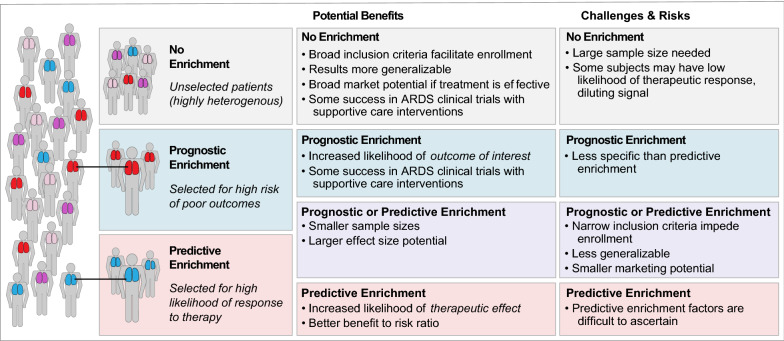
Enrichment strategies have both advantages and disadvantages (Fig. 1). The major theoretical advantage of both prognostic and predictive enrichment is to increase the signal-to-noise ratio, reducing sample size and increasing the likelihood of detecting a therapeutic benefit. Predictive enrichment also may lead to a larger effect size. By excluding patients less likely to benefit from a specific treatment, predictive enrichment may also improve the benefit-to-risk ratio of a trial since patients who are unlikely to benefit from a therapy are still at risk of its adverse effects.
Fig. 1.
Potential benefits, challenges and risks of different enrichment strategies for clinical trials in ARDS
The major disadvantage of both prognostic and predictive enrichment is reduction in generalizability. As an example, the proning strategy applied in the PROSEVA trial cannot be generalized to all ARDS, since the trial enriched for more severe ARDS (PaO2/FiO2 < 150 mmHg). Similarly, if a therapy were found to be effective in patients with hyperinflammatory ARDS, this finding would apply only to the smaller subset (~ 25–30%) of ARDS patients in the hyperinflammatory class. The potential for a more restricted indication for a new pharmacologic therapy may reduce enthusiasm from the pharmaceutical industry, a major funder of large phase 3 clinical trials. Another disadvantage is that enrichment strategies, by design, exclude many patients from enrollment. Such restrictions may make enrollment challenging and hinder timely completion of trials. In addition, regulators (such as the FDA) or patient associations might request that the therapeutic effects be assessed in the marker-negative or non-enriched population in order to demonstrate benefits of predictive enrichment strategies [10]; these concerns are best addressed once a treatment benefit has been identified in a target population. Finally, it should be noted that identification of reliable enrichment factors is challenging. Although this is most true for predictive enrichment, where proposed mechanisms are often theoretical, it can also be true for prognostic enrichment. An example is the LIPS-A study of aspirin for prevention of ARDS [11]. In that study, the lung injury prevention score (LIPS) was used to prognostically enrich for patients more likely to develop ARDS. However, the actual rate of ARDS in the study was far less than predicted by LIPS which may have contributed to the negative outcome of the trial.
As we enter the decade of the 2020s, we have the opportunity to design clinical trials in ARDS that are more likely to demonstrate a beneficial treatment effect. Prognostic and predictive enrichment can improve the signal-to-noise ratio, allowing smaller sample sizes and increased effect sizes. These enrichment approaches represent one of the most promising ways to improve clinical trial design in ARDS in the coming decade and can also be applied to trials in patients with ARDS due to SARS-CoV-2 infection as new data emerges around the pathogenesis of this pandemic disease.
Compliance with ethical standards
Conflicts of interest
Dr. Ware reports personal fees from Bayer, CSL Behring, Quark, Merck, Citius, Foresee and Boehringer Ingelheim and research contracts with Genentech and CSL Behring, all outside the submitted work. Dr. Mebazaa reports personal fees from Orion, Servier, Otsuka, Philips, Sanofi, Adrenomed, Epygon and Fire 1 and grants and personal fees from 4TEEN4, Abbott and Sphyngotec, outside the submitted work. Dr. Matthay reports research contracts from Bayer and Roche-Genentech outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, Rice TW, Matthay MA, Calfee CS, Ware LB. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha P, Delucchi KL, McAuley DF, O'Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med. 2020;8:247–257. doi: 10.1016/S2213-2600(19)30369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney TE, Perumal TM, Henao R, Nichols M, Howrylak JA, Choi AM, Bermejo-Martin JF, Almansa R, Tamayo E, Davenport EE, Burnham KL, Hinds CJ, Knight JC, Woods CW, Kingsmore SF, Ginsburg GS, Wong HR, Parnell GP, Tang B, Moldawer LL, Moore FE, Omberg L, Khatri P, Tsalik EL, Mangravite LM, Langley RJ. A community approach to mortality prediction in sepsis via gene expression analysis. Nat Commun. 2018;9:694. doi: 10.1038/s41467-018-03078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harhay MO, Casey JD, Clement M, Collins SP, Gayat E, Gong MN, Jaber S, Laterre PF, Marshall JC, Matthay MA, Monroe RE, Rice TW, Rubin E, Self WH, Mebazaa A. Contemporary strategies to improve clinical trial design for critical care research: insights from the First Critical Care Clinical Trialists Workshop. Intensive Care Med. 2020;46:930–942. doi: 10.1007/s00134-020-05934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Festic E, Carr GE, Cartin-Ceba R, Hinds RF, Banner-Goodspeed V, Bansal V, Asuni AT, Talmor D, Rajagopalan G, Frank RD, Gajic O, Matthay MA, Levitt JE. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goligher EC, Amato MBP, Slutsky AS. Applying precision medicine to trial design using physiology. Extracorporeal CO2 removal for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:558–568. doi: 10.1164/rccm.201701-0248CP. [DOI] [PubMed] [Google Scholar]
- 8.Goligher EC, Combes A, Brodie D, Ferguson ND, Pesenti AM, Ranieri VM, Slutsky AS, Investigators S, for the International EN Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med. 2019;45:1219–1230. doi: 10.1007/s00134-019-05708-9. [DOI] [PubMed] [Google Scholar]
- 9.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O'Kane CM, McAuley DF, Grp ICCT. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Food and Drug Agency (2019) Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Guidance for Industry. https://www.fda.gov/media/121320/download. Accessed 7 June 2020.
- 11.Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN, Illness USC, Injury Trials Group: Lung Injury Prevention with Aspirin Study G Effect of aspirin on development of ards in at-risk patients presenting to the emergency department: the LIPS-A randomized clinical trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]