Abstract
PURPOSE
Little is known, or has been published previously, regarding consolidated data on the epidemiology of gynecologic cancers (GC) in Brazil. This article describes the incidence, morbidity, and mortality of women in Brazil affected with GC between the years of 2000 and 2017.
METHODS
Incidence, morbidity, and mortality data from patients with a diagnosis of one out of the five most common GC, cervical (CC), uterine (UC), ovarian (OC), vulvar (VvC), and vaginal (VgC), were obtained from three governmental sources of data.
RESULTS
From 2000 to 2015 CC, OC, and VgC incidence rates (IRs) decreased, whereas the IRs for UC and VvC remained relatively stable. Data from 382,932 women with GC were analyzed. Most patients presented with locally advanced or advanced disease at diagnosis: 60.1% of patients with CC, 31.2% of patients with UC, 67.2% of patients with OC, 45.2% of patients with VvC, and 67.0% of patients with VgC. Time from diagnosis to first treatment was ≥ 60 days in 58.0% of patients with CC, 58.5% of patients with UC, 27.0% of patients with OC, 55.3% of patients with VvC, and 52.7% of patients with VgC. Regarding mortality rates (MRs), with the exception of CC, UC, and VvC, which showed a slight decrease, MRs remained stable between 2000 and 2017.
CONCLUSION
A comparison with international data indicates that Brazilian patients are diagnosed with more advanced disease and face a longer delay between diagnosis and first treatment. Despite advances in screening and treatment, GC mortality has not decreased satisfactorily in this country.
INTRODUCTION
Most clinical and epidemiologic data on gynecologic cancers (GC) come from high-income countries (HICs), and there is a dearth of information on low- and middle-income countries (LMICs).1 Every 3 years, the Brazilian National Cancer Institute (INCA) publishes incidence data. According to the latest publication, cervical cancer (CC), ovarian cancer (OC), and uterine cancer (UC) were the third, seventh, and eighth most common cancers in Brazil, with 16,590, 6,650, and 6,540 new cases, respectively, expected in 2020.2 Brazil is a continental country with diverse and highly admixed populations. The North (N)/Northeast (NE) and South (S)/Southeast (SE)/Middle-West (MW) are distinct regions in terms of the development index, in which the N/NE is the poorest (Human Development Index [HDI] = 0.667 and 0.663, respectively) and the S/SE/MW, the richest (HDI = 0.754, 0.766, and 0.757, respectively), with better access to cancer prevention and treatment.3
CONTEXT
Key Objective
What is the panorama of gynecologic cancer (GC) in a developing country? Little is known regarding the epidemiology of GC in low- and low-middle-income countries, places where patients can face many obstacles in receiving preventive care and treatment for many types of cancer. The main purpose of this article was to discuss the current epidemiologic panorama of the five major gynecologic tumors in Brazil.
Knowledge Generated
Compared with international data, Brazilian patients are diagnosed with more advanced disease and face a longer delay between diagnosis and first treatment. Despite advances in screening and treatment, GC mortality has not decreased satisfactorily in this country.
Relevance
These data may also mirror those of other low- and low-middle-income countries, because most barriers are not common to Brazil.
To date, there has been no population-based analysis of the basic characteristics of GC in Brazil. The purpose of this study was to describe the whole scenario of women with GC in Brazil, including incidence, morbidity, and mortality, which could mirror other LMICs around the world, to inform future clinical management and local policy decisions.
METHODS
Ethical committee approval was not required because only secondary data available on the Internet were used in this study. The ecological study was conducted based on three governmental sources of data: population-based cancer registries (PBCRs), hospital-based cancer registries (HBCRs), and the National Mortality Information System. Cases were identified according to the International Classification of Diseases (10th revision) codes: C53 (CC), C54/55 (UC), C56 (OC), C51 (vulvar cancer [VvC]), and C52 (vaginal cancer [VgC]).
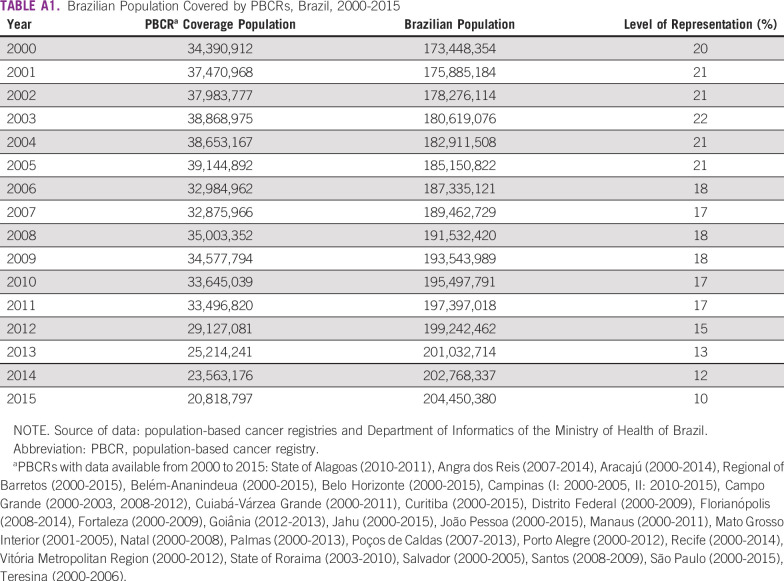
For incidence analysis, data from 30 Brazilian PBCRs with at least 2 years of consolidated information between 2000 and 2015 were obtained in February 2020. All new cancer cases diagnosed in permanent residents in a clearly defined geographic area were considered incident. The raw data are available on the Internet at INCA’s site.4 Population coverage by the PBCRs is displayed in Appendix Table A1. Crude incidence rates (IRs) of GC per 100,000 women by age group and year of diagnosis were calculated by dividing the number of new cases by the female population. Rates were adjusted according to the female world standard population. The mean IRs allowed the estimation of an annual IR for the country as a whole.
