EPIDEMIOLOGY
Over the previous 2 decades, use of opioids grew dramatically across the United States. In 2015, 37% of American adults were prescribed at least one opioid pain reliever (OPR)1—3 times as many as in 1999.2 Although OPR use remains elevated across the United States, more recently consumption of heroin and fentanyl and deaths from overdose of these drugs increased substantially.3 Rising opioid use across the United States has been associated with complications among many populations, including pregnant women and infants.4 Neonatal abstinence syndrome (NAS) is a postnatal withdrawal syndrome that manifests shortly after birth in infants born to women with opioid use (including heroin, use or misuse of prescription painkillers, or maternal treatment medications such as methadone or buprenorphine) during pregnancy. Concurrent with the increase in opioid use among pregnant women, the number of infants diagnosed with NAS grew nearly 7-fold from 2000 to 2014.4–6 By 2014, more than 30,000 infants were diagnosed with the syndrome, accounting for more than $500 million in hospitalization costs.6 Increases in NAS occurred disproportionately in rural areas7 and in US states with high rates of other opioid-related complications including overdose death.8
NAS is a complex disorder that is variably expressed, both in types of signs and severity, among different infants, and in the same infant over time. Every opioid-exposed infant is unique and resides along a continuum of signs of withdrawal.9 Currently, there is no way to accurately predict the severity of NAS expression in any given infant. NAS is typically associated with maternal use or misuse of opioids (heroin, prescription painkillers), or with maternal treatment medications such as methadone or buprenorphine. However, its expression can be modified by many maternal, infant, and environmental factors (Table 1).10–27 Timing of exposure during gestation, maternal stress associated with opioid use disorder (OUD), poor maternal nutrition, or lack of medical or obstetric care can affect the fetus and the intrauterine environment. Infant cò-morbid medical conditions can also complicate the postnatal picture of NAS.
Table 1.
Maternal, infant, and/or environmental factors that can alter infant neonatal abstinence syndrome expression
| Maternal Factors | |
| Illicit substance use: heroin, cocaine, marijuana | In general, polysubstance exposure alters NAS expression by increasing its severity, or causes neurobehavioral signs consistent with a withdrawal phenomenon.10 |
| Licit substance use/misuse: oxycodone, benzodiazepines, gabapentin, nicotine | Oxycodone and benzodiazepines increase NAS expression.11–13 |
| Gabapentin produces an atypical NAS display.14 | |
| Cigarette smoking can increase NAS severity.15,16 | |
| Licit medications: psychotropics, OUD treatment medications (eg, methadone, buprenorphine) | Psychotropic exposure can alter or increase NAS display.17 |
| OUD treatment medications can predispose the exposed infant to NAS, but benefits associated with maternal comprehensive treatment that includes medications for OUD are paramount for the dyad. | |
| Genetics/epigenetics | Infants with particular genotypes (SNPs) at the OPRM1 and COMT gene sites had less severe NAS expression.18 |
| Hypermethylation at the same sites was associated with more severe NAS, consistent with gene silencing.19 | |
| Breastfeeding | Can reduce NAS severity.20 |
| Infant factors | |
| Sex | Male infants have been reported to have more severe NAS expression.21,22 |
| Gestational age | Preterm infants have less severe expression of NAS (notably, NAS measurement tools were designed for term infants. As such, NAS may not be adequately assessed in preterm infants).23 |
| Fetal programming | The fetus adapts to an unfavorable intrauterine environment by altering ANS set points. These changes can be adaptive in utero and maladaptive ex utero and may be expressed as NAS.22 Alterations from these changes may not be evident until the affected neurosystem matures, potentially later in life.24 |
| Environmental factors | |
| Physical environment | NICU care can exacerbate NAS severity, while maternal rooming-in can reduce NAS severity.25,26 |
| Caregiver (parent or medical staff) handling and communication | Misinterpretation of or inappropriate responses to infant cues or insensitive handling can exacerbate NAS expression.27 |
Abbreviations: ANS, autonomic nervous system; NICU, newborn intensive care unit; OUD, opioid use disorder; SNP, single nucleotide polymorphism.
