Abstract
PURPOSE
Cervical cancer remains a major health challenge in low- to middle-income countries. We present the experiences of two centers practicing in variable resource environments to determine predictors of improved radiochemotherapy treatment.
METHODS AND MATERIALS
This comparative review describes cervical cancer presentation and treatment with concurrent chemoradiotherapy with high-dose-rate brachytherapy between 2014 and 2017 at the National Radiotherapy Oncology and Nuclear Medicine Center (NRONMC) in Korle-Bu Teaching Hospital, Accra, Ghana, and Moffitt Cancer Center (MCC), Tampa, FL.
RESULTS
Median follow-up for this study was 16.9 months. NRONMC patients presented with predominantly stage III disease (42% v 16%; P = .002). MCC patients received para-aortic node irradiation (16%) and interstitial brachytherapy implants (19%). Median treatment duration was longer for NRONMC patients compared with MCC patients (59 v 52 days; P < .0001), and treatment duration ≥ 55 days predicted worse survival on multivariable analysis (MVA; P = .02). Stage ≥ III disease predicted poorer local control on MVA. There was a difference in local control among patients with stage III disease (58% v 91%; P = .03) but not in survival between MCC and NRONMC. No significant difference in local control was observed for stage IB, IIA, and IIB disease.
CONCLUSION
Although there were significant differences in disease presentation between the two centers, treatment outcomes were similar for patients with early-stage disease. Longer treatment duration and stage ≥ III disease predicted poor outcomes.
INTRODUCTION
Cervical cancer remains a disease of significant global health importance. It ranks fourth for both incidence and mortality from cancer in women worldwide.1 The disease accounts for approximately 80% of human papillomavirus (HPV)-related cancers in India and sub-Saharan Africa, which is estimated to account for more than 20% of the global HPV-related cancer burden.2 The incidence of cervical cancer in developed countries has decreased after the institution of HPV vaccination and cervical cancer screening programs to detect premalignant lesions. In sharp contrast, patients with cervical cancer in low- to middle-income countries (LMICs) typically present with symptoms and/or more advanced stage. In LMICs lacking well-resourced treatment centers with geographic and financial barriers, treatment outcomes have been less than desired.3
Cisplatin-based concurrent chemoradiotherapy remains the mainstay of cervical cancer treatment of locally advanced disease and a curative option for early-stage palpable disease.4,5 Disparity exists in the distribution of radiation oncology infrastructure, with a greater capacity in developed countries.6 This is also true of diagnostic imaging used in the pretreatment evaluation, as well as radiation treatment planning and follow-up surveillance. Although global efforts at improving access to early detection strategies and HPV vaccination are anticipated to lead to a long-term reduction in the incidence of cervical cancer in LMICs, in the short term, this will not materialize early enough for present-day patients who present with advanced disease. Feasible strategies to improve treatment delivery are therefore needed.
CONTEXT
Key Objective
Cervical cancer remains a major health challenge in low- to middle-income countries (LMICs). The experiences of two centers practicing in variable resource environments were analyzed to determine predictors of improved radiochemotherapy treatment.
Knowledge Generated
Significant differences between the two centers in treatment and disease presentation that led to poor outcomes included stage ≥ III disease, as well as treatment duration ≥ 55 days. There was a difference in local control among patients with stage III disease but not in survival between the two centers. No significant difference in local control was observed for stage IB, IIA, and IIB disease.
Relevance
Longer treatment duration and stage ≥ III disease predicted poor outcomes and may be of particular importance in improving clinical outcomes in LMICs.
The National Radiotherapy Oncology and Nuclear Medicine Center of Korle-Bu Teaching Hospital (NRONMC) treats a high volume of patients with cervical cancer in West Africa.7 Moffitt Cancer Center (MCC) is a National Cancer Institute Comprehensive Cancer Center offering sophisticated cervical cancer treatment delivery. The objective of this study was to evaluate differences in patterns of care and disease characteristics between the two institutions to identify differences that affect clinical outcomes and opportunities for treatment optimization.
METHODS AND MATERIALS
Patient Selection and Study Design
The databases of NRONMC and MCC were queried for patients with cervical cancer treated between January 2014 and December 2017 after clearance from the respective institutional review boards. A total of 226 patients were identified. Patients were required to have histologic confirmation of cervical cancer and have undergone curative-intent treatment. Patients with International Federation of Gynecologic Oncology (FIGO) stage IVB disease, receipt of palliative treatment, lack of brachytherapy, and prior surgical management, and who did not receive concurrent chemotherapy were excluded from the analysis. A total of 179 patients met criteria for this study assessment.
All patients were clinically staged at presentation with detailed clinical history and physical examination. Presence of lung or liver metastasis or hydronephrosis were assessed with chest radiographs and abdominopelvic ultrasound scans, respectively, at NRONMC. Positron emission tomography (PET)-computed tomography (CT) and pelvic magnetic resonance imaging (MRI) were used to characterize disease extent at MCC. All patients were staged based on FIGO 2008 clinical staging guidelines irrespective of the imaging modality used in their evaluation.8
External Beam Radiation
National Radiotherapy Oncology and Nuclear Medicine Center.
