Abstract
PURPOSE
Several factors affect how medical oncologists in the Philippines use biomarkers in real-world practice. This study describes patterns of biomarker testing for the management of breast, colorectal, and lung cancers among medical oncologists in the Philippines.
METHODS
A cross-sectional survey was performed among practicing medical oncologists in the Philippines from November to December 2019. The questionnaire determined the ideal and practical use of biomarkers as perceived by the respondents. Responses were summarized. Associations between biomarker use across select conditions were determined.
RESULTS
A total of 127 respondents (38% of medical oncologists in the Philippines) participated in this study. In actual practice, 97% of the respondents requested estrogen receptor/progesterone receptor testing, and 93% requested human epidermal growth factor receptor 2 testing. For colorectal cancer, the respondents would use KRAS and mismatch repair/microsatellite instability, but 59.84% had never used BRAF. For lung cancer, 97.64% of respondents would test for epidermal growth factor receptor (EGFR), 88.19% would test for PD-L1, 80.31% for anaplastic lymphoma kinase, 58.27% for ROS1, and 33.07% for BRAF. In actual practice, EGFR was the most frequently ordered test (67.72%), while 44.80% of medical oncologists had never used ROS1. The most common reason for testing was adherence to international guidelines (96%). The most commonly cited barrier to biomarker use was patients’ financial constraints (94.49%). Overall, the respondents’ use of biomarkers was not significantly associated with institutional affiliation, the number of patients they saw monthly, and the availability of biomarker tests in their areas of practice.
CONCLUSION
Medical oncologists in the Philippines would use biomarkers in treating breast, colorectal, and lung cancers if these were clinically indicated and if cost were not a factor. Financial difficulty experienced by patients was the most commonly cited barrier to biomarker use.
INTRODUCTION
Cancer management is shifting toward an era of precision oncology, with personalized cancer treatment as the ultimate goal. The ongoing development of targeted small molecules and immuno-oncology biopharmaceuticals largely drives this paradigm shift. Precision oncology relies on validated biomarkers and their companion diagnostics. The presence or absence of a biomarker determines whether a treatment strategy is appropriate. Biomarkers can classify patients according to their disease risk and prognosis.1
CONTEXT
Key Objective
The study described real-world biomarker testing practices among medical oncologists in the Philippines in the management of breast, colorectal, and lung cancers, the top three malignancies in the country.
Knowledge Generated
Medical oncologists in the Philippines would use biomarkers if these were clinically indicated and if cost were not a factor. Testing was driven most frequently by guideline recommendations. Patients’ limited finances and refusal to undergo testing, and the unavailability of biomarkers, were the most commonly cited barriers to testing.
Relevance
Filipino medical oncologists treat patients in a resource-limited context where health expenditures are generally out-of-pocket and where biomarker tests are not readily accessible. Improved access to biomarker testing may be accomplished through programs that lower the cost of the tests, provide financial assistance, and increase the number of capable laboratories.
Personalized treatment strategies lead to improved clinical outcomes in many cancers. For instance, superior clinical outcomes were demonstrated for human epidermal growth factor receptor 2 (HER2)–directed therapy in HER2-positive metastatic breast cancer,2 epidermal growth factor receptor (EGFR)–directed therapy in metastatic colorectal cancer with wild-type RAS,3,4 and EGFR-directed therapy in non–small-cell lung cancer (NSCLC) with actionable EGFR driver mutations.5,6 As a result, many clinical practice guidelines now recommend biomarker-driven approaches. Table 1 lists the key biomarkers recommended by the National Comprehensive Cancer Network (NCCN), ASCO, and the European Society for Medical Oncology (ESMO) in breast, colorectal, and lung cancers.
TABLE 1.
Guideline Recommendations for Biomarker Use in Specific Cancers by NCCN, ASCO, and ESMO, and Cost of Test in the Philippines
Although precision oncology holds promise in improving clinical outcomes, there are many regions in the world where its use remains limited. In such places, the potential of biomarker-driven treatment strategies may be hindered by several factors, including the countries’ health policies and care delivery systems.20 For example, in the Philippines, a low- and middle-income country (LMIC) in Southeast Asia, patients often have to pay out-of-pocket for the tests. Laboratories are concentrated in highly urbanized cities, which limits access to testing for patients residing in remote areas. Because medical oncologists need to discuss the costs of biomarker tests and subsequent therapeutic options with their patients before treatment, the use of biomarkers as the lynchpin of treatment planning may prove difficult in the setting of LMICs. This is an important consideration in the effort to harmonize treatment guidelines on molecular diagnostics and patient-tailored treatment options between more progressive and developing regions of the world.21
The practice of medical oncology in the Philippines is governed by the Philippine Society of Medical Oncology (PSMO). In 2019, PSMO had 332 members (275 board-certified members and 57 fellows-in-training). The aim of this study was to describe the patterns of biomarker testing among medical oncologists in the Philippines for the management of breast, colorectal, and lung cancers, the country’s top three malignancies.22 In addition, we aimed to identify the driving factors and barriers to biomarker use in the country.