SIGNS OF NEONATAL ABSTINENCE SYNDROME
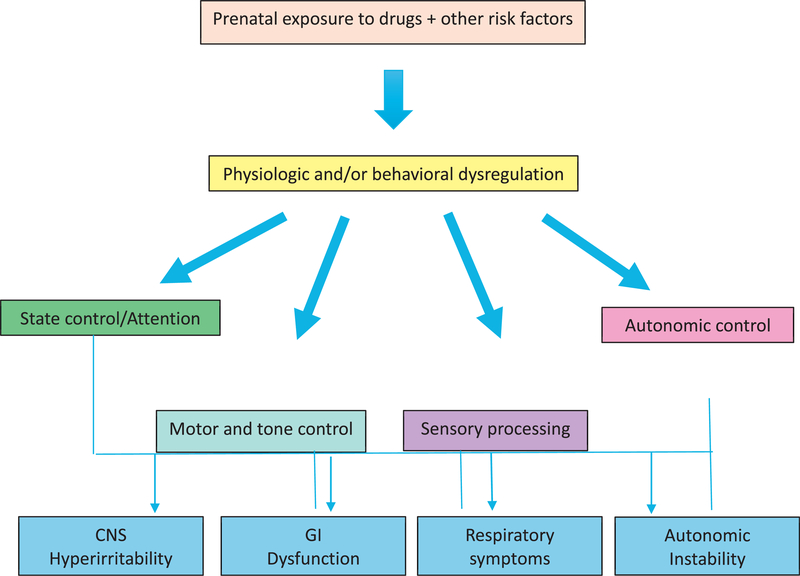
NAS is a disorder of neurobehavioral dysregulation; hence, it is important to consider the development of regulatory capacity in understanding this disorder. Each infant has a specific functional repertoire and neurobehavioral competencies that are unique. According to Als’ Model of Synactive Organization of Behavioral Development,28 development fundamentally represents the emergence of more complex and integrated forms of self-regulation over the lifespan. This self-regulatory capacity serves to regulate the infant’s own functioning as well as caregiver behaviors and responses. Each of 4 behavioral subsystems (autonomic control, motor and tone control, state control and attention, and sensory processing) supports the others and interacts with the infant’s environment. When newborn development is disturbed, as with substance exposure or inappropriate caregiver responses, disturbances in self-regulation and altered trajectories of development may occur.29 For example, an opioid-exposed infant who spends an exorbitant amount of energy in one subsystem, such as tone in hypertonic infants, may have little energy to spend in other subsystems, such as attention/interaction. This dysregulatory imbalance is the hallmark of infants affected by NAS.
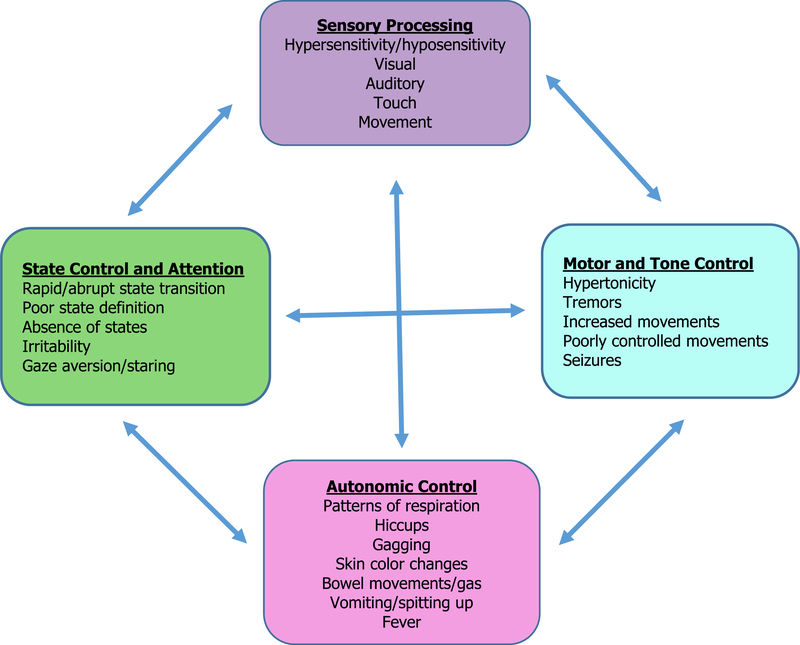
The signs of NAS can be thought of as arising from dysregulation in these 4 behavioral domains. Dysfunction in one domain can influence regulation in another domain (Fig. 1).27 Specific symptoms include irritability and crying; poor state control;hypertonicity; tremors and jitteriness and accompanying skin breakdown; failure to thrive; hyper- or hyposensitivity to ordinary stimuli; vomiting/diarrhea; and autonomic signs such as hiccups, gagging, color changes, tachypnea, or fever. For example, an infant who is hypersensitive to stimuli may have problems with state control and difficulties achieving a quiet alert state necessary for feeding, leading to weight loss (Fig. 2).27
Fig. 1.
Signs of NAS arise from dysregulation of 4 domains of functioning. (Adapted from Velez M, Jansson LM. Non-pharmacologic care of the opioid dependent mother and her newborn. J Addiction Med 2008;2:113–20.)
Fig. 2.
Signs of NAS expression in each of the 4 domains of functioning can influence expression in other domains. (Adapted from Velez M, Jansson LM. Non-pharmacologic care of the opioid dependent mother and her newborn. J Addiction Med 2008;2:113–20.)
IDENTIFICATION OF NEONATAL ABSTINENCE SYNDROME AND DIFFERENTIAL DIAGNOSIS
Identifying the infant at risk for NAS is important for initiating nonpharmacologic care, scoring for NAS, and maternal treatment and is not intended to subject the mother to punitive action. Screening for opioid use or misuse in all pregnant women should be performed periodically and can be done by using one of many available screening instruments30 (eg, 4Ps Plus tool).31 Ideally, the infant presenting with signs of NAS has a clear and defined history of opioid exposure, and the mother is receiving comprehensive care and maintenance treatment with methadone or buprenorphine for an OUD. The utility of universal infant urine toxicologic screening for maternal substance use or misuse is debated today.