Demographic and clinical data of women with GC who were diagnosed and treated between 2000 and 2016 in 336 Brazilian hospitals (including the 26 states and the Federal District) came from the HBCR Integrator System available on the Internet.4 Data were downloaded in December 2019. The following variables were collected: age at diagnosis, self-reported ethnicity/skin color according to the Brazilian Institute of Geography and Statistics,5 schooling, marital status, time between diagnosis and first treatment (defined as the time interval between the GC diagnosis in a histopathologic report and the beginning of the first treatment, labeled as < 60 days v ≥ 60 days, using the Brazilian Law of Sixty Days as a reference), Federation of Gynecology and Obstetrics (FIGO) stage, and early death (defined as death occurring before the end of the first line of treatment, that is the first set of therapies that has been applied for antineoplastic drugs uninterrupted or all cancer-directed treatment administered to the patient within 4 months after the initiation of therapy).
Mortality data for GC in Brazil between 2000 and 2017, disaggregated by age and year of diagnosis for the country as a whole, were retrieved on March 2020 from the National Mortality Information System.6 Crude annual mortality rates (MRs) of GC per 100,000 women were calculated by dividing the number of deaths by the female population. Rates were adjusted for the female world standard population. The population used as a denominator for IRs and MRs was estimated by the Interagency Network of Information for Health for the period between 2000 and 2017, taken from the Website of the Department of Informatics of the Ministry of Health of Brazil.6
Data were analyzed using SPSS version 21.0. Descriptive statistics were used for demographic, epidemiologic, and clinical characteristics. The analysis was based on valid data; missing data were excluded from the analysis, but they are displayed in the tables. Kolmogorov-Smirnov was used to check the normal distribution of age, and analysis of variance was used to compare mean age between distinct gynecologic cancer subtypes. The χ2 test was performed to compare the distribution of the categoric variables. To identify changes in incidence and mortality trends, Joinpoint regression analysis was performed. Annual percent changes (APCs) with 95% CIs were calculated. Mean IRs and interquartile ranges were used as measures of central tendency to synthesize IRs for each PBCR and for the Brazilian macroregions. Differences were considered significant when the P value was < .05.
RESULTS
Results refer to 2000-2015 incidence data from 30 PCBRs covering 10% to 22% of the Brazilian population, morbidity characteristics of 382,932 patients with GC registered by the Integrator System from 2000 to 2017, and analysis of 203,975 GC deaths recorded between 2000 and 2017. The most relevant characteristics of each are described later in the text and summarized in the tables and figures.
CC
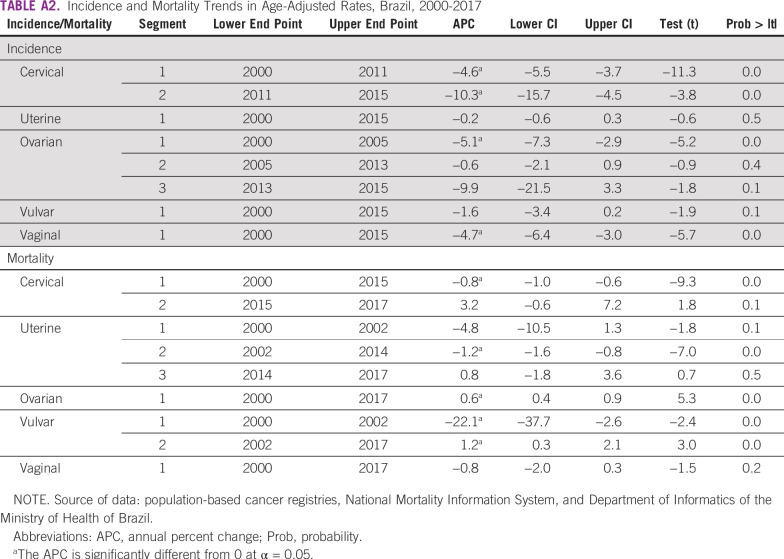
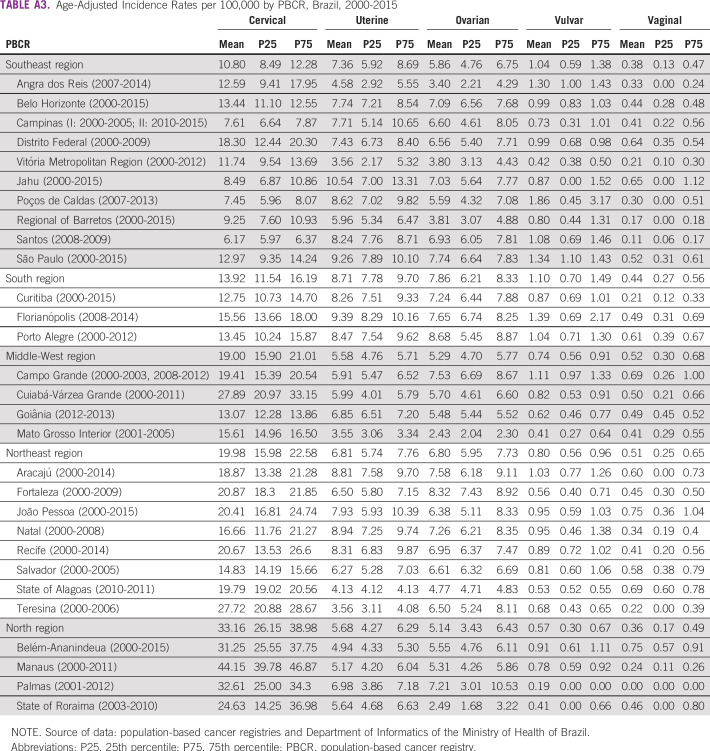
The CC adjusted IR for the whole population fell from 21.15 per 100,000 women in 2000 to 11.44 per 100,000 women in 2015, an APC of −4.6 from 2000 to 2011 and −10.3 from 2011 to 2015 (Fig 1A; Appendix Table A2). IRs were higher in the N region (33.16 per 100,000; Appendix Table A3).