METHODS
Study Design and Participant Eligibility
From November to December 2019, a cross-sectional survey among medical oncologists was conducted. All members of PSMO were eligible to participate. Nonconsent for participation was the only exclusion criterion. This study received ethical approval from the University of the Philippines Manila Research Ethics Board (UPM REB code 2019-414-01).
Survey Questionnaire
Before the survey, the authors developed a 15-item instrument, formatted as an online Google form (Google, Mountain View, CA) and printed questionnaire (Data Supplement). Of the 15 questions, four were devoted to demographics, nine to biomarker use for each cancer type, and two to factors that drive or hinder biomarker testing. On a Likert scale of always, sometimes, often, and never, respondents were asked to indicate how often they used these tests in actual practice. These categorizations were subjective. Several questions allowed multiple responses to be given, including (1) the respondent’s institutional affiliation, (2) tests used in actual clinical practice and those that would be ordered if cost did not play a role, and (3) factors that drive or hinder biomarker testing from the respondents’ perspective.
The instruments were pretested among eight medical oncologists for cultural acceptability, ease of use, and overall appeal. These oncologists were staff consultants and trainees from a government hospital and academic medical center in Manila who were preselected because of their knowledge and experience in cancer treatment.
E-mail invitations to participate in the study were sent to all society members and fellows-in-training through the PSMO secretariat. Printed questionnaires were also distributed during the 2019 PSMO Annual Convention held in November for which 91.3% of the members were registered. The printed questionnaires were distributed in the convention’s registration booth. Invitations to participate in the study were announced before the plenary sessions. Participants could choose between printed or online instruments. Follow-up e-mails were sent by the PSMO secretariat to all members, including nonresponders.
Data Analysis
The responses were summarized using descriptive statistics using Stata 13.0 software (StataCorp, College Station, TX). A series of χ2 tests of association and Fisher’s exact tests were performed to determine the presence of association between the frequency of physicians’ use of biomarkers across select conditions, such as public/private institutions, number of patients they saw monthly, availability of the said biomarkers in their areas of practice, and whether their patients experienced financial constraints. The level of significance for all sets of analysis was set at P < .05, using two-tailed comparisons.
RESULTS
A total of 127 unique responses were collected. These comprised 38% of the 332 medical oncologists affiliated with PSMO. Eighty-two (65%) of respondents completed the printed questionnaire, and 45 (35%) answered the online form.
Sixty-three percent of respondents were staff consultants. Two thirds of the medical oncologists were affiliated with private hospitals or clinics, and 56.8% were in their first 3 years of practice. More than half of the medical oncologists practiced in Metro Manila (Table 2). Table 3 lists the average number of patients seen monthly by the respondents for each cancer type.
TABLE 2.
Respondent Demographic Characteristics (N = 127)

TABLE 3.
Number of Patients Seen in a Month According to Cancer Diagnosis

Biomarker Testing for Breast Cancer
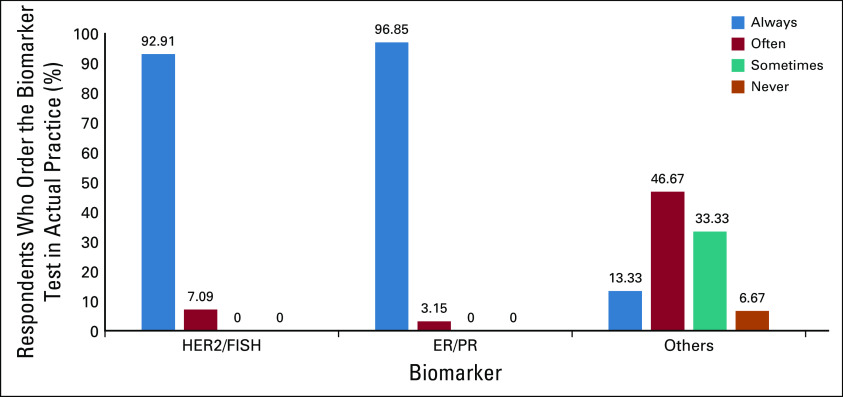
If cost were not an issue, almost all respondents would order estrogen receptor (ER)/progesterone receptor (PR) and HER2 testing (Fig 1). Twenty-five percent would include protein encoded by the MKI67 gene (Ki-67). Other volunteered responses for testing were BRCA mutations (four respondents), Oncotype Dx (Genomic Health, Redwood City, CA; two respondents), and androgen receptor (one respondent). In actual practice, ER/PR and HER2 testing were almost always used (Fig 2).