If an infant born to a mother who denies opioid use or misuse exhibits signs of NAS, toxicology screening using urine (infant or maternal), meconium, umbilical cord blood, or maternal plasma may be necessary. There are drawbacks to each method, which are summarized in Table 2. Drawbacks common to all include the following:
Negative results do not rule out an OUD.
Positive results may be obtained from prescribed pain medications and do not represent an OUD.
Positive results do not quantify use; alcohol, which arguably has the greatest impact on the fetus, is not detected due to a short half-life.
Detection of OUD at birth has no value in mitigating teratogenic effects in early pregnancy.
Table 2.
Screening for opioid exposure
| Biomatrix | Detection of Exposure |
Properties | Drawbacks |
|---|---|---|---|
| Maternal or infant urine toxicology testing | Generally 1–3 d; longer for THC and benzodiazepines | Easy to collect Inexpensive | Women with OUD may have negative results by abstaining from use just before delivery or “rigging” (providing urine not their own) |
| Results readily available | |||
| Reflects only recent exposure | |||
| Maternal plasma | 12–72 h; longer for THC and benzodiazepines | Relatively inexpensive | Bruising |
| Results readily available | Reflects only recent exposure | ||
| Meconium | During 2nd-3rd trimester | May be difficult or impossible to collect (can be lost in utero) | Information about opioid use in the 2nd and 3rd trimester may not reflect abstinence closer to term, and may not be appropriate tests, particularly for women in OUD treatment |
| May take up to 5 d to be available | |||
| Expensive | |||
| Umbilical cord | During 2nd-3rd trimester | Easy to collect | Results may be delayed for several days |
| Expensive | |||
Abbreviation: THC, tetrahydrocannabinol.
NAS should be a considered a diagnosis of exclusion, and considering other diagnoses is important, because many infants with NAS are at elevated risk for infections and other comorbidities (Table 3). Further, some clinical signs of NAS (eg, irritability) can be present with other conditions, including sepsis. It is also important to avoid attributing every aspect of infant adaptation in the early postnatal period to NAS. Insensitive handling, pain, toxidromes related to substance or medication exposures, hunger, suboptimal physical environments, and transient tachypnea of the newborn are factors that may be misinterpreted as signs of NAS; however, all can occur concomitantly in an infant experiencing NAS.
Table 3.
Neonatal abstinence syndrome differential diagnosis
| Specific NAS Sign | Differential Diagnosis |
|---|---|
| Irritability | GE reflux |
| Pain/discomfort | |
| Sepsis | |
| Brain injury | |
| Fever | Sepsis (especially herpes simplex virus) |
| Hyperthyroidism | |
| Feeding problems | Oromotor dysfunction |
| Anomalies (eg, cleft palate, micrognathia, Pierre Robin sequence, genetic syndromes such as Prader Willi) | |
| Polycythemia | |
| Immaturity, including late preterm birth | |
| Brain injury | |
| Sepsis | |
| Jitteriness | Hypoglycemia |
| Hypocalcemia | |
| Immaturity | |
| Injury of the nervous system | |
| Myoclonic jerkinga | Not uncommon in opioid-exposed infants and can be mistaken for seizure activity |
| Seizures (rare in infants with NAS) | Hypocalcemia |
| Hypoglycemia | |
| Hypoxic-ischemic encephalopathy | |
| Brain hemorrhage/stroke | |
Abbreviation: GE, gastroesophageal.
Myoclonic jerks can be unilateral or bilateral, occur during sleep, and do not stop when the extremity or affected body part is held. They may be medication related. Electroencephalograms are not warranted in infants with myoclonic jerks. They generally do not respond to medications used to treat NAS.
The onset of signs of NAS varies with the maternal substance used and its half-life. Heroin-exposed infants exhibit signs of withdrawal the earliest, typically at 12 to 24 hours of age32; whereas, methadone- and buprenorphine-exposed infants begin to show symptoms at 48 to 72 hours of age.33 The onset of NAS can be delayed in some infants, beginning at 5 to 7 days of age, sometimes after the infant has been discharged from the hospital.34
ASSESSMENT FOR NEONATAL ABSTINENCE SYNDROME
Periodic and frequent assessment of the infant with NAS using standardized assessment tools, such as a modification of the Finnegan scale, is currently the standard of care in the United States. There are no empirically derived data to inform the use of any one tool as superior, and there is wide variation in tools used today. These tools are designed to frequently assess/reassess the infant and to determine whether initiation of medication therapy is needed (in approximately 50%−60% of opioid-exposed infants), dosing parameters, and eligibility for weaning. Ideally, infant scoring should include maternal input when appropriate. Drawbacks of these tools are the subjective reporting of signs/symptoms by caregivers; therefore, it is recommended that periodic interrater reliability training occur. Further, assessment tools do not consider dyadic communication and synchrony (ie, the mother’s ability to read, interpret, and respond appropriately to infant cues and the ability of the infant to effectively relay needs to the mother), which can be an important aspect of the infant’s functioning in the immediate postnatal period and beyond.
