Abstract
PURPOSE
The purpose of this study was to evaluate the prognostic value of Immunoscore in patients with stage III colon cancer (CC) and to analyze its association with the effect of chemotherapy on time to recurrence (TTR).
METHODS
An international study led by the Society for Immunotherapy of Cancer evaluated the predefined consensus Immunoscore in 763 patients with American Joint Committee on Cancer/Union for International Cancer Control TNM stage III CC from cohort 1 (Canada/United States) and cohort 2 (Europe/Asia). CD3+ and cytotoxic CD8+ T lymphocyte densities were quantified in the tumor and invasive margin by digital pathology. The primary end point was TTR. Secondary end points were overall survival (OS), disease-free survival (DFS), prognosis in microsatellite stable (MSS) status, and predictive value of efficacy of chemotherapy.
RESULTS
Patients with a high Immunoscore presented with the lowest risk of recurrence, in both cohorts. Recurrence-free rates at 3 years were 56.9% (95% CI, 50.3% to 64.4%), 65.9% (95% CI, 60.8% to 71.4%), and 76.4% (95% CI, 69.3% to 84.3%) in patients with low, intermediate, and high immunoscores, respectively (hazard ratio [HR; high v low], 0.48; 95% CI, 0.32 to 0.71; P = .0003). Patients with high Immunoscore showed significant association with prolonged TTR, OS, and DFS (all P < .001). In Cox multivariable analysis stratified by participating center, Immunoscore association with TTR was independent (HR [high v low], 0.41; 95% CI, 0.25 to 0.67; P = .0003) of patient’s sex, T stage, N stage, sidedness, and microsatellite instability status. Significant association of a high Immunoscore with prolonged TTR was also found among MSS patients (HR [high v low], 0.36; 95% CI, 0.21 to 0.62; P = .0003). Immunoscore had the strongest contribution χ2 proportion for influencing survival (TTR and OS). Chemotherapy was significantly associated with survival in the high-Immunoscore group for both low-risk (HR [chemotherapy v no chemotherapy], 0.42; 95% CI, 0.25 to 0.71; P = .0011) and high-risk (HR [chemotherapy v no chemotherapy], 0.5; 95% CI, 0.33 to 0.77; P = .0015) patients, in contrast to the low-Immunoscore group (P > .12).
CONCLUSION
This study shows that a high Immunoscore significantly associated with prolonged survival in stage III CC. Our findings suggest that patients with a high Immunoscore will benefit the most from chemotherapy in terms of recurrence risk.
INTRODUCTION
The anatomically based American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM classification system provides useful yet incomplete prognostic information.1 New ways to classify cancer focusing on tumor cells1,2 have only shown moderate prediction accuracy and limited clinical usefulness. Chemotherapy is recommended for all stage III colon cancer (CC); however, real-life data on > 200,000 patients show that only 62%-64% of patients with stage III disease are receiving chemotherapy.3,4 Only 20% of patients with stage III CC really benefit from adjuvant chemotherapy, exposing 80% of patients to unnecessary hazards and toxicity of multi-agent chemotherapy.3,4 A recent international study (IDEA; ClinicalTrials.gov identifier: NCT00958737)5,6 evaluated adjuvant chemotherapy regimens in > 12,000 patients with stage III CC. Overall, the study did not meet the primary end point of noninferiority of 3 versus 6 months chemotherapy. It was concluded that age, risk assessment, and more precise risk categories could be used to inform the duration of adjuvant chemotherapy.
CONTEXT
Key Objective
To test the prognostic value of Immunoscore in Stage III patients and its predictive value in response to chemotherapy.
Knowledge Generated
Independently of age, Immunoscore significantly predicted time to recurrence and overall survival. Immunoscore had the highest contribution to survival, was significant in Cox multivariable analysis, and predicted the likelihood of response to chemotherapy.
Relevance
The benefit of chemotherapy may be dependent upon a proper pre-existing immunity, and Immunoscore may help to predict and stratify patients who will benefit from treatment.
Extensive literature demonstrated a favorable prognostic impact of the in situ immune-cell infiltrate in tumors.1,7-19 In CC we have shown that time to recurrence (TTR) and overall survival (OS) strongly correlated with the strength of in situ adaptive immune reaction9,14,18,20,21 at the center of tumor (CT) and invasive margin (IM).22 We characterized immune subtypes infiltrating tumors23 and proposed that intratumoral immune contexture of tumors could be a dominant determinant of clinical outcome.8,9 We showed the usefulness of a derived immune score—Immunoscore—to predict clinical outcome in patients with early21 and advanced24-28 stage CC. Recently, an international consortium validated the prognostic value of the consensus Immunoscore in patients with TNM stage I-III29 and stage II CC.29 However, the prognostic value of Immunoscore to predict risk of recurrence and death in stage III CC and the predictive value of Immunoscore regarding response to chemotherapy remain unclear. Herein, the international Society for Immunotherapy of Cancer Immunoscore consortium aimed at validating the predefined consensus Immunoscore for patients with stage III CC. We report the final results of Immunoscore to stratify patients with stage III CC and examined its potential to further refine T and N risk categories, with implications for adjuvant therapy.