FIG 1.
Biomarker use in breast cancer if cost were not an issue. ER/PR, estrogen receptor/progesterone receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2.
FIG 2.
Biomarker testing practices in breast cancer in actual practice. ER/PR, estrogen receptor/progesterone receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2.
Biomarker Testing for Colorectal Cancer
Approximately half of the respondents saw three to 10 patients with colorectal cancer monthly (Table 2). If cost were not an issue and if the tests were clinically indicated, almost all respondents would test for KRAS mutation. Only 63.78% would test for BRAF mutations (Fig 3). In actual practice, < 50% of respondents routinely requested KRAS/NRAS, BRAF, and microsatellite instability (MSI)/mismatch repair (MMR) tests (Fig 4). Sixty percent of the respondents never tested for BRAF mutations.
FIG 3.
Biomarker use in colorectal cancer if cost were not an issue. MMR, mismatch repair; MSI, microsatellite instability.
FIG 4.
Biomarker testing practices in colorectal cancer in actual practice. MMR, mismatch repair; MSI, microsatellite instability.
Biomarker Testing for Lung Cancer
Almost all respondents would request EGFR mutation testing if cost were not an issue and if clinically indicated. PD-L1 and anaplastic lymphoma kinase (ALK) were also common responses (Fig 5), and 54.33% of respondents would test for T790M mutation. In actual clinical practice, 67.72% of the respondents always ordered EGFR testing (Fig 6).
FIG 5.
Biomarker use in lung cancer if cost were not an issue.
FIG 6.
Biomarker testing practices in lung cancer in actual practice.
Driving Factors for and Barriers to Biomarker Testing
The most frequent reason cited by respondents for why they would pursue biomarker testing was that the tests are recommended by clinical practice guidelines (Table 4). The guidelines used by the respondents were NCCN (32.28%), ASCO (4.72%), ESMO (1.57%), and all three of these (61.47%). Other factors that drove them to use biomarkers were their patients’ advanced or metastatic stage (76.38%) and their patients’ inclusion in clinical trials that made use of such biomarkers (49.61%; Table 4).
TABLE 4.
Driving Factors for and Barriers to Biomarker Use

The respondents reported several barriers that hindered them from pursuing biomarker testing for their patients (Table 4). They would not pursue testing if their patients reported financial difficulties (94.49%), if their patients stated that they did not wish to be tested (60%), if the tests were not available in their areas of practice (58.27%), and if there was insufficient tissue sample for the tests to be reliably performed (47.24%).
Factors Associated With Biomarker Use
The respondents’ use of biomarkers was not significantly associated with their institutional affiliation (Appendix Table A1), their patients’ financial difficulties (Appendix Table A2), the availability of biomarkers in their areas of practice (Appendix Table A3), and the number of patients they saw monthly (Appendix Table A4). However, there were several exceptions. Respondents affiliated with academic institutions tested for EGFR T790M for lung cancer (P = .02) and NRAS (P = .01) for colorectal cancer more frequently than respondents who were not affiliated with academia (Appendix Table A5). There was a nonsignificant trend for an association between the use of EGFR for lung cancer among respondents affiliated with private hospitals compared with those affiliated with government-run hospitals (P = .08).
A significant association between the respondents’ use of biomarkers and the number of patients seen monthly was observed only in ROS1 for lung cancer (P = .04). It seemed that respondents who saw more patients with lung cancer monthly ordered EGFR (P = .08), PD-L1 (P = .11), and EGFR T790M (P = .08) tests, although the associations were not significant. Regardless of the number of patients seen, the medical oncologists tended to prescribe NTRK testing for patients with lung cancer less frequently (P = .07). The association between biomarker use and its availability in the area of practice was only significant for KRAS/NRAS in colorectal cancer (P < .01).