In general, the infant at risk for experiencing NAS is assessed with a score every 3 to 4 hours during the entire hospital stay, with the score representing the period since the last evaluation. A rescore is recommended for the institution or escalation of medication for NAS, to allow for assessment of the infant’s external (ie, soiled diapers, improper handling) or internal (ie, hunger) environments and their potential contribution to the infant’s display. In these cases, the infant is rescored within the 4-hour time frame after care for the noxious stimuli has been completed. When short-acting medications such as morphine are used for treatment, scoring intervals longer than 4 hours can result in more severe or rebound NAS expression as the medication is metabolized.35 Therefore, it is recommended to start scoring the infant at closer to 3 hours, when warranted, to avoid going beyond the 4-hour treatment window.
Scoring may need to be adjusted for older infants to reflect their progress developmentally. For example, the sleep item may be eliminated to allow the older infant to sleep for shorter periods between feedings so it does not “count against” the infant and result in a higher score than necessary. Recently, the Eat, Sleep, Console method for evaluation and treatment has resulted in less medication and shorter hospitalizations for infants with NAS.36 However, it remains to be seen if less appreciation for the widely variable infant expression of NAS, as would occur with assessment reduced to only 3 infant functions, will result in improved care. The impact of this approach on maternal comprehension of the infant and her ability to deliver appropriate care and on infant development, which should be the paramount goal of any intervention (instead of shorter hospitalization), is also unknown.
MANAGEMENT OF NEONATAL ABSTINENCE SYNDROME
Optimal management of the infant with NAS includes the following:
Nonpharmacologic management of the infant, beginning at birth and continuing throughout hospitalization and after discharge
Pharmacologic treatment for the subset of infants that cannot thrive with non-pharmacologic care alone
Comprehensive care of the mother
Nonpharmacologic Care
This has traditionally been thought of as dimming lights, swaddling, pacifier use, and gentle handling. However, it is actually more complex, requiring a focus on each unique mother-infant dyad and, when effectively implemented, can reduce or eliminate the need for medication for NAS treatment. Nonpharmacologic care is a set of interventions, ideally applied prenatally as well as in the postpartum period, which leads to a thorough understanding of the infant, the mother, and their interaction, resulting in modifications to care and the environment to optimize regulation of the dyad (Table 4).27 Nonpharmacologic care is provided to the infant and mother at all times, independent of initiation of medication, during the postnatal period and beyond.
Table 4.
Nonpharmacologic care of the maternal-infant dyad affected by neonatal abstinence syndrome
| Assessment Functioning of the: |
With the Goal of: |
|---|---|
| Infant | Implementing comforting techniques and environmental modifications that decrease signs of neurobehavioral dysregulation |
| Promoting the infant’s self-regulation | |
| Nurturing healthy development and interactive capabilities | |
| Mother | Promoting maternal self-regulation |
| Encouraging and supporting parenting confidence | |
| Fostering maternal ability to support her child’s healthy development and to maximize her interactional capacity | |
| Dyad | Bidirectional communication and dyadic synchrony |
Data from Velez M, Jansson LM. The opioid dependent mother and her newborn. J Addiction Med 2008;2:113-20.
Additional aspects of nonpharmacologic care include education of physicians, nurses, therapists, and the infant’s care providers regarding the unique features of NAS in the infant. Systematic observation of the newborn before, during, and after interventions can help to define these features, as well as differentiating them from typical competencies of infants of different gestational and chronologic ages. Based on the observed infant’s cues and behaviors, an approach is developed that includes modification of the environment and caregiver (mother, nurses, doctors) interactions to support the infant’s self-regulation.27
Observing the infant’s capacities in the 4 domains (see Fig. 2) and how they interact with each other, as well as how the infant responds to environmental and handling modifications, will help to guide nonpharmacologic interventions.27 For infants who display problems with sensory integration (ie, those infants who become dysregulated with ordinary stimuli), observing the infant’s responses (eg, changes in tone, autonomic function, etc) to careful introduction of visual, auditory, and touch stimuli can help to guide nonpharmacologic interventions. For example, if an infant becomes easily overstimulated to auditory but not visual stimuli, a provider might make the environment quiet, but not necessarily dim the lights. Swaddling is helpful for infants with hypertonicity, but may need to be modified for infants who have more autonomic features, such as fever. Teaching the handler to recognize infant signs that signal dysregulation (eg, signs of stress, such as color changes, hiccups, mottling, gas, vomiting, tachypnea, etc.) and ways to intervene to allay those symptoms, such as eliminating or reducing the stimuli that caused the signs, can be simple but important information to relay.
State control issues are common in infants with NAS, and can be reflected as poorly defined or absent states (Table 5). Infants with difficulties with state control can fluctuate between crying and sleeping, which can be problematic because a state 4 is necessary for feeding and interaction.37 Helping the infant to achieve both quiet sleep (state 1) and a quiet alert state (state 4) is important for the regulation of infant functioning in all domains. To attain this goal, caregivers need to be taught how to gently move the infant from sleep to awake states by eliminating all triggers of dysregulation and keeping the infant from stressful stimuli that will cause the infant to go directly to a state 5 or 6. Similarly, allowing the infant to fall back to a sleep state by reducing the same stimuli can promote more regular and prolonged sleep. Failing to intervene for early neuroregulatory problems can result in altered trajectories of development that can affect the child’s behavioral, cognitive, emotional, and social capacities later in life.