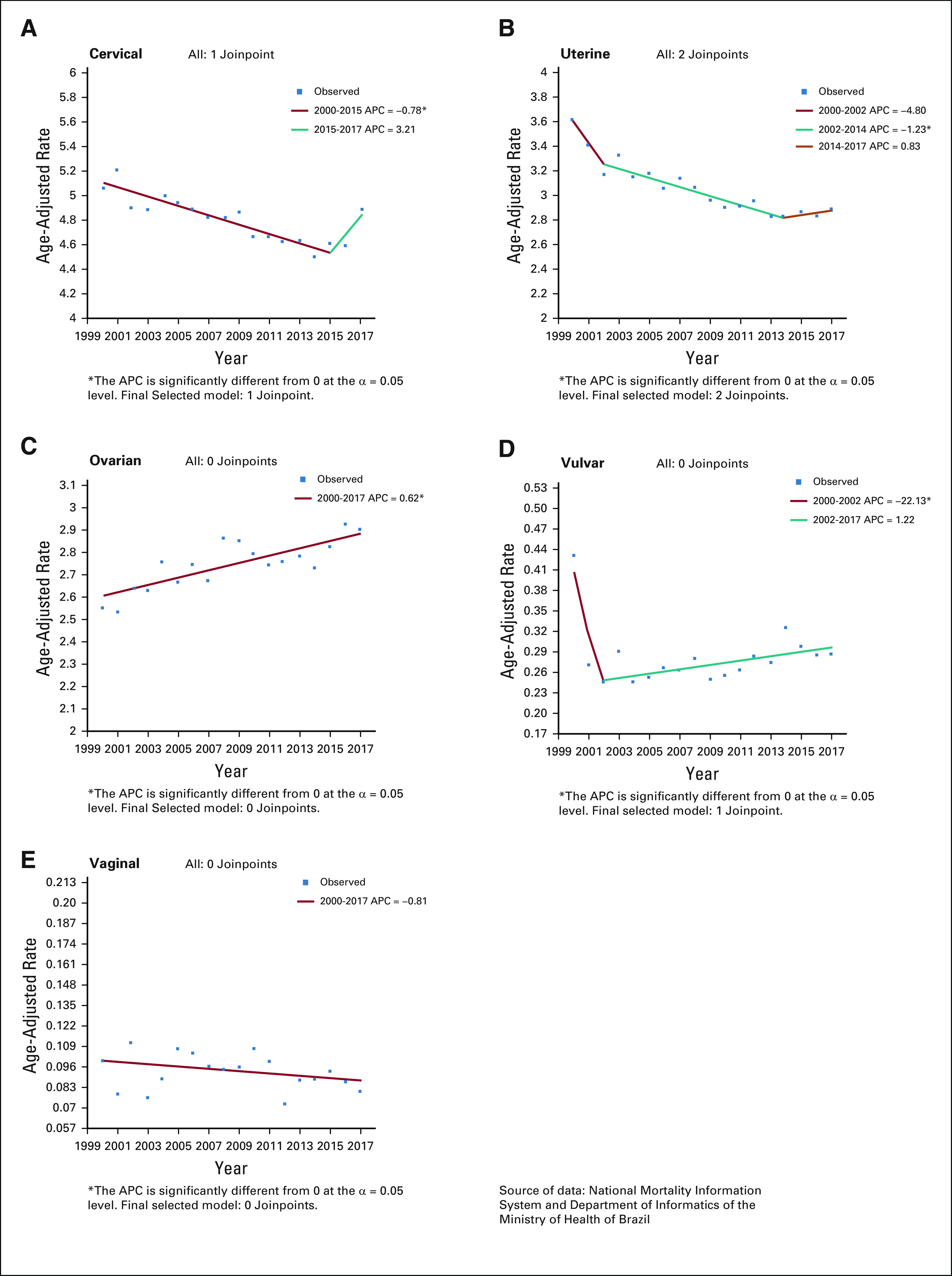
FIG 1.