DISCUSSION
To our knowledge, this study is the first to describe real-world practices of medical oncologists in the Philippines with regard to precision medicine in cancer management. In actual practice, patterns of biomarker use seemed heterogenous. Testing was driven most frequently by guideline recommendations. Patients’ limited finances and refusal to undergo testing, as well as the unavailability of biomarkers in oncologists’ areas of practice, were the most common barriers that hindered the respondents from pursuing the recommended tests.
This study was important to carry out for three reasons:
Filipino medical oncologists treat patients in a setting where health expenditures are generally out-of-pocket.
Filipino patients may opt to defer treatment to spare their families from economic and emotional hardships.23
Some medical oncologists practice in areas where biomarker tests may not be readily available. In this study, particular focus was given to breast, colorectal, and lung cancers because these were the most common malignancies in the Philippines.22
In breast cancer, we showed that the respondents used ER/PR and HER2 testing routinely. A quarter of respondents would test for Ki-67, despite the controversy about its value because of a lack of standardized assessments.24 It seemed that the respondents did not rely exclusively on guideline recommendations.
In colorectal cancer, the respondents would use KRAS, NRAS, BRAF, and MSI/MMR status testing if these were indicated and if cost were not an issue (Table 4). Approximately 40% of respondents, however, answered that they would not order BRAF testing despite guideline recommendations for its use in metastatic disease. In actual clinical practice, < 30% of respondents requested all four tests routinely. A prominent finding was that 59.84% of respondents had never used BRAF. It seemed that on top of cost, other issues serve as barriers to biomarker use.
In lung cancer, almost all respondents (97.64%) would test for EGFR mutations. In actual practice, however, only 67.72% tested for EGFR routinely. Of note, 55.2% and 44.8% of respondents had never used BRAF and ROS1, respectively, in real-world practice.
Guideline recommendation was the most frequent motivation for biomarker testing. This mirrored the results of a similar survey of oncologists from other countries.25 In the absence of local guidelines, medical oncologists in the Philippines refer to guidelines developed by NCCN, ASCO, and ESMO. Updated regularly, these reflect the standards of care in developed regions with ready access to cutting-edge molecular tests and targeted treatments that may not be available in the Philippines. Recently, regional guidelines have emerged to adapt western guidelines, taking into account ethnic differences associated with the treatment of metastatic NSCLC cancer in Asian patients. PSMO was not part of the consensus panel for these guidelines. Even these Pan-Asian guidelines might not be directly applicable in the Philippine setting.26
For the three cancers, evidence shows that biomarkers can guide treatment planning for the first and subsequent lines. Related to this, our results showed that medical oncologists used biomarker testing to explore alternative treatment options.
Of note, almost half reported using biomarker tests because their patients were included in clinical trials (Table 4). Testing was likely required before enrollment. Oncologists who practiced in academic medical centers would have more exposure to clinical trials. We showed a significant association between the use of EGFR T790M and NRAS with affiliation to academia.
Almost all the respondents indicated that they would not pursue biomarker testing if their patients reported financial difficulties (Table 4). Biomarker tests are expensive (Table 1). Given that the average monthly income of Filipino households is 26,000 Philippine pesos (US $500),27 the cost of cancer diagnosis and treatment would be prohibitive for many. According to one study, 40.6% of Filipinos with cancer will experience financial catastrophe that arises from their illness.23 The costs of cancer care consist of expenditures for medications and diagnostics, including biomarkers. This issue is not limited to LMICs.25
Sixty percent of the respondents would not pursue testing if their patients refused to be tested. Patients might not comprehend the benefits of testing,25 they might want to proceed with best supportive care instead, or they might be daunted by the cost of treatment.
Approximately 60% of respondents reported that the unavailability of tests in their areas of practice hindered testing (Table 4), similar to the findings of a multinational study.25 As of 2019, the tests for BRCA mutation, Oncotype Dx, KRAS, NRAS, BRAF, MSI, EGFR, ALK, BRAF, PD-L1, T790M, and ROS1 were only available in six centers in Metro Manila (Fig 7).
FIG 7.
Tests for BRCA mutation, Oncotype Dx, KRAS, NRAS, BRAF, microsatellite instability, epidermal growth factor receptor, anaplastic lymphoma kinase, BRAF, PD-L1, T790M, and ROS1 are only available in the National Capital Region (Metro Manila, Philippines), which is highlighted in red.