Table 5.
Infant sleep-wake states
| Infant State | Description |
|---|---|
| 1. Quiet sleep | Nearly still, with the occasional startle or twitch |
| 2. Active sleep | Some body movements |
| Rapid eye movements | |
| May smile or make fussy sounds | |
| 3. Drowsy | Variable activity level; the infant may startle and open and close eyes |
| 4. Quiet alert | Attentive with eyes wide and bright |
| Regular breathing | |
| 5. Active alert | Variable activity |
| Eyes open but dull or glazed | |
| Irregular breathing | |
| May have periods of fussiness | |
| 6. Crying | Increased activity |
| Skin color changes present | |
| Eyes tightly closed or open | |
| Responsive to stimuli | |
Adapted from Brazelton TB. Neonatal behavioral assessment scale. Clinics in developmental medicine No 50, 1973. p. 5–8. with permission. Available at: http://nidcap.org/wp-content/uploads/2013/12/Brazelton-1973-BNBAS.pdf.
Pharmacologic Care
Medication used to treat more severe NAS expression generally begins when the infant reaches a certain numerical score based on a scoring tool, indicating that the infant is not thriving with nonpharmacologic care alone. Although optimal scoring and treatment paradigms have not been scientifically defined, there are 2 general methods of approach, symptom-based and weight-based treatment algorithms. The weight-based approach treats infants on an mg per kg basis, providing higher initial doses of medication and higher doses to infants of larger weight. In the symptom-based approach, lower starting doses can be administered to infants with less severe expression, theoretically allowing mildly affected infants to be treated with less medication and to be discharged sooner from the hospital. Conversely, infants with more significant symptoms of NAS receive higher initial doses. One example of a symptom-based approach has been published previously.38
Optimal medications for NAS have not been defined, although first-line medications for opioid-induced abstinence should consist of opioids, such as morphine or methadone.39 Clonidine in combination with an opioid40 or as monotherapy41 may be as effective, but clinical trials are needed. Buprenorphine has been suggested as a first-line medication, and recent data for this medication are promising.42 Second-line medication use is reserved for those cases of severe or complex (usually polysubstance-induced) abstinence and is instituted when the infant is unable to be managed by one medication alone. These medications are usually clonidine or phenobarbital. Medications no longer used include paregoric, diazepam, or chlorpromazine.
Weaning an infant from medications used to treat NAS is done in a step-wise fashion, slowly over time, with an observation period at the end, before discharge. Occasionally, an infant who is undergoing weaning from medication experiences an escalation of signs of NAS, necessitating an increase in medication. These increases are generally at lower doses than initial escalation dosing (see Table 4). Some institutions have elected to discharge home infants on medication weans; however, although this practice does shorten hospital stays, it is associated with prolonged lengths of treatment that may not be necessary and potentially have a negative effect on development.43 In general, home medication weans should be avoided unless in a rigorous, closely monitored, and comprehensive program for infants with NAS.
CARE OF THE MOTHER AND BREASTFEEDING
Although maternal care is often not provided by pediatric providers, it is important to recognize that the mother’s well-being for the high-risk opioid-exposed dyad is imperative for the infant to thrive and develop optimally. Women with OUDs are at high risk for psychiatric comorbidities, most often depression but also anxiety, posttraumatic stress disorder and attention deficit hyperactivity disorder, abuse (physical, sexual, and emotional),44 and medical concerns (related to substance use, prostitution, or other factors associated with lifestyles of women with substance use disorders). Pediatricians are well-positioned to evaluate the dyad and to observe difficulties that interfere with the well-being of both mother and infant (Table 6).45,46 Providers should never assume that all family members or significant others are aware of maternal OUD or methadone or buprenorphine treatment and maternal confidentiality should be strictly maintained at all times. Judgmental or punitive attitudes have no place in the care of the woman with OUD. Pediatric providers may also provide the only link to any type of care for the dyad once the infant is discharged from the hospital. As such, the responsibility of care for the at-risk dyad as opposed to only the pediatric patient should be paramount. Pediatric providers should be able to provide links to appropriate maternal OUD treatment (ideally gender specific, comprehensive care programs that accept the infant’s presence) and psychiatric care if necessary.
Table 6.