External beam radiation treatment (EBRT) plans were generated by conventional simulation with the superior, lateral, and inferior borders set to the L4/L5 intervertebral space, 2 cm lateral to the pelvic inlet, and 3 cm distal to the inferior-most tumor extent or ischial tuberosity, whichever was lower, delivered by opposed anterior-posterior and posterior-anterior (AP-PA) fields. Some patients who had an AP-PA separation > 21 cm were treated with a four-field box technique setting the anterior and posterior borders of the lateral fields to the anterior pubic symphysis and to include the entire sacrum, respectively. The superior and inferior borders of the lateral fields were the same as the AP-PA fields. Customized cerrobend molds were used to shape the fields and to reduce the volume of the small bowel, femoral heads, and soft tissues in the target volume.
All radiation was delivered by cobalt-60 teletherapy, with two-dimensional (2D) planning weighted heavier in the AP-PA fields for four-field box plans to a target dose of 46 Gy in 23 fractions over 4.5 weeks prescribed to midline. No patient received para-aortic treatment. Patients with stage IIB disease received bilateral sidewall and parametrial boost doses of 6-10 Gy over 3-5 fractions with a mid- line block of 4 cm width. Concurrent weekly cisplatin 40 mg/m2 (maximum weekly dose of 70 mg) was prescribed with the course of EBRT.
Moffitt Cancer Center.
CT simulation for radiation treatment planning was performed. The CT dataset was transferred to the treatment planning system for treatment target and organs at risk volume delineation aided by MRI and PET scans based on Radiation Therapy Oncology Group recommendations.9 The planning target volume was prescribed at 45-50.4 Gy in 25-28 daily fractions delivered with multi-energy linear accelerators using intensity-modulated radiation therapy (IMRT). Extended field para-aortic radiation was administered in the setting of PET-avid nodes, which were boosted simultaneously or sequentially to 54-64 Gy based on bowel tolerance. All patients received weekly concurrent cisplatin 40 mg/m2 chemotherapy.
Brachytherapy
National Radiotherapy Oncology and Nuclear Medicine Center.
All brachytherapy was delivered with a high-dose-rate (HDR) remote afterloader using a cobalt-60 source at NRONMC. All brachytherapy at NRONMC was performed with tandem and ovoid or tandem and cylinder applicators. Treatment was prescribed to point A using orthogonal radiographs while ensuring the International Commission on Radiation Units Report 38 recommendations for rectal and bladder tolerance doses were respected. The majority of patients were prescribed 28 Gy in 4 fractions (n = 64; 76%) and 18 Gy in 2 fractions (n = 10; 12%) to point A.
Moffitt Cancer Center
Brachytherapy at MCC was performed with tandem and ovoid, ring or cylinder applicators, and in select patients, interstitial catheters were used. Treatments were delivered with an iridium-192 source. Three-dimensional treatment planning using fused CT Digital Imaging and Communications in Medicine data and MRI for treatment target and critical structure definition after applicator insertion following the Groupe Européen de Curiethérapie–European Society for Radiotherapy and Oncology recommendations.10 In patients receiving tandem and ovoid or ring treatment, CT simulation and MRIs with applicators in place were repeated before delivery of each fraction with the treatment replanned before the delivery of each fraction. For those patients receiving interstitial treatment, patients underwent one CT simulation and MRI, and were admitted for the duration of their brachytherapy treatment delivery. A high-risk clinical target volume (HR-CTV) was delineated, together with organs-at-risk volumes and the treatment dose prescribed to the 100% isodose line.
Follow-Up
Patients were seen at 2-6 weeks postbrachytherapy for initial treatment assessment and vaginal dilator education and adherence. NRONMC patients were subsequently evaluated clinically at 3-6-month clinic visits. MCC patients were evaluated at approximately 3 months postbrachytherapy with PET-CT for evaluation of persistent or recurrent disease, with a repeat scan at 6 months if the initial scan results were inconclusive.
Local failure was defined as recurrence of disease at the location of initial disease after treatment. Distant failure was defined as evidence of disease recurrence outside the pelvis after treatment. Overall survival (OS) was measured from the date of treatment start to the date of death.