Insufficient tissue sample was another barrier. This reason should merit clarification from respondents because in at least some instances, it would seem remediable. Certainly, the quantity and quality of the tumor material is a limiting factor for adequate biomarker analysis.24
We determined the presence of an association between the physician’s use of biomarkers and select conditions. In general, we noted no statistically significant associations, likely because of our study’s sample size. Nevertheless, there were several key exceptions for which the association was significant—EGFR T790M and NRAS with academic center affiliation, ROS1 with more patients with lung cancer seen monthly, and KRAS/NRAS with its availability in the area of practice. Several points might explain these findings: (1) physicians from academic and private institutions would have greater access to the biomarker tests, (2) physicians from private hospitals would have more contact with patients who could afford treatment, and (3) physicians who saw more patients monthly would have more opportunities to pursue testing.
Our study had several limitations. First, there was a risk of social desirability bias; respondents might have felt pressured to give more acceptable answers. While the effect of this bias could not be eliminated completely, maintaining respondent anonymity would mitigate it.28 In this study, we used anonymized questionnaires. Online submissions could not be traced back to the sender, and paper submissions were through a third party (PSMO secretariat). Another concern was nonresponse bias possibly as a result of indifference or busyness. Furthermore, studies have reported that physician response rates to surveys tend to be low.29,30 Regardless of the reasons, interventions that could improve response rates include personalizing cover letters, incentivizing survey response, and implementing a thorough follow-up system for nonresponders.29-32 In our study, we aggressively pursued an advertising campaign through e-mail sent to the entire PSMO and supplemented by frequent announcements during plenary sessions. In addition, we made electronic and paper formats of the survey available to make participation more convenient. Despite these interventions, the response rate was only 38%, and respondents tended to be younger members of the society. Second, although we attempted to include all medical oncologists, our study had a response rate of < 40%, which is comparable to other similarly conducted studies.9.30,33,34 A strength of the study is the participation of medical oncologists from various institutions, including oncology trainees and attending physicians (Table 2). Because all medical oncologists affiliated with PSMO were invited, the probability of a differential response bias could have been lessened. Nevertheless, there was a higher response rate among respondents who were from Metro Manila and were in private practice. On the basis of the PSMO membership data, 43% of consultants practice in Metro Manila. The concentration of medical oncologists affiliated with private hospitals/clinics in urbanized areas was reflected in the distribution of respondents of this study. Third, we did not perform qualitative analysis (interviews or focus group discussions) to probe the underlying reasons. The study relied solely on a printed instrument to elicit responses. Nevertheless, the questionnaire provided the respondents an opportunity to volunteer other reasons if they saw fit to do so. Fourth, by leaving some questions open to interpretation due to the vagueness of the statement (ie, “as clinically indicated”), the survey might have assumed that the respondents were fully aware of the indications for each of the tests. Fifth, the survey did not attempt to measure the baseline knowledge of the respondents with respect to biomarker testing guidelines. This would have allowed better contextualization of the study’s results. Finally, the driving factors and barriers to testing were not analyzed separately for each cancer type; the responses could certainly vary depending on the cancer. In the end, the study described the general factors affecting physicians’ biomarker use in the Philippines.
In summary, medical oncologists in the Philippines would use biomarkers in the management of breast, colorectal, and lung cancers if these were clinically indicated and if cost were not an issue. Almost all the respondents indicated that they would not pursue testing if their patients reported financial difficulties.
Given our findings, we have the following recommendations. First, additional patient access programs may need to be developed and existing ones strengthened. These programs may involve lowering the costs of the tests through government regulations, increasing the number of certified and capable laboratories throughout the Philippines, and giving patients financial subsidies. Second, patient-centered education on the value of the tests in cancer treatment may need to be implemented in the clinics. Third, medical societies may provide avenues for continuing medical education on precision medicine. Finally, hospitals ought to engage in clinical trials whenever possible because these may allow free access to molecular diagnostics.
ACKNOWLEDGMENT
We thank PSMO for the help in the conduct of the study.
Appendix
TABLE A1.
Frequency Distribution of Responses Across Type of Institution

TABLE A2.
Frequency Distribution of Responses Across Presence of Financial Constraints

TABLE A3.
Frequency Distribution of Responses Across Availability of Biomarker Tests

TABLE A4.
Frequency Distribution of Responses Across Number of Patients Seen
TABLE A5.