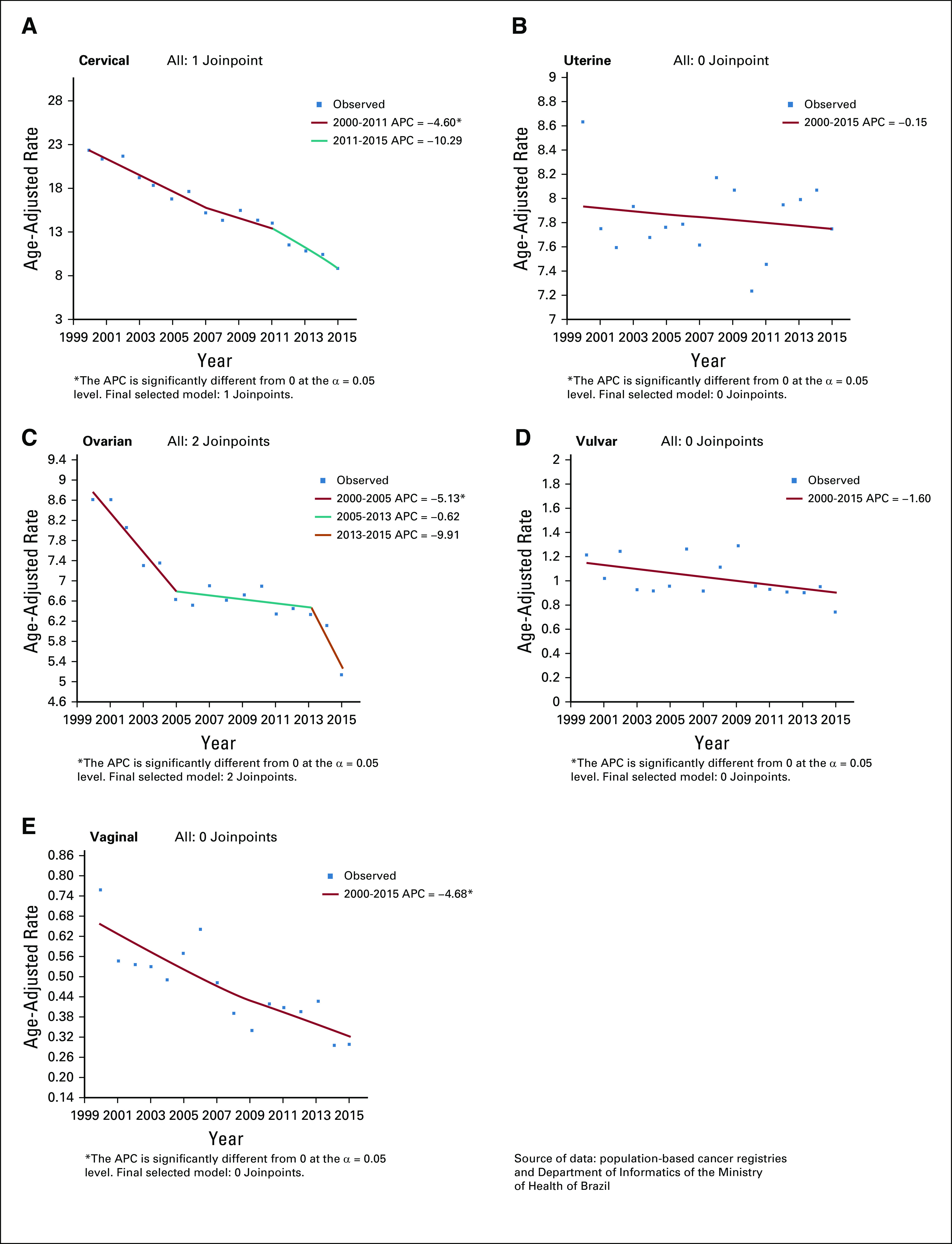
Age-adjusted incidence rates (2000-2015) per 100,000 of gynecologic cancer in Brazil. (A) Cervical cancer. (B) Uterine cancer. (C) Ovarian cancer. (D) Vulvar cancer. (E) Vaginal cancer. APC, annual percent change.
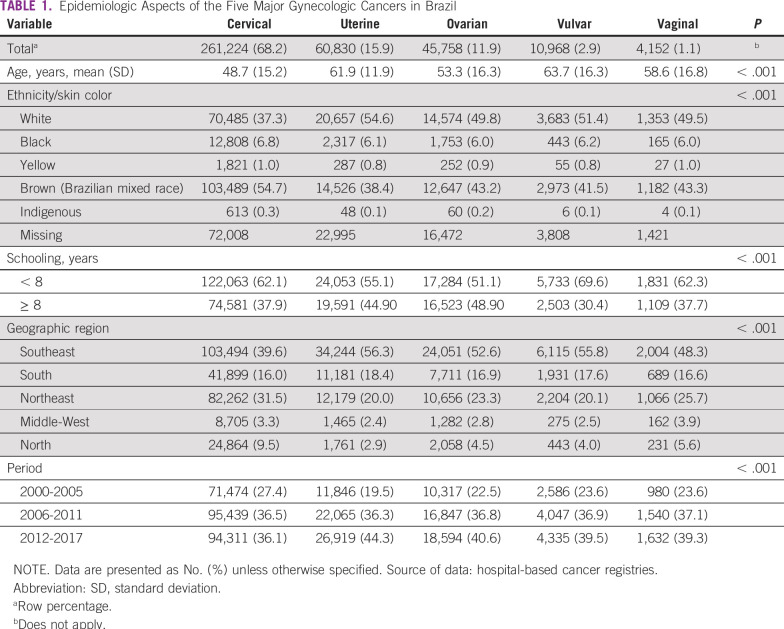
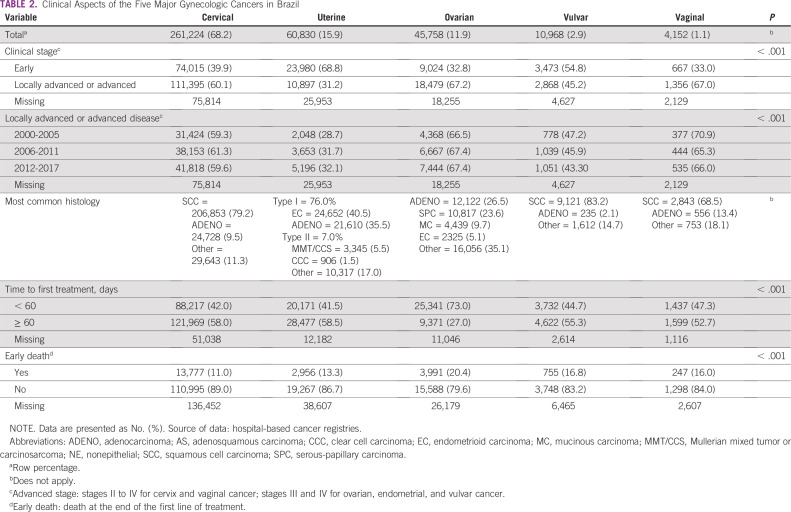
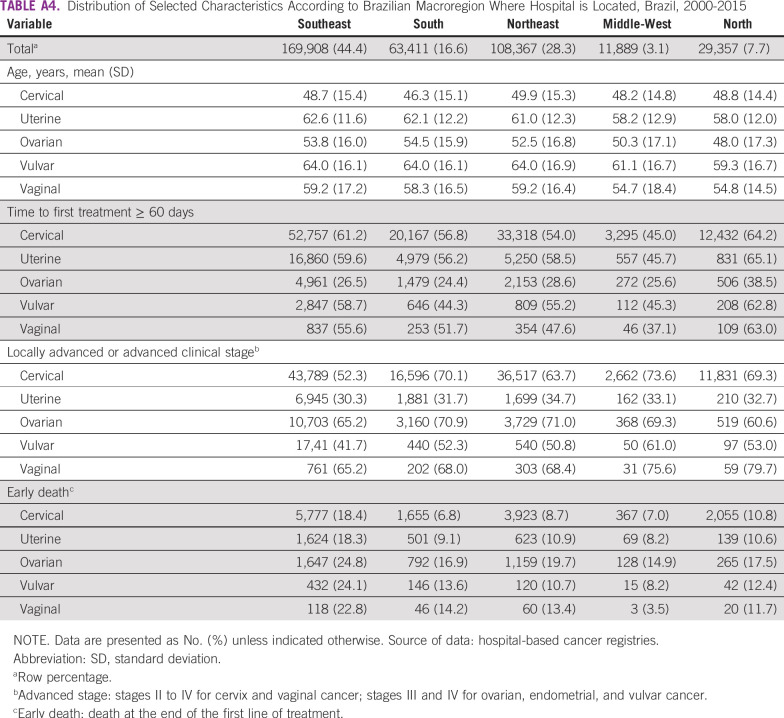
A total of 261,224 new cases were identified from the HBCR Integrator System between 2000 and 2017, corresponding to 68.2% of all GC analyzed. Mean age at diagnosis was 48.7 (±15.2) years (Table 1). Most patients (60.1%) presented with advanced CC or locally advanced CC (LACC) at diagnosis, and squamous cell carcinoma (SCC) represented 79.2% of the cases (Table 2). Comparing the SE region with other regions, fewer patients were diagnosed with advanced CC or LACC (52.3% in SE v 63.7% to 73.6% in the other regions; Appendix Table A4). The period between diagnosis and first treatment was more than 60 days in 58.0%. Early deaths were as high as 11.0% (Table 2). Regarding mortality, between 2000 and 2015, there was a slight decrease in CC MRs (APC, −0.8), from 5.05 per 100,000 women in 2000 to 4.61 per 100,000 women in 2015; the MR remained stable from 2015 through 2017 (Fig 2A; Appendix Table A2).
TABLE 1.
Epidemiologic Aspects of the Five Major Gynecologic Cancers in Brazil
TABLE 2.
Clinical Aspects of the Five Major Gynecologic Cancers in Brazil
FIG 2.
Age-adjusted mortality rates (2000-2017) per 100,000 of gynecologic cancer in Brazil. (A) Cervical cancer. (B) Uterine cancer. (C) Ovarian cancer. (D) Vulvar cancer. (E) Vaginal cancer. APC, annual percentage change.
UC
Between 2000 and 2015, the UC adjusted IR for the whole population remained relatively stable, with the highest incidence in 2008 (8.14 per 100,000 women) and the lowest in 2010 (7.23 per 100,000 women; Fig 1B; Appendix Table A2). The S and SE regions showed the highest IRs: 8.71 and 7.36 per 100,000 women, respectively (Appendix Table A3).