Statistics
Baseline demographic and clinicopathologic characteristics were detailed using summary statistics. To test differences between cohorts, the Wilcoxon, Pearson’s χ2, and Fisher’s exact tests were used when appropriate. Local control, distant control, and OS were estimated using Kaplan-Meier curves. The association between these end points and patient, disease, and treatment characteristics were evaluated using univariable (UVA) and multivariable (MVA) Cox proportional hazard models. Statistical analyses were performed using JMP 13 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
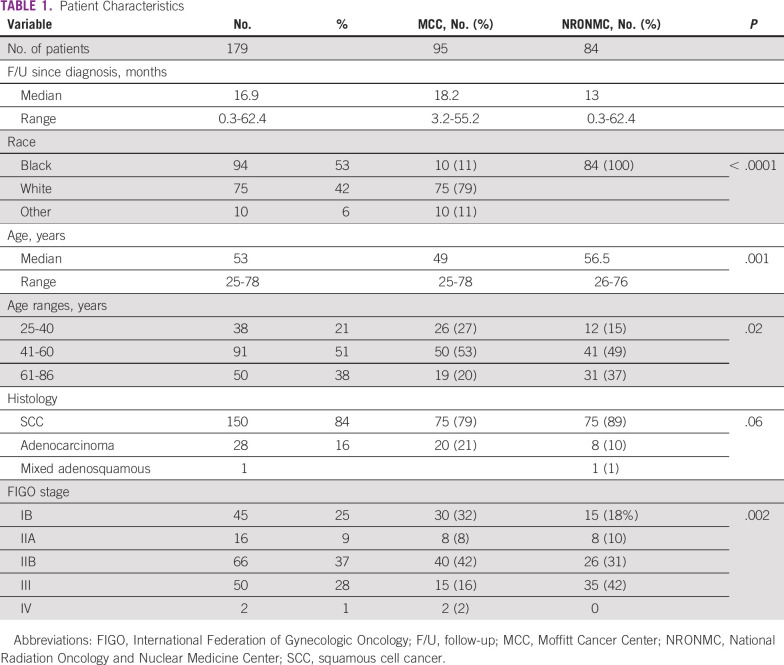
Patient characteristics are detailed in Table 1. A total of 179 patients were treated with definitive chemotherapy and radiation (84 from NRONMC; 95 from MCC) and met criteria to be included in this analysis. The median follow-up for the study since the date of diagnosis was 16.9 months (NRONMC, 13 months; MCC, 18.2 months), with NRONMC patients presenting at an older median age compared with MCC patients (56.5 v 49 years; P = .001). Squamous cell carcinoma was the predominant histologic subtype at both institutions (NRONMC, 89% v MCC, 79%; P = .06). NRONMC patients tended to present with higher stage (stage III at NRONMC, 42% v MCC, 16%; P = .002).
TABLE 1.
Patient Characteristics
Treatment Characteristics
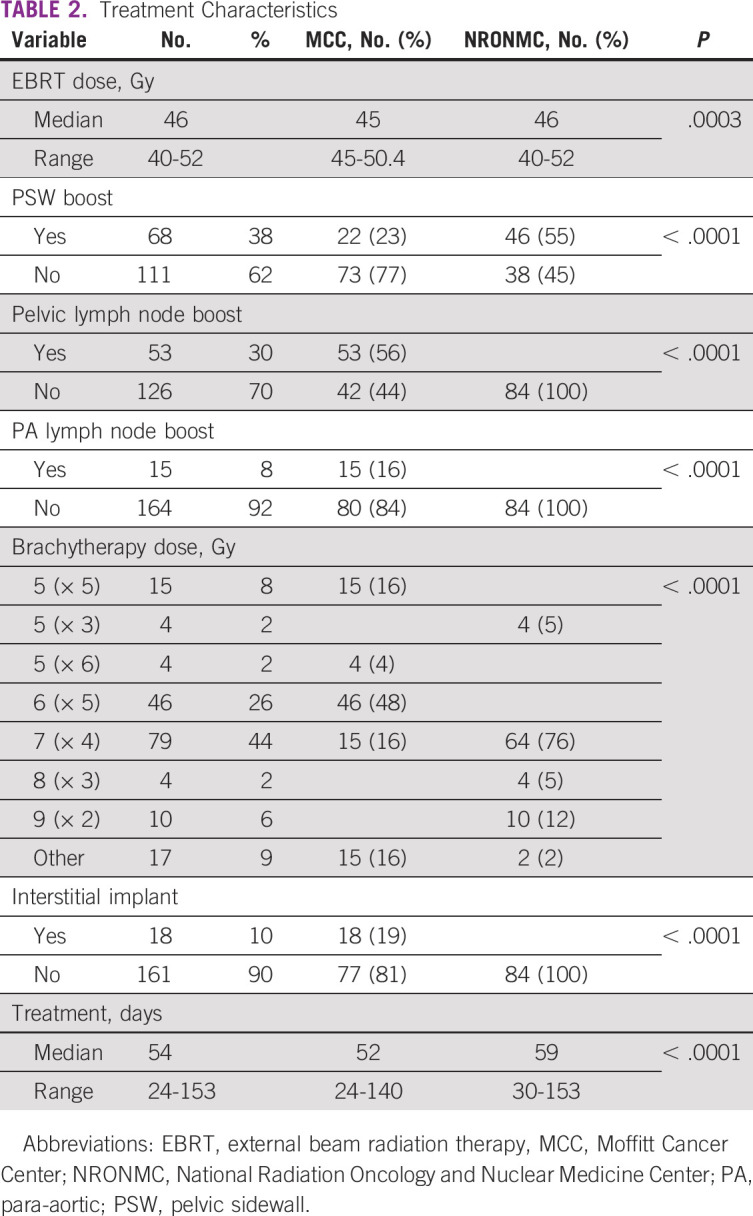
Treatment characteristics are detailed in Table 2. The median EBRT dose prescribed at NRONMC was 46 Gy in 23 fractions and 45 Gy in 25 fractions over 5 weeks at MCC (P = .0003). Isolated pelvic and para-aortic lymph node boosts were not used by NRONMC, but were received by 56% and 16% of MCC patients, respectively. Interstitial implants were received by 19% of MCC patients but not by NRONMC patients. The median duration of treatment was longer for NRONMC patients compared with MCC patients (59 days v 52 days; P < .0001).