METHODS
Patients
An international consortium composed of 14 pathology expert centers from 13 countries evaluated the consensus Immunoscore in primary tumors from 763 patients with stage III CC.29 Clinical data from North America and Europe plus Asia data sets are presented in the Data Supplement (online only). These retrospective cohorts include untreated patients and also patients treated with fluorouracil (FU), folinic acid, and oxaliplatin (FOLFOX) and other combinations of chemotherapy drugs such as oxaliplatin and capecitabine (XELOX) and folinic acid, fluorouracil, and irinotecan (FOLFIRI). These patients did not receive any immune checkpoint therapy. The primary end point was TTR, defined as time from surgery to disease recurrence. Secondary end points were OS, disease-free survival (DFS), and prognosis in microsatellite-stable (MSS) status. All end points were prespecified. The study was approved by the ethics committees from each center.
Immunohistochemistry
Within each center, a pathologist selected for each patient a tumor block containing CT and IM. Two tissue paraffin sections of 4 μm were processed for standardized immunohistochemistry as previously described.29
Image Analysis
CD3 and CD8 cell densities were determined in CT and IM regions using dedicated Immunoscore software.29 The mean and the distribution of the staining intensities were monitored, providing an internal quality control of each slide.
Immunoscore Determination
CD3 and CD8 densities in CT and IM regions were converted into percentiles as previously described.29 The mean of the four percentiles obtained was calculated and translated into Immunoscore scoring system (HalioDx, Marseille, France). Immunoscore categories were previously defined independently of clinical data.29 These predefined categories were used herein, with three Immunoscore categories (0%-25%, low [Lo]; > 25%-70%, intermediate [Int], > 70%-100% high [Hi]). Additional categories are described in the Data Supplement.
Monitoring of the Study
The biomarker reference center (Hôpital-Européen-Georges-Pompidou AP-HP, INSERM, Paris) optimized immunostaining protocols, provided Immunoscore software user’s manual, and validated data from each cohort analyzed.29 Exclusion criteria included missing counts at either tumor region or low staining intensity (≤ 152 Arbitrary Unit [AU]).
Microsatellite Instability and Mutation Status
For patients with enough sample material available (n = 476), genomic DNA was extracted from paired tumor and normal colonic tissue from formalin-fixed paraffin-embedded slides. Microsatellite-instability (MSI) status was assessed with the molecular new Bethesda panel as previously described.29 Patients with deficient mismatch repair and proficient mismatch repair were denoted MSI and MSS, respectively. Mutations were tested in patients with available material (BRAF, APC, TP53, n = 109; KRAS, n = 119 patients).
Statistics
Demographics and disease characteristics were compared across the North America and Europe plus Asia cohorts by t test, Fisher’s exact test, and χ2 test when applicable. Bivariable association between Immunoscore and time-to-event outcomes was evaluated by the log-rank test and by participating center–stratified Cox proportional hazards model. Multivariable Cox models stratified by center were used to assess the associations between Immunoscore and outcomes adjusting for potential confounders. The comparison of the performance of risk prediction models was performed using the likelihood-ratio test P value. Survival time distribution was done using the restricted mean survival time (RMST).30 See Data Supplement for details.
RESULTS
Immune Densities and Immunoscore in Relation to the Age of the Patients
Biomarker data from 763 patients with CC with AJCC/UICC TNM stage III who were part of the Immunoscore international validation study29 were investigated. Patients were divided into two data sets: cohort 1 (North America), cohort 2 (Europe and Asia; Fig 1A), with balanced demographic and clinical characteristics (Data Supplement). Overall, patients were 48.8% male, with a median age of 66.9 ± 13 years. The mean number of lymph nodes (LN) examined was 18.9 ± 14.3. Across all patients, 251 (32.9%) relapses, and 342 (44.8%) deaths were observed. The median follow-up time for censored patients was 69.6 months (95% CI, 63.5 to 73.9 months), and median survival time from surgery to death due to any cause was 102.9 months (95% CI, 92.6 to 109 months).
FIG 1.