A total of 60,830 UC cases were identified from the HBCR Integrator System between 2000 and 2017, corresponding to 15.9% of GC cases analyzed and the second most common GC in this series. Mean age at diagnosis was 62.9 (±11.9) years (Table 1). Endometrioid (40.5%) and adenocarcinoma NOS (35.5%) tumors were the most common morphologic subtypes. In general, patients were diagnosed more frequently in stages I and II (68.8%). However, in the N, MW, and NE regions, patients were more likely to be diagnosed at stages III and IV than they were in the S and SE regions (32.7% to 34.7% v 30.3% to 31.7%; Appendix Table A4). Time between diagnosis and first treatment exceeded 60 days in 58.5%. Early deaths occurred in 13.3% of patients (Table 2). Regarding mortality, rates decreased between 2002 and 2014, from 3.17 to 2.81 per 100,000 women (APC, −1.2 between 2002 and 2014), and then remained stable until the end of the period (Fig 2B; Appendix Table A2).
OC
For OC, between 2000 and 2005, the adjusted IR for the whole population fell from 8.62 to 6.62 (APC, −5.1), remaining without significant changes until 2015 (Fig 1C; Appendix Table A2). The highest IR was observed for the S region (7.86 per 100,000 women; Appendix Table A3)
Over the studied period, 45,758 cases of OC were found in the HBCR Integrator System between 2000 and 2017. Mean age at diagnosis was 53.3 (±16.3) years (Table 1). Adenocarcinoma NOS and serous histologies were the most common types, representing 26.5% and 23.6%, respectively. Stages III and IV represented 67.2% of the cases. Patients were more likely to be diagnosed with advanced-stage disease in the S, NE, and MW regions (69.3% to 71.0%) than in the other regions (60.6% to 65.2%; Appendix Table A4). Time between diagnosis and first treatment received was greater than 60 days in 27.0%. Early deaths occurred in 20.4% of patients (Table 2).