The role of the pediatrician in the care of the mother with an opioid use disorder as part of an at-risk dyad
| Recommendation | Things to Consider | Actions |
|---|---|---|
| Think outside of the box | Consider the mother and her unique needs | Refer to comprehensive OUD treatment program that will accept the infant’s presence, if needed |
| Refer for trauma informed care, if warranted | ||
| Refer for psychiatric care, when warranted | ||
| Consider the environment in which the dyad resides | Discuss the needs of significant others (eg, substance use disorder treatment) and physical needs of the mother (eg, legal, housing, etc.) | |
| Provide multidisciplinary care, which is the gold standard for opioid-exposed dyads | Discuss dyadic care with obstetric providers Discuss the dyad with substance use treatment providers, if present (with maternal written consent) |
Ensure appropriate postpartum care, including contraceptive services, if desired All women on methadone or buprenorphine for OUD treatment have an addiction treatment provider and a written care plan. Discuss necessary and specific care of the infant as part of comprehensive care for the mother |
| Beware of overtly or covertly undermining dyadic attachment and communication, both within the dyad and with caregivers | Recognize that language and terms used are important | Avoid use of stigmatizing terms such as “addicted newborn,” “NAS baby,” “methadone baby,” “withdrawal baby,” “if your baby were a normal baby,” etc. All of these terms are pejorative and can negatively affect the interest of the mother with OUD to seek and engage in necessary medical and psychosocial treatment for herself and her infant |
| Provide trauma-informed care | Be aware that sexual, physical, and emotional trauma are common in women with OUD and are often not diagnosed before delivery | Refer for specialized psychiatric care as soon as a history of trauma is suspected |
| Modify the environment and care to assure the comfort of the mother based on her unique experiences. This may include only female nursing staff, no nighttime visitors, being aware of exposing the mother’s body, avoiding standing IVs that “tether” her to the bed | ||
| Recognize that there may be obstacles to breastfeeding,45 including early cessation46 | ||
Abbreviation: IV, intravenous.
Breastfeeding may provide particular benefit for the opioid-exposed dyad and is not contraindicated in most women with OUD47; formula feeding should not be the default choice. Medications used to treat maternal OUD (such as methadone, buprenorphine, and some psychiatric medications) are not incompatible with breastfeeding. In general, breastfeeding is recommended for women who are not in active use patterns for any substance of abuse or misuse (including marijuana) and who have maintained abstinence from substance use for a period of time before delivery.48 Similar to other breastfeeding women in the United States, breastfeeding is contraindicated for women who are positive for human immunodeficiency virus or are hepatitis C virus positive with cracked or bleeding nipples.49 Breastfeeding an infant with NAS can be affected by the infant’s signs and maternal appreciation of these signs, and women should receive support from an experienced lactation specialist.
FOLLOW-UP CARE FOR THE INFANT WHO EXPERIENCES NEONATAL ABSTINENCE SYNDROME
It is important to recognize that the infant affected by NAS, regardless of the need for pharmacotherapy for NAS, will be discharged from the hospital with residual signs that may last for months. In addition, in some instances, late-onset signs of abstinence can occur (particularly for opioid- and benzodiazepine-exposed infants), necessitating rehospitalization.50 As such, reliable, timely, and knowledgeable pediatric care should be instituted before delivery. This care should be accessible to the mother, who may not have transportation or who has programmatic obligations to receive medications daily. In addition, this pediatric care should be instituted immediately after hospital discharge to avoid complications of NAS that may include rehospitalization. The woman should be satisfied with their provider, because many women with OUD may have issues trusting medical professionals due to harmful past experiences, and providers not infrequently possess biases against this group of mothers. Ideally, this relationship should develop before hospital discharge, and the mother should be provided with information to be able to access care when she needs it.
Federal law requires that Plans of Safe Care be created for substance-exposed infants who should include ensuring a safety plan for the infant as well as connection of the mother with treatment resources. It is important to know your state’s reporting requirements for substance-exposed infants. Although the goal of child welfare involvement is to provide services to allow the family to stay together, sometimes alternative custody arrangements are needed. Communications about child welfare are stressful to the mother who is likely dealing with an infant experiencing NAS, causing additional guilt and anxiety. It is important to provide information to mothers in clear and consistent, nonthreatening language, while maintaining her confidentiality (other family members may not know about methadone maintenance, OUD treatment, etc.). It is important to provide mothers with follow-up care for OUD, including medication-assisted treatment, trauma services, psychiatric care, contraceptive services, and any other interventions that she needs, regardless of infant custody.
Pediatric care for opioid-exposed children should be frequent (ie, monthly in the first 6 months, every 1–2 months until 1 year, every 3 months in the second year, and biannually or more frequently afterward as needed) to enhance detection of failures of communication or outstanding needs of the mother. It should include periodic developmental assessment to allow for early detection of problems that warrant intervention, such as expressive language concerns, common in this population of children. A good rapport with the mother and a trusting mother-provider relationship is important to be able to discuss issues about maternal OUD treatment, violence exposure, or relapse that are multiply important for child health and development. Child welfare services should be used when warranted (such as in cases of child maltreatment, neglect, or harm) but not automatically for instances of maternal relapse or psychiatric concerns, where the more appropriate response is maternal engagement in or enhancement of services. Engagement of significant others can also be important, if appropriate. Asking about maternal OUD treatment, exposure to violence, contraceptive care, and medical and psychiatric health care should all be a part of these visits. A current list of referrals for treatment in any arena should be available, and close follow-up to assure that the mother is able to obtain this treatment is important. The dyad affected by opioid exposure is often challenging for the pediatric provider but should be considered among the highest risk children for myriad reasons; as such, these children should receive pediatric care of the highest quality with compassionate consideration for the mother and the environment. Pediatricians play a large role in addressing the opioid epidemic faced by the United States today by providing comprehensive care to affected children and their families.