Frequency Distribution of Responses Across Type of Institution
PRIOR PRESENTATION
Presented at the ASCO 2020 Virtual Annual Meeting, May 29-31, 2020.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Lance Isidore Catedral, Danielle Benedict Sacdalan, Dennis L. Sacdalan
Collection and assembly of data: Lance Isidore Catedral, Harold Nathan Tan, Alfredo Chua Jr
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Danielle Benedict Sacdalan
Honoraria: Merck, OEP
Travel, Accommodations, Expenses: Qualimed
Dennis L. Sacdalan
Honoraria: Fresenius Kabi (I), Abbott Nutrition (I), Menarini (I)
Consulting or Advisory Role: Unilab, Pfizer, Roche
Speakers’ Bureau: MSD, Eli Lilly
Research Funding: Amgen, Novartis
Travel, Accommodations, Expenses: Unilab, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vargas AJ, Harris CC. Biomarker development in the precision medicine era: Lung cancer as a case study. Nat Rev Cancer. 2016;16:525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;2014:CD006242. doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 4.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7. doi: 10.6004/jnccn.2018.0088. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: Breast cancer version 2.2019. www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 8.Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 9.Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2015;33:2695–2704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krop I, Ismaila N, Andre F, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017;35:2838–2847. doi: 10.1200/JCO.2017.74.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.6004/jnccn.2018.0088. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: Colon cancer version 2.2019. www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 14. doi: 10.6004/jnccn.2018.0088. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: Rectal cancer version 2.2019. www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 15.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453–1486. doi: 10.1200/JCO.2016.71.9807. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.6004/jnccn.2017.0050. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: Non-small cell lung cancer version 5.2019. www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 18.Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36:911–919. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
- 19.Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 20.Huntington SF, Davidoff AJ, Gross CP. Precision medicine in oncology II: Economics of targeted agents and immuno-oncology drugs. J Clin Oncol. 2020;38:351–358. doi: 10.1200/JCO.19.01573. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch FR, Zaric B, Rabea A, et al. Biomarker testing for personalized therapy in lung cancer in low- and middle-income countries. Am Soc Clin Oncol Educ Book. 2017;37:403–408. doi: 10.1200/EDBK_175243. [DOI] [PubMed] [Google Scholar]
- 22. doi: 10.3322/caac.21492. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [Erratum: CA Cancer J Clin 70:313, 2020] [DOI] [PubMed] [Google Scholar]
- 23. Ngelangel CA, Lam HY, Rivera AS, et al: Philippine Costs in Oncology (PESO): Describing the economic impact of cancer on Filipino cancer patients using the ASEAN costs in oncology study dataset. Acta Med Philipp 52:125-133, 2018. [Google Scholar]
- 24.Reck M, Hermes A, Tan EH, et al. Tissue sampling in lung cancer: A review in light of the MERIT experience. Lung Cancer. 2011;74:1–6. doi: 10.1016/j.lungcan.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Ciardiello F, Adams R, Tabernero J, et al. Awareness, understanding, and adoption of precision medicine to deliver personalized treatment for patients with cancer: A multinational survey comparison of physicians and patients. Oncologist. 2016;21:292–300. doi: 10.1634/theoncologist.2015-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. doi: 10.1093/annonc/mdq189. D’addario G, Früh M, Reck M, et al: Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v116-v119, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Philippine Statistics Authority: Statistical Tables on 2018 Family Income and Expenditure Survey, 2020. https://psa.gov.ph/content/statistical-tables-2018-family-income-and-expenditure-survey.
- 28.Larson RB. Controlling social desirability bias. Int J Mark Res. 2019;61:534–547. [Google Scholar]
- 29.Weaver L, Beebe TJ, Rockwood T. The impact of survey mode on the response rate in a survey of the factors that influence Minnesota physicians’ disclosure practices. BMC Med Res Methodol. 2019;19:73. doi: 10.1186/s12874-019-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32. doi: 10.1186/s12874-015-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman DM, Tan LL, Cheng TL. Surveys and response rates. Pediatr Rev. 2015;36:364–366. doi: 10.1542/pir.36-8-364. [DOI] [PubMed] [Google Scholar]
- 32. doi: 10.1200/JOP.2014.001484. Mazzarello S, Clemons M, Graham ID, et al: Surviving surveys. J Oncol Pract 11:44-46, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Holdhoff M, Ye X, Blakeley JO, et al. Use of personalized molecular biomarkers in the clinical care of adults with glioblastomas. J Neurooncol. 2012;110:279–285. doi: 10.1007/s11060-012-0968-3. [DOI] [PubMed] [Google Scholar]
- 34.Sung MR, Ellis PM, Verma S, et al. Approach to biomarker testing: Perspectives from various specialties. Curr Oncol. 2016;23:178–183. doi: 10.3747/co.23.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]