Regarding mortality, between 2000 and 2017, rates increased by 0.6% a year (APC, +0.6) with the lowest rate in 2001 (2.53 per 100,000 women) and the highest rate in 2016 (2.94 per 100,000 women; Fig 2C; Appendix Table A2).
VvC
For VvC, between 2000 and 2015, the adjusted IR for the whole population remained stable, ranging from 1.22 to 0.73 per 100,000 women (Fig 1D; Appendix Table 2). IRs were 1.10 and 1.04 per 100,000 women in the S and SE regions, respectively (Appendix Table A3).
A total of 10,968 cases of VvC were found in the HBCR Integrator System between 2000 and 2017, corresponding to 2.9% of GC. The mean age at diagnosis was 63.7 (±16.3) years (Table 1). Patients were generally diagnosed at stages II-IV (67.0%), and SCC was the most common histology (83.2%). Time between diagnosis and first treatment exceeded 60 days in 52.7%. Early deaths occurred in 16.0% of patients (Table 2).
Regarding mortality, between 2000 and 2002, the rates decreased from 0.43 in 2000 to 0.24 in 2002 (APC, −22.1) and then increased, reaching 0.29 per 100,000 women in 2017 (APC, +1.2; Fig 2D; Appendix Table A2).
VgC
For VgC, between 2000 and 2015, adjusted IR for the world population fell by 4.7% per year (APC, −4.7); over this period, the highest incidence was 0.76 per 100,000 women in 2000 and the lowest incidence was 0.29 per 100,000 women in 2014 and 2015 (Fig 1E; Appendix Table A2). The NE (0.51 per 100,000 women) and MW (0.52 per 100,000 women) regions had the highest IRs (Appendix Table A3).
A total of 4,152 cases of VgC were identified from the HBCR Integrator System between 2000 and 2017. Mean age at diagnosis was 58.6 (±16.8) years; Table 1). VgC was diagnosed at locally advanced stages (stages II-IV) in 67.0% of patients, and SCC corresponded to 68.5% of cases. Patients were more likely to be diagnosed at advanced-stage disease in the N region (79.7%; Appendix Table A4). Time between diagnosis and first treatment exceeded 60 days in 52.7% of cases. Early deaths occurred in 16.0% of cases (Table 2).
The VgC MR between 2000 and 2017 remained relatively stable, ranging from 0.10 per 100,000 women in 2000 to 0.08 per 100,000 women in 2017 (Fig 2E; Appendix Table A2).
DISCUSSION
To our knowledge, this is the largest study to be conducted in Brazil evaluating the current demographic and clinical aspects of GC. The severity of the global divide in cancer morbidity and mortality between LMICs and HICs is well known, and it is expected to increase.7 Added to that, cancer incidence in LMICs, GC included, may be underestimated, because it is rare for LMICs to have reliable cancer registries and reporting systems. The aim of the current analysis was to collect Brazilian GC data not reported previously and to inform future clinical management and local policy decisions, which may help other LMICs with limited knowledge of their own numbers.
According to the GLOBOCAN database, the prevalence of OC and UC is higher in HICs, particularly northern Europe and North America. In contrast, IRs are much higher for CC in LMICs, where 80% of all cases occur.7
Mainly because of its epidemiologic relevance, more information regarding CC in LMICs is now available. The current worldwide estimated age-standardized incidence, according to the WHO, is divided into the following four tiers: 7.3-11.5, 11.5-18.1, 18.1-26, and > 26 cases per 100,000 women18. According to this analysis, Brazil is at the second lowest tier, but significant heterogeneity exists within the country, and approximately one third of the Brazilian states, all of them located in the northern areas, have an incidence higher than 18.1 cases per 100,000 women, similar to the rates of low-income and lower-middle-income countries (despite the fact that the World Bank currently classifies Brazil as an upper intermediate-income country). In regions with low socioeconomic indexes, such as the N/NE states, CC represented 85.5% and 75.1% of GC, respectively, compared with 65.9% and 57.5%, respectively, in the wealthier S and SE of Brazil. This distribution is in line with the worldwide incidence, with more CC in poorer areas and a higher proportion of UC and OC in richer and more developed regions.7 Addressing these internal specificities in LMICs may play a key role in cancer control. For example, the WHO CC Elimination Modeling Consortium predicted that countries that have weaker health systems and a CC incidence of > 20 cases per 100,000 women-years that start human papillomavirus (HPV) vaccination alone will progress more slowly toward elimination, but that adding screening to HPV vaccination can significantly accelerate CC incidence decline. Tailored strategies are fundamental.
A rising problem in Brazil is EC. The estimated age-standardized IRs vary from one to 30 cases/100,000 women across countries globally, with the highest rates in HICs, where almost two thirds of all cases occur. The incidence of EC is increasing in Brazil, potentially because of more Westernized lifestyles and higher rates of obesity, as well as an increase in life expectancy. Currently, 20.7% of Brazilian women are obese, and 53.9% are overweight.8 Therefore, it is imperative to implement EC prevention and early detection policies and improve radiotherapy access.
Most Brazilian patients with GC, with the exception of EC, are diagnosed with locally advanced disease. When analyzing the different time periods, it is remarkable to note that there was no significant impact on tumor stage and GC mortality over time. One possible explanation for the persistent high MR is that prevention and screening strategies have yet to reach the most vulnerable populations. Treatment delays also probably contribute. Compared with other LMICs, considering CC FIGO stage, the Brazilian pattern is similar to North Africa and the Middle East, where approximately 70% of patients present with locally advanced disease at diagnosis.7 Because OC is a rapidly proliferating tumor, most patients across the world are at stage III or IV at diagnosis; in Brazil, this figure is 70%.