TABLE 2.
Treatment Characteristics
Local and Distant Control
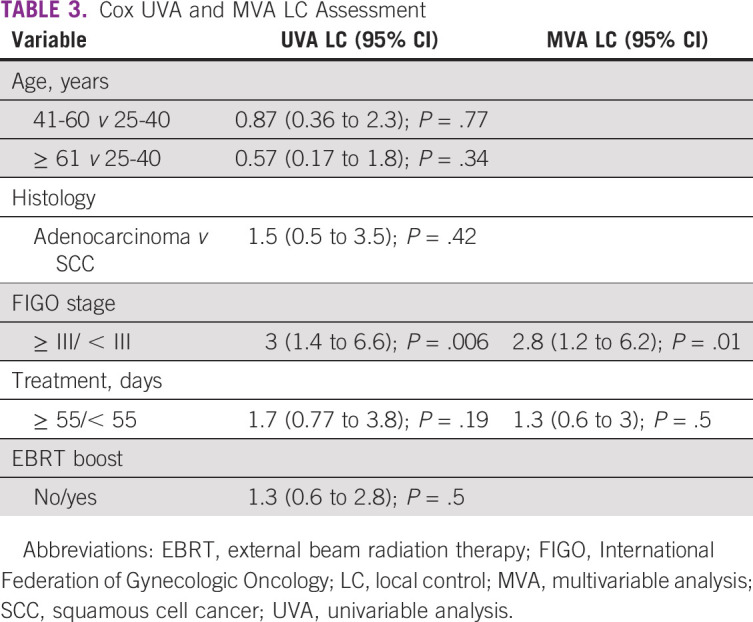
Significant differences were noted in local control between the two institutions for patients with stage III disease only. For stages IB, IIA, IIB, and III, 12-month local control rates for NRONMC versus MCC patients were 70% v 93% (P = .14), 86% v 100% (P = .29), 83% v 91% (P = .59), and 58% v 91% (P = .03), respectively (Fig 1). Factors found to predict for worse local control on UVA included FIGO ≥ III/< III disease (3; 95% CI, 1.4 to 6.6; P = .001; Table 3). Factors that continued to predict for local failure on MVA were FIGO ≥ III/< III disease (2.8; 95% CI, 1.2 to 6.2; P = .01). Distant control rates did not differ between NRONMC and MCC, and at 12 months, were 90% versus 89% (P = .76), respectively.
FIG 1.
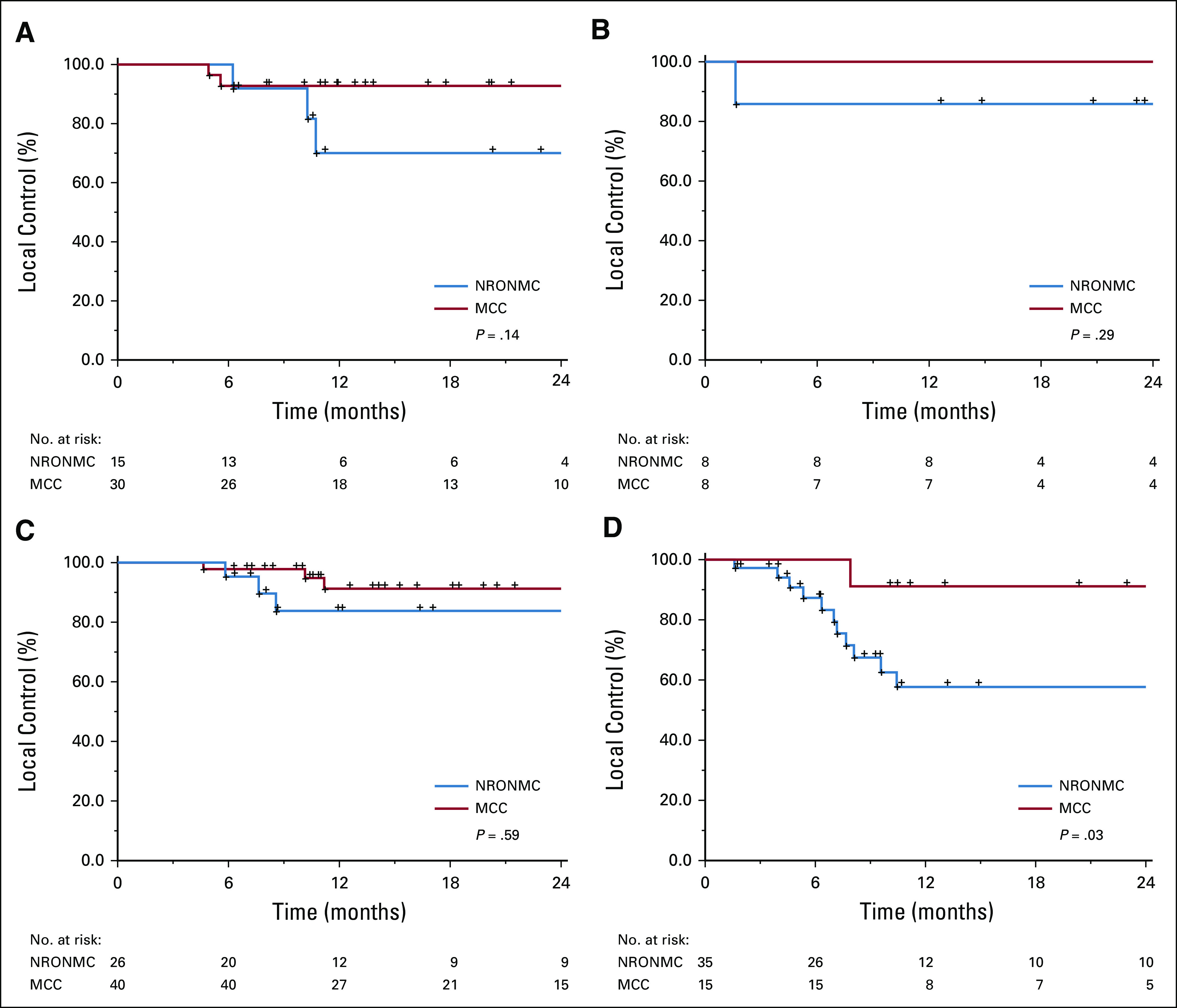
Kaplan-Meier local control between Moffit Cancer Center (MCC) and National Radiation Oncology and Nuclear Medicine Center (NRONMC) for International Federation of Gynecologic Oncology stages (A) IB; (B) IIA; (C) IIB; and (D) III.
TABLE 3.
Cox UVA and MVA LC Assessment
Overall Survival