SUMMARY
NAS is not simply a complex array of clinical signs expressed by a substance-exposed infant. It also represents complicated social, physical, and psychological stressors for the mother-infant dyad. Optimal care for the at-risk infant must be inclusive of the mother’s needs, beginning with standardized approaches to diagnosis and treatment, and emphasizing the importance of nonpharmacologic care and ensuring adequate follow-up for the child and the mother after hospital discharge.
KEY POINTS.
Neonatal abstinence syndrome (NAS) is a complex and variable disorder of neuroregulatory dysfunction in the infant; no one common problem can explain all signs.
Primary management of NAS should be nonpharmacologic assessment and care, which begins prenatally or at birth and continues throughout the infant’s hospitalization, regardless of the requirement for pharmacologic treatment for NAS.
Treatment of NAS necessitates assessment of and treatment for the mother.
Acknowledgments
Disclosures: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number RO1DA0413671 (Jansson) K23DA038720 (Patrick), R01DA045729 (Patrick), The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 national survey on drug use and health. Ann Intern Med 2017;167:293–301. [DOI] [PubMed] [Google Scholar]
- 2.Guy GP, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017;66:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedegaard H, Warner M, Minino AM. Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief 2017;294:1–8. [PubMed] [Google Scholar]
- 4.Patrick SW, Schumacher RE, Benneyworth BD, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 2012;307:1934–40. [DOI] [PubMed] [Google Scholar]
- 5.Patrick SW, Davis MM, Lehmann CU, et al. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015;35:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelman TNA, Villapiano N, Kozhimannil KB, et al. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004–2014. Pediatrics 2018;141(4) [pii:e20173520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villapiano NL, Winkelman TN, Kozhimannil KB, et al. Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr 2017;171:194–6. [DOI] [PubMed] [Google Scholar]
- 8.Ko JY, Patrick SW, Tong VT, et al. Incidence of neonatal abstinence syndrome - 28 States, 1999–2013. MMWR Morb Mortal Wkly Rep 2016;65:799–802. [DOI] [PubMed] [Google Scholar]
- 9.Jansson LM, Velez M. Neonatal abstinence syndrome. Curr Opin Pediatr 2012; 24:252–8. [DOI] [PubMed] [Google Scholar]
- 10.Hudak ML, Tan RC, AAP The Committee on Drugs, Committee on fetus and newborn. Neonatal drug withdrawal. Pediatrics 2012;129(2):e540–60. [DOI] [PubMed] [Google Scholar]
- 11.Seligman NS, Salva N, Hayes EJ, et al. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol 2008;199:396.e1–7. [DOI] [PubMed] [Google Scholar]
- 12.Wachman EM, Newby PK, Vreeland J, et al. The relationship between maternal opioid agonists and psychiatric medications on length of hospitalization for neonatal abstinence syndrome. J Addict Med 2011;5:293–9. [DOI] [PubMed] [Google Scholar]
- 13.Pritham UA, Paul JA, Hayes MJ. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. J Obstet Gynecol Neonatal Nurs 2012; 41:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loudin S, Murray S, Prunty L, et al. An atypical withdrawal syndrome in neonates prenatally exposed to gabapentin and opioids. J Pediatr 2017;37:1108–11. [DOI] [PubMed] [Google Scholar]
- 15.Jones HE, Heil SH, Tuten M, et al. Cigarette smoking in opioid-dependent pregnant women: neonatal and maternal outcomes. Drug Alcohol Depend 2013;131: 271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction 2012;107(Suppl 1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huybrechts KF, Bateman BT, Desai RJ, et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids an psychotropic medications: cohort study. BMJ 2017;358:j3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013;309:1821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wachman EM, Hayes MJ, Lester BM, et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J Pediatr 2014;165: 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welle-Strand GK, Skurtveit S, Jansson LM, et al. Breastfeeding among women in opioid maintenance treatment in Norway and it’s influence on neonatal abstinence syndrome. Acta Paediatr 2013;102:1060–6. [DOI] [PubMed] [Google Scholar]
- 21.Charles MK, Cooper WO, Jansson LM, et al. Male sex associated with increased risk for neonatal abstinence syndrome. Hosp Pediatr 2017;7:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson LM, DiPietro JA, Elko A, et al. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonatal Med 2007;20:677–85. [DOI] [PubMed] [Google Scholar]
- 23.Gibson KS, Stark S, Kumar D, et al. The relationship between gestational age and the severity of neonatal abstinence syndrome. Addiction 2017;112:711–6. [DOI] [PubMed] [Google Scholar]
- 24.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95: 115–28. [PubMed] [Google Scholar]