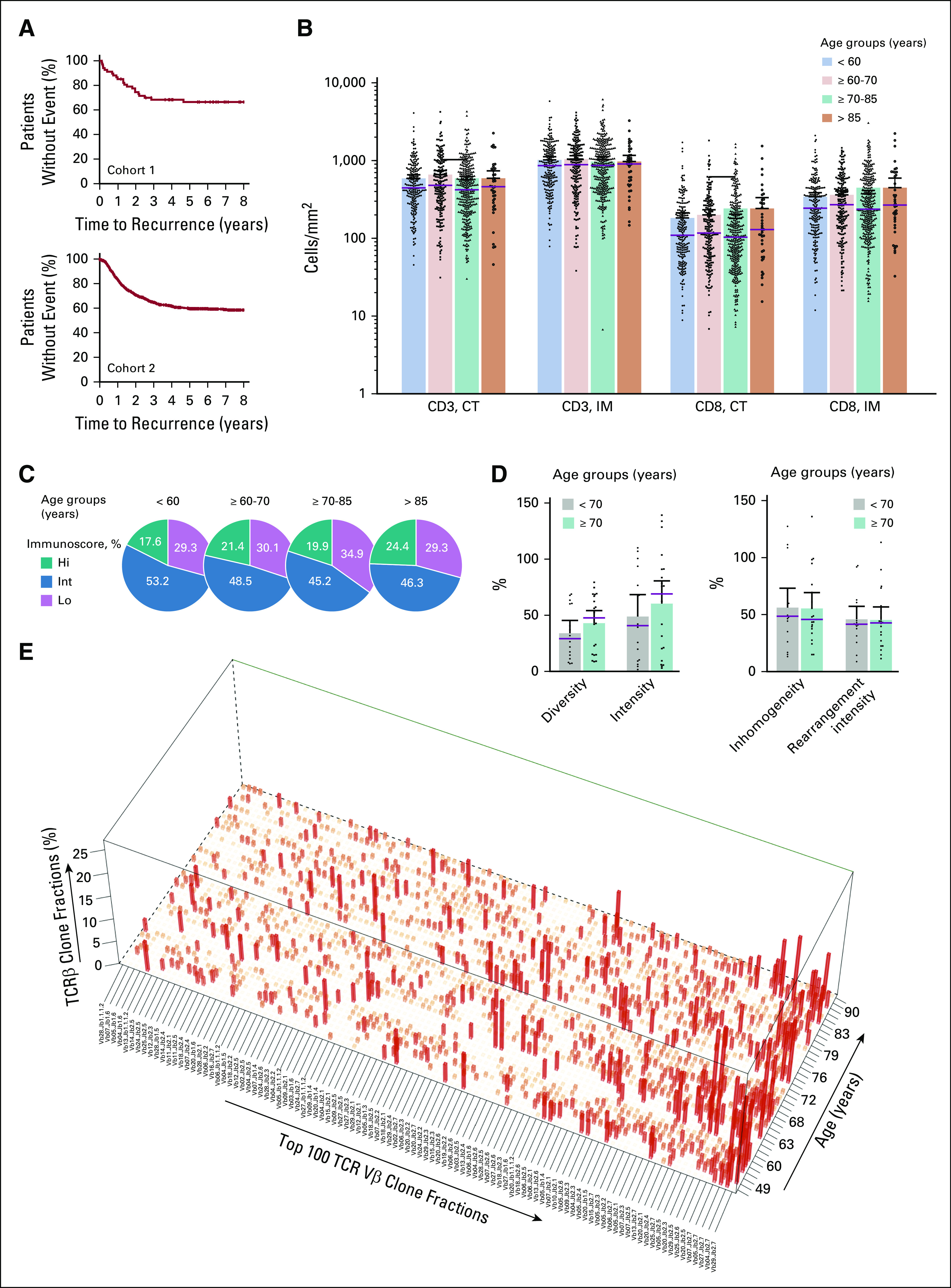
The immune infiltrate and Immunoscore in patients with different ages. (A) Kaplan-Meier curves for patients with colon cancer from cohorts 1 (upper panel) and 2 (lower panel). (B) Patient groups based on age: < 60 years (light blue), ≥ 60-70 (light orange), ≥ 70-85 (green) and ≥ 85 (orange)and CD3 and CD8 immune densities quantified in the tumor core (CT) and invasive margin (IM). Each dot represents a mean whole-slide quantification in 1 patient. (C) The Society for Immunotherapy of Cancer study cutoff of the Immunoscore scoring system applied to convert immune densities into percentiles. The Immunoscore classified patients (grouped by age) into three categories: low (Lo, mean percentile 0%-25%, purple), intermediate (Int, > 25%-70%, blue), and high (Hi, > 70%-100%, green). (D) T-cell receptor diversity, intensity, inhomogeneity, and rearrangement intensity in patient groups based on age (< 70, gray; ≥ 70, green). (E) Clonal configuration of T-cell receptor (TCR) transcripts (quantitative polymerase chain reaction) in patients with different ages. The top 100