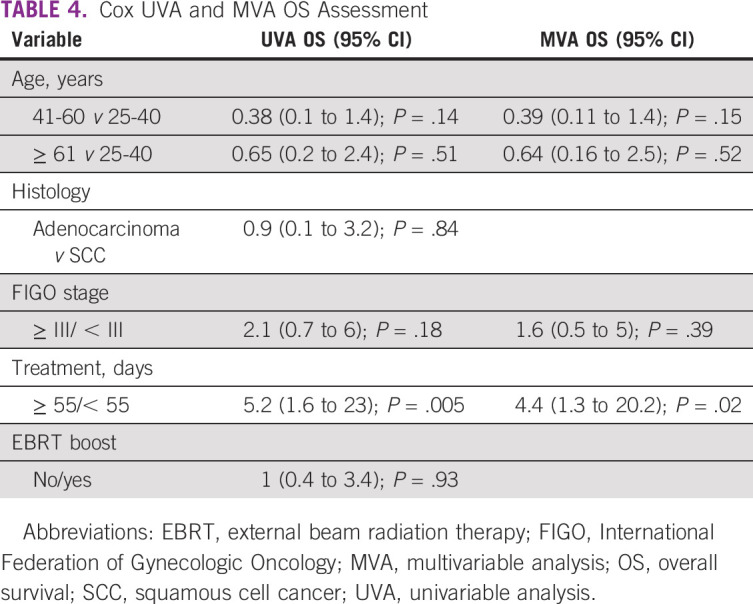
For stages IB, IIA, IIB, and III, 12-month OS rates for NRONMC versus MCC patients were 86% versus 100% (P = 0.05), 100% versus 100%, 95% versus 100% (P = .14), and 90% versus 91% (P = .48), respectively. Factors found to predict for OS on UVA included longer treatment duration ≥ 55/< 55 days (5.2; 95% CI, 1.6 to 23; P = .005; Table 4). Factors that continued to predict for OS on MVA included longer treatment ≥ 55/< 55 days (4.4; 95% CI, 1.3 to 20.2; P = .02).
TABLE 4.
Cox UVA and MVA OS Assessment
DISCUSSION
In this study, we compared cervical cancer outcomes between patients at MCC and NRONMC to identify differences in patient and disease characteristics as well as treatment delivery to identify opportunities for treatment optimization. We noted significant differences in presentation, with NRONMC patients more likely to present with more advanced stage and older age and to have longer treatment durations. The receipt of radiation therapy boosts to gross nodal disease with the availability of interstitial implantation likely led to improved outcomes in patients with stage III disease.