- 25.Abrahams RR, Kelly SA, Payne S, et al. Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Can Fam Physician 2007;53: 1722–30. [PMC free article] [PubMed] [Google Scholar]
- 26.MacMillan KDL, Rendon CP, Verma K, et al. Association of rooming-in with outcomes for neonatal abstinence syndrome: a systematic review and meta-analysis. JAMA Pediatr 2018. 10.1001/jamapediatrics.2017.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velez M, Jansson LM. Non-pharmacologic care of the opioid dependent mother and her newborn. J Addict Med 2008;2:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Als H. Toward a synactive theory of development: promise for the assessment and support of infant individuality. Infant Ment Health J 1982;3:229–43. [Google Scholar]
- 29.Als H. Neurobehavioral organization of the newborn: opportunity for assessment and intervention. NIDA Res Monogr 1991;114:106–16. [PubMed] [Google Scholar]
- 30.World Health Organization. Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. (Annex 3 Screening instruments for substance use in prenatal or pregnant women). Switzerland (Geneva): WHO Document Production Services; 2014. Available at: http://apps.who.int/iris/bitstream/handle/10665/107130/9789241548731_eng.pdf;jsessionid=AC00997ED4A94B7074D04344600E7A00?sequence=1. [PubMed] [Google Scholar]
- 31.Chasnoff IJ, McGourty RF, Bailey GW, et al. The 4P’s Plus screen for substance use in pregnancy: clinical application and outcomes. J Perinatol 2005;25:368–74. [DOI] [PubMed] [Google Scholar]
- 32.Zelson C, Rubio E, Wasserman E. Neonatal narcotic addiction: 10 year observation. Pediatrics 1971;48:178–89. [PubMed] [Google Scholar]
- 33.Gaalema DE, Heil SH, Badger GJ, et al. Time to initiation to treatment for neonatal abstinence syndrome in neonates exposed in utero to buprenorphine or methadone. Drug Alcohol Depend 2013;133:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandall SR, Gartner LM. Late presentation of drug withdrawal symptoms in newborns. Am J Dis Child 1974;127:58–61. [DOI] [PubMed] [Google Scholar]
- 35.Jones HC. Shorter dosing interval for opiate solution shortens hospital stay for methadone babies. Fam Med 1999;31:327–30. [PubMed] [Google Scholar]
- 36.Grossman MR, Lipshaw MJ, Osborn RR, et al. A novel approach to assessing infants with neonatal abstinence syndrome. Hosp Pediatr 2017;8(1). 10.1542/hpeds.2017-0128. [DOI] [PubMed] [Google Scholar]
- 37.Als H, Tronick E, Lester BM, et al. The Brazelton neonatal behavioral assssment scale. J Abnorm Child Psychol 1977;5:215–31. [DOI] [PubMed] [Google Scholar]
- 38.Jansson LM, Velez M, Harrow C. The opioid exposed newborn: assessment and pharmacologic management. J Opioid Manag 2009;5(1):47–58. [PMC free article] [PubMed] [Google Scholar]
- 39.Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev 2005;(3):CD002059. [DOI] [PubMed] [Google Scholar]
- 40.Agthe AG, Kim GR, Mathias KB, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized controlled trial. Pediatrics 2009; 123:e849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streetz VN, Gildon BL, Thompson DF. Role of clonidine in neonatal abstinence syndrome: a systematic review. Ann Pharmacother 2016;50:301–10. [DOI] [PubMed] [Google Scholar]
- 42.Kraft WK, Adeniyi-Jones SC, Chernova I, et al. Buprenorphine for the treatment of neonatal abstinence syndrome. N Engl J Med 2017;377:997–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maalouf FI, Cooper WO, Slaughter JC, et al. Outpatient pharmacotherapy for neonatal abstinence syndrome. J Pediatr 2018;141(4) [pii:e20173520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velez ML, Montoya ID, Jansson LM, et al. Exposure to violence among substance-dependent pregnant women and their children. J Subst Abuse Treat 2006;30:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kendall Tackett K. Breastfeeding and the sexual abuse survivor. Unit 10 lactation consultant series two. North Carolina (US): La Leche League International publication; 2003. No. 1561. Available at: http://breastfeedingmadesimple.com/wp-content/uploads/2016/02/LCSA.pdf. [DOI] [PubMed] [Google Scholar]
- 46.Sorbo MF, Lukasse M, Brantsaeter AL, et al. Past and recent abuse is associated with early cessation of breastfeeding: results from a large prospective cohort in Norway. BMJ Open 2015;5(12):e009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansson LM, Velez M. Lactation and the substance-exposed dyad. J Perinat Neonatal Nurs 2015;29:277–86. [DOI] [PubMed] [Google Scholar]
- 48.Jansson LM. Academy of breastfeeding medicine protocol #21: guidelines for breastfeeding and the drug-dependent woman. Breastfeed Med 2009;4:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jansson LM, Patrick SW. Breastfeeding and the substance-exposed dyad In: Wright TE, editor. Opioid use disorders in pregnancy: management guidelines for improving outcomes. Cambridge (United Kingdom): Cambridge University Press; 2018. p. 127–38. [Google Scholar]
- 50.Patrick SW, Burke JF, Biel TJ, et al. Risk of hospital readmission among infants with neonatal abstinence syndrome. Hosp Pediatr 2015;5:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]