Focusing on CC, the high illiteracy rate of the current population, coupled with the lower than expected screening coverage in the Brazilian population (79% of patients in the target population were screened in 2013, which is less than the 85% goal), help illustrate the core of the problem.9 It has been reported previously that screening acceptance is higher in women with better access to education, and that delivery strategies should be aimed at encouraging older and less educated women to get screened.10
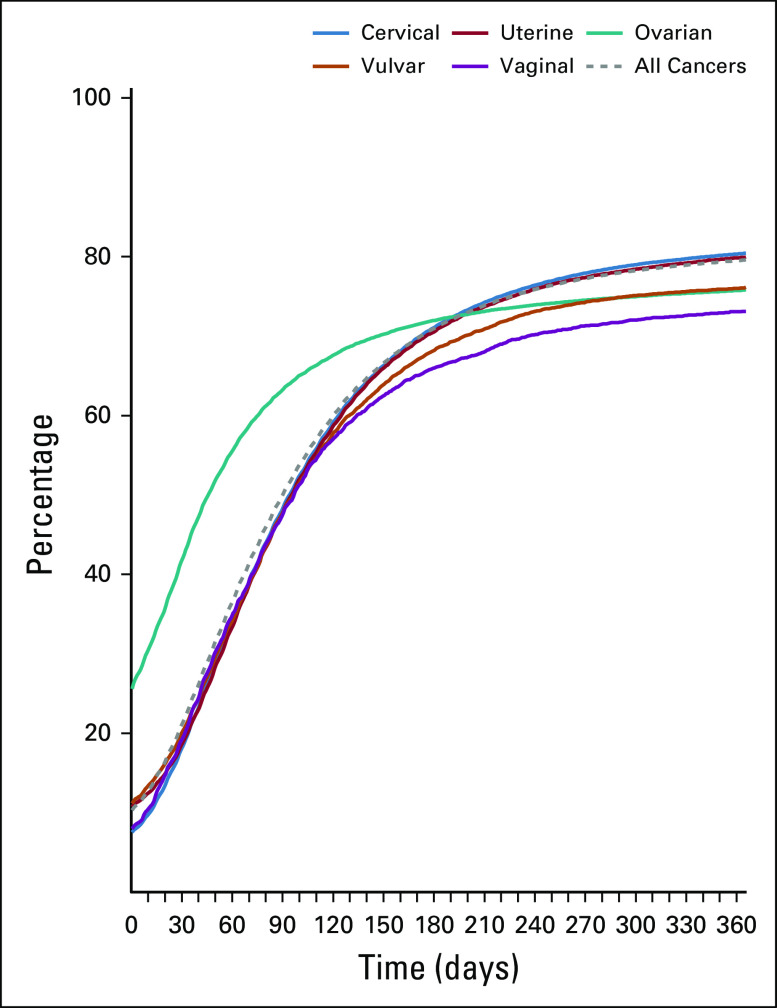
Regarding access to treatment, Brazilian patients with GC face significant hurdles. Even after the so-called Law of Sixty Days, which guarantees treatment of patients within 60 days of diagnosis, no significant advances have been achieved, with as many as 39% of patients with GC starting treatment after 60 days of diagnosis, and 24% after 90 days (Appendix Fig A1).11 Treatment delay, together with advanced stage at diagnosis (46% in CC compared with 20.6% in American women), may help explain the high percentage of early deaths (13.2%) found in this cohort. In a recent article regarding barriers in primary surgery in OC in Latin America, a number of obstacles, such as access barriers and inadequate resources, are identified.12 In recent years, Brazil has suffered a shortage of radiation machines as well as human resources. A Brazilian poll about radiotherapy needs in 2012 showed that 135 machines are necessary to cover the whole population, and to meet these needs, a planned expansion of 80 machines was proposed for 2015.13 However, to date, only 22 additional machines are in operation and 16 are in process.14,15 Another concern is that there is no formal subspecialization in gynecology oncology in Brazil. Some patients are treated by general gynecologists or general surgeons, who may not be ideally trained in oncologic practices.
The current data emphasize the need for preventive strategies in Brazil. Considering the high CC burden and the fact that it often has the same etiologic factor (HPV) as VvC and VgC, it would be of value if our leaders increased efforts to improve adherence to the national HPV vaccination program. According to the Ministry of Health, there was a 23% decrease in vaccination coverage from 2014 to 2015, a dramatic reduction in 1 year.16 An article regarding vaccination rates in Latin America confirms this trend.17 Furthermore, because the vaccine is not therapeutic and patients who will be diagnosed with CC in the next 15 years are already HPV infected, Papanicolau test screening method adherence must also be reinforced.
This analysis has several limitations inherent to retrospective studies, such as the inadequacy of the collected information, confounding factors, and missing information. Because this is partly a hospital-based study, the cohort does not represent the entire Brazilian population; hospital-based studies are more susceptible to selection bias than are studies that are based on population registries, which were used here only for incidence data. Moreover, over the period of this study, changes in GC classification and staging have taken place. In the current study, the IRs of CC and EC were estimated without the elimination of hysterectomized women from the population at risk, because of a lack of these specific data. Conversely, strengths should also be mentioned, such as the high number of patients included, the use of trained professionals for data collection, the coverage of all regions in Brazil, and the use of incidence data from VvC and VgC, which, to our knowledge, had never been reported before in this country.
GC causes significant burden in Brazil, and these data could mirror those of other LMICs. Most patients present with advanced-stage disease at diagnosis and face long waiting times to start treatment, and therefore, a high percentage of early deaths occur. In addition, GC mortality has not been decreasing satisfactorily in this country. It is necessary to restructure GC control in Brazil, including technology incorporation and doctor training. Special emphasis should be placed on HPV vaccination and Papanicolau test adherence, because CC continues to be at the core of the GC problem in Brazil.