Concurrent cisplatin chemotherapy and radiation treatment of cervical cancer has been available at NRONMC since 2001. Brachytherapy was delivered using two cesium136 low-dose-rate (LDR) afterloader brachytherapy sources before transitioning to an HDR cobalt-60 brachytherapy program 5 years ago. LDR brachytherapy was typically delivered in two 20-Gy fractions over 24-30 hours each. We reported our experience using LDR brachytherapy with a 3-year locoregional recurrence rate of 19%, 3-year OS of 86%, and median treatment completion time of 73 days.7 The center transitioned to an HDR brachytherapy program in 2014, which offers outpatient treatment, higher treatment capacity, and the potential to complete treatment within 9 weeks, which has been realized in this study.
We found approximately a quarter of NRONMC patients presented with early-stage disease compared with 40% at MCC. It is also striking to note that nearly half of NRONMC patients presented with stage III disease compared with less than 20% of MCC patients. Cervical cancer screening has been instituted in the United States for decades, with a precipitous decline in the incidence of advanced cervical cancer.11,12
The introduction of vaccines aimed at building immunity against the most common HPV strains is anticipated to further reduce the incidence of the disease in the United States.13,14 A cross-sectional epidemiologic study detailing HPV subtypes among patients with invasive cervical cancer from three sub-Saharan countries including Ghana indicates similar HPV subtype prevalence between the two populations.14,15 A holistic approach to improve cervical cancer outcomes may include screening and vaccination programs, as well as increasing access to radiotherapy (RT) services.
For stage IB, IIA, and IIB, there were no statistically significant differences in outcome between the two centers in terms of local control and OS. A difference in local control, however, was noted for patients with stage III disease. This observation is probably due to differences in staging procedures, radiation delivery technique, and the radioisotope used for brachytherapy. Differences in receipt of follow-up imaging between the two centers makes interpretation of distant control more difficult.
MCC patients received para-aortic node radiation and interstitial boosts as appropriate based on pretreatment MRI and PET. Tumor volume delineation with MRI is more accurate and more likely to ensure more accurate dose delivery relative to prescription to midline and fluoroscopic 2D planning. MRI-based target volume delineation in adaptive brachytherapy planning has been reported to offer improved disease control and OS benefits compared with historical controls treated with point A brachytherapy dose prescription.16,17 A French prospective nonrandomized study also found 3D brachytherapy dosimetry to be associated with improved relapse-free survival and toxicity profiles.18
Intracavitary brachytherapy generally has a higher dose inhomogeneity compared with EBRT, because of the rapid dose fall-off due to the inverse square law as one moves away from the radiation source. This inhomogeneity has been shown to be compounded by the radial dose function or the tissue attenuation factor of the radioactive isotope used.19 It has been shown that the radial dose function of iridium-192 is fairly constant and does not fall off sharply within the first 10 cm of radial distance; therefore, its dose distribution is fairly homogenous, especially in bulky tumors.19-21 However, the radial dose function of cobalt-60 is rather steep within the first 10 cm of radial distance, and hence, the dose distribution, especially in bulky tumors, may not be homogenous.19,20
Thus, even though the dose to the side walls is adequate as recommended by the American Brachytherapy Society (55-60 Gy for bulky disease),22 the dose distribution to bulky disease may not be homogeneous in the NRONMC cohort. This probably accounted in part for the differences in outcome observed for patients with stage III disease, with larger-volume disease likely to harbor occult metastasis to pelvic and para-aortic nodes.
Para-aortic nodal irradiation alone (without chemotherapy) results in inferior overall and failure-free survival compared with pelvic radiation concurrent with fluorouracil and cisplatin chemotherapy.23 However, there are nonrandomized studies that suggest improved nodal recurrence and survival rates when para-aortic nodal radiation is administered in the setting of concurrent chemoradiotherapy in both the pre-PET and PET imaging eras.23-26 Para-aortic nodal irradiation is mandated in prespecified patient subsets in the ongoing prospective EMBRACE II study (ClinicalTrials.gov identifier: NCT03617133) with the hope of improving nodal control rates.27
Radiation dose coverage of the CTV can be optimized with interstitial implants during brachytherapy. Demanes et al28 reported their experience with transperineal interstitial cervical cancer brachytherapy and documented local control rates of 95% and 75% for patients with stage III and IVA disease at 5 years, respectively. The rate of interstitial implant use to augment HR-CTV coverage during brachytherapy was approximately 20% in the EMBRACE I study (ClinicalTrials.gov identifer: NCT04467411). This approach is also being explored in EMBRACE II to improve CTV coverage in patients with bulky disease.27 The use of para-aortic nodal radiation and interstitial implants in MCC patients may have contributed to local control differences among patients with stage III disease.
Although IMRT was used at MCC, a traditional 2D planning technique was used at NRONMC to plan out the EBRT. RTOG 1203 ClincicalTrials.gov identifier: NCT01672892) revealed significantly less acute GI and urinary toxicity with pelvic IMRT compared with standard RT.29 In addition, IMRT reduces GI and urinary adverse events at 1 and 3 years of follow-up.30 No differences have been noted in OS, disease-free survival, or local regional failure.30 The EMBRACE II study incorporates IMRT EBRT into its treatment planning.27 Although we were unable to make meaningful comparisons regarding toxicity in our study, we did not find a difference in clinical outcomes in patients with early-stage disease treated between the two centers using IMRT or 2D treatment planning.
Avoiding treatment interruptions during cervical cancer treatment with EBRT and brachytherapy has been demonstrated in multiple studies to be associated with improved disease control.31,32 A report of the Mallinckrodt Institute’s experience identified an overall treatment time of 7 weeks to be associated with improved OS compared with longer treatment periods among patients with stage IB-II cervical cancer.33 Another report from Japan documents similar findings among patients with stage IIB-III cervical cancer treated with HDR brachytherapy.34
A recent review of patients with cervical cancer undergoing chemoradiation from the National Cancer Database suggests that an overall treatment time of approximately 9 weeks may not be detrimental to disease-free survival and OS compared with the 7 weeks with no chemotherapy.35 In our study, we found NRONMC patients experienced longer median overall treatment completion times of 59 days compared with 52 days at MCC. Prolongation of treatment time at NRONMC was due to factors such as delays in brachytherapy scheduling, patient comorbidities, logistics, and correction of severe anemia. Even though this treatment duration is well within the recommended 9 weeks, our study suggests added local control benefit to completing treatment over a shorter duration.
Although the findings in our study are intriguing and hypothesis generating, they are not without limitations. The study was affected by the limitations common to retrospective studies and was limited by patient numbers. We are unable to draw meaningful comparisons of treatment toxicity between the two centers, given the retrospective evaluation of data in this series. In addition, it was difficult to identify all factors that could contribute to predictors of poor clinical outcome between the two centers due to inherent differences between the two sites.
In conclusion, differences in staging technique with the use of PET imaging and subsequent radiation treatment of identified disease in the para-aortic region, MRI for contouring of local disease, interstitial implants to optimize treated volume coverage, and the radioisotope type used for brachytherapy most likely accounted for the difference in outcome for stage III disease between the two centers. Efforts should also be made to improve early screening to avoid advanced disease presentation. In addition, we note that minimizing treatment breaks leads to improved outcomes, and all attempts should be made to minimize gaps in treatment.
SUPPORT
This work was made possible in part due to the American Cancer Society Audrey Myer Mars Clinical Fellowship Grant.
AUTHOR CONTRIBUTIONS
Conception and design: Francis Adumata Asamoah, Joel Yarney, Michael E. Montejo, Mian M. Shahzad, Kosj Yamoah, Kamran A. Ahmed
Administrative support: Aba Scott, Kwabena Anarfi, Charles Aidoo, Kosj Yamoah
Provision of study materials or patients: Francis Adumata Asamoah, Joel Yarney, Aba Scott, Daniel C. Fernandez, Mervin Agyeman, Samuel Ntiamoah Boateng
Collection and assembly of data: Francis Adumata Asamoah, Joel Yarney, Aba Scott, Zhigang Yuan, Daniel C. Fernandez, Mervin Agyeman, Samuel Ntiamoah Boateng, Kwabena Anarfi, Kamran A. Ahmed
Data analysis and interpretation: Francis Adumata Asamoah, Joel Yarney, Aba Scott, Verna Vanderpuye, Zhigang Yuan, Samuel Ntiamoah Boateng, Charles Aidoo, Mian M. Shahzad, Jing-Yi Chern, Hye-Sook Chon, Robert M. Wenham, Kosj Yamoah, Kamran A. Ahmed
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Joel Yarney
Honoraria: Roche, Johnson & Johnson, AstraZeneca
Speakers' Bureau: Johnson & Johnson
Travel, Accommodations, Expenses: Roche
Daniel C. Fernandez
Employment: HCA Healthcare (I)
Leadership: Memorial Hospital of Tampa (HCA Healthcare; I)
Stock and Other Ownership Interests: HCA Healthcare (I)
Honoraria: Janssen (I), Sage Therapeutics (I)
Consulting or Advisory Role: Janssen (I), Sage Therapeutics (I)
Speakers' Bureau: Janssen (I), Sage Therapeutics (I)
Travel, Accommodations, Expenses: Sage Therapeutics (I), Janssen (I)
Michael E. Montejo
Stock and Other Ownership Interests: CRSPR, Editas Medicine, Fate Therapeutics, Bristol Myers Squibb, ION Pharma, Pfizer, Intellia
Kwabena Anarfi
Employment: National Center for Radiotherapy and Nuclear Medicine, Korle-Bu, Accra, Ghana
Charles Aidoo
Employment: Korle-Bu
Jing-Yi Chern
Consulting or Advisory Role: NCCN/AstraZeneca, Tesaro
Robert M. Wenham
Stock and Other Ownership Interests: Ovation Diagnostics
Honoraria: Tesaro
Consulting or Advisory Role: Mersana, Merck, Tesaro, Clovis Oncology, Genentech, Regeneron, AbbVie, AstraZeneca, GlaxoSmithKline
Speakers' Bureau: Tesaro, Clovis Oncology, Genentech
Research Funding: Merck (Inst), Prescient Therapeutics (Inst)
Travel, Accommodations, Expenses: TapImmune
Other Relationship: AstraZeneca
Kamran A. Ahmed
Honoraria: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.3322/caac.21492. Bray F, Ferlay J, Soerjomataram I, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [Erratum: CA Cancer J Clin 70:313, 2020] [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nartey Y, Hill PC, Amo-Antwi K, et al. Factors contributing to the low survival among women with a diagnosis of invasive cervical cancer in Ghana. Int J Gynecol Cancer. 2017;27:1926–1934. doi: 10.1097/IGC.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 4.Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87:111–119. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 7.Vulpe H, Asamoah FA, Maganti M, et al. External beam radiation therapy and brachytherapy for cervical cancer: The experience of the National Centre for Radiotherapy in Accra, Ghana. Int J Radiat Oncol Biol Phys. 2018;100:1246–1253. doi: 10.1016/j.ijrobp.2017.12.270. [DOI] [PubMed] [Google Scholar]
- 8.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Lim K, Small W, Jr, Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 10.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Devesa SS. Descriptive epidemiology of cancer of the uterine cervix. Obstet Gynecol. 1984;63:605–612. [PubMed] [Google Scholar]
- 12.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: A 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031–1037. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 14.Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Denny L, Adewole I, Anorlu R, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer. 2014;134:1389–1398. doi: 10.1002/ijc.28425. [DOI] [PubMed] [Google Scholar]
- 16.Rijkmans EC, Nout RA, Rutten IH, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol. 2014;135:231–238. doi: 10.1016/j.ygyno.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013;52:1510–1519. doi: 10.3109/0284186X.2013.818253. [DOI] [PubMed] [Google Scholar]
- 18.Charra-Brunaud C, Harter V, Delannes M, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol. 2012;103:305–313. doi: 10.1016/j.radonc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Venselaar JL, van der Giessen PH, Dries WJ. Measurement and calculation of the dose at large distances from brachytherapy sources: Cs-137, Ir-192, and Co-60. Med Phys. 1996;23:537–543. doi: 10.1118/1.597811. [DOI] [PubMed] [Google Scholar]
- 20.Strohmaier S, Zwierzchowski G. Comparison of (60)Co and (192)Ir sources in HDR brachytherapy. J Contemp Brachytherapy. 2011;3:199–208. doi: 10.5114/jcb.2011.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meigooni AS, Nath R. A comparison of radial dose functions for 103Pd, 125I, 145Sm, 241Am, 169Yb, 192Ir, and 137Cs brachytherapy sources. Int J Radiat Oncol Biol Phys. 1992;22:1125–1130. doi: 10.1016/0360-3016(92)90819-4. [DOI] [PubMed] [Google Scholar]
- 22.Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 23.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 24.Malfetano JH, Keys H, Cunningham MJ, et al. Extended field radiation and cisplatin for stage IIB and IIIB cervical carcinoma. Gynecol Oncol. 1997;67:203–207. doi: 10.1006/gyno.1997.4865. [DOI] [PubMed] [Google Scholar]
- 25.Vargo JA, Kim H, Choi S, et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90:1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Liang JA, Chen SW, Hung YC, et al. Low-dose, prophylactic, extended-field, intensity-modulated radiotherapy plus concurrent weekly cisplatin for patients with stage IB2-IIIB cervical cancer, positive pelvic lymph nodes, and negative para-aortic lymph nodes. Int J Gynecol Cancer. 2014;24:901–907. doi: 10.1097/IGC.0b013e31829f4dc5. [DOI] [PubMed] [Google Scholar]
- 27.Pötter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demanes DJ, Rodriguez RR, Bendre DD, et al. High dose rate transperineal interstitial brachytherapy for cervical cancer: High pelvic control and low complication rates. Int J Radiat Oncol Biol Phys. 1999;45:105–112. doi: 10.1016/s0360-3016(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 29.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol. 2018;36:2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung AR, Pugh S, Klopp AH, et al. IMRT improves late toxicity compared to conventional RT: An update on NRG Oncology-RTOG 1203. Int J Radiat Oncol Biol Phys. 2019;105:S50. [Google Scholar]
- 31.Eifel PJ, Moughan J, Erickson B, et al. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: A patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144–1153. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 32.Girinsky T, Rey A, Roche B, et al. Overall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys. 1993;27:1051–1056. doi: 10.1016/0360-3016(93)90522-w. [DOI] [PubMed] [Google Scholar]
- 33.Perez CA, Grigsby PW, Castro-Vita H, et al. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;32:1275–1288. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- 34.Chatani M, Matayoshi Y, Masaki N, et al. High-dose rate intracavitary irradiation for carcinoma of the uterine cervix. The adverse effect of treatment prolongation. Strahlenther Onkol. 1997;173:379–384. doi: 10.1007/BF03038241. [DOI] [PubMed] [Google Scholar]
- 35.Tergas AI, Neugut AI, Chen L, et al. Radiation duration in women with cervical cancer treated with primary chemoradiation: A population-based analysis. Cancer Invest. 2016;34:137–147. doi: 10.3109/07357907.2015.1131291. [DOI] [PMC free article] [PubMed] [Google Scholar]