Appendix
FIG A1.
Cumulative percentage of women treated per day after diagnosis, by cancer type.
TABLE A1.
Brazilian Population Covered by PBCRs, Brazil, 2000-2015
TABLE A2.
Incidence and Mortality Trends in Age-Adjusted Rates, Brazil, 2000-2017
TABLE A3.
Age-Adjusted Incidence Rates per 100,000 by PBCR, Brazil, 2000-2015
TABLE A4.
Distribution of Selected Characteristics According to Brazilian Macroregion Where Hospital is Located, Brazil, 2000-2015
PRIOR PRESENTATION
Presented at the Annual Meeting of the Gynecological Oncology Society, March 12-15, 2017, National Harbor, MD.
AUTHOR CONTRIBUTIONS
Conception and design: Eduardo Paulino, Paul Goss, Angelica Nogueira-Rodrigues
Administrative support: Andreia Cristina de Melo
Provision of study material or patients: Andreia Cristina de Melo, Paul Goss, Angelica Nogueira-Rodrigues
Collection and assembly of data: Eduardo Paulino, Andreia Cristina de Melo, Agnaldo Lopes Silva-Filho, Luiza de Freitas Maciel, Luiz Claudio Santos Thuler
Data analysis and interpretation: Eduardo Paulino, Andreia Cristina de Melo, Agnaldo Lopes Silva-Filho, Luiza de Freitas Maciel, Luiz Claudio Santos Thuler, Angelica Nogueira-Rodrigues
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eduardo Paulino
Honoraria: AstraZeneca, MSD, Roche
Consulting or Advisory Role: AstraZeneca, MSD, Roche
Speakers' Bureau: MSD, AstraZeneca, Roche
Travel, Accommodations, Expenses: AstraZeneca, Roche, MSD
Andreia Cristina de Melo
Honoraria: MSD Oncology, Novartis, BMS Brazil
Speakers' Bureau: BMS Brazil, MSD Oncology
Research Funding: Roche (Inst), MSD Oncology (Inst), BMS Brazil (Inst), Novartis (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Angelica Nogueira-Rodrigues
Honoraria: Roche, MSD, AstraZeneca
Consulting or Advisory Role: Roche, AstraZeneca, MSD, Eisai
No other potential conflicts of interest were reported.
REFERENCES
- 1. American Cancer Society: Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society, 2018.
- 2.Instituto Nacional de Câncer Estatísticas de câncer [in Portugese] https://www.inca.gov.br/numeros-de-cancer
- 3.Atlas do Desenvolvimento Humano no Brasil [in Portugese]. http://atlasbrasil.org.br/2013/
- 4.Instituto Nacional de Câncer https://www.inca.gov.br/
- 5.Instituto Brasileiro de Geografia e Estatistica https://www.ibge.gov.br/
- 6.DATASUS https://datasus.saude.gov.br/
- 7.International Agency for Research on Cancer Cancer today. http://gco.iarc.fr/today/home
- 8.Ministério da Saúde http://portalarquivos2.saude.gov.br/
- 9.de Oliveira MM, Andrade SSC de A, de Oliveira PPV, et al. Cobertura de exame Papanicolaou em mulheres de 25 a 64 anos, segundo a Pesquisa Nacional de Saúde e o Sistema de Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico, 2013 [in Portugese] Rev Bras Epidemiol[epub ahead of print on August 27, 2018]http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-790X2018000100413&lng=pt&tlng=pt [DOI] [PubMed]
- 10.Croso C, Vóvio C, Masagão V. Latin America: Literacy, adult education and the international literacy benchmarks. AED. 2008;71 https://www.dvv-international.de/en/adult-education-and-development/editions/aed-712008/national-and-regional-reflections-on-operationalising-the-benchmarks/latin-america-literacy-adult-education-and-the-international-literacy-benchmarks/ [Google Scholar]
- 11.Paulino E, de Melo AC, Nogueira-Rodrigues A, et al. Gynecologic cancer in Brazil and the law of sixty days. J Gynecol Oncol. 2018;29:e44. doi: 10.3802/jgo.2018.29.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulino E, Nogueira Rodrigues A, Strasser-Weippl K, et al. Barriers to primary debulking surgery for advanced ovarian cancer in Latin America. Int J Gynecol Cancer. 2017;27:1645–1649. doi: 10.1097/IGC.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 13.Ministério da Saúde Portaria No. 931, de 10 de Maio de 2012 [in Portugese] https://bvsms.saude.gov.br/bvs/saudelegis/gm/2012/prt0931_10_05_2012.html
- 14.Ministério da Saúde Plano de expansão da radioterapia no SUS [in Portugese] https://www.saude.gov.br/ciencia-e-tecnologia-e-complexo-industrial/complexo-industrial/plano-de-expansao-da-radioterapia-no-sus
- 15.de Araújo LP, de Sá NM, Atty ATdM. Necessidades atuais de radioterapia no SUS e estimativas para o ano de 2030 [in Portugese] Rev Bras Cancerol. 2016;62:35–42. [Google Scholar]
- 16.Ministério da Saúde PNI-Programa Nacional de Imunizações [in Portugese] https://www.saude.gov.br/images/pdf/2017/julho/28/Boletim-informativo.pdf
- 17.Nogueira-Rodrigues A, Bukowski A, Paulino E, et al. An alert to Latin America: Current human papillomavirus vaccination trends highlight key barriers to successful implementation. Cancer. 2017;123:2193–2199. doi: 10.1002/cncr.30647. [DOI] [PubMed] [Google Scholar]
- 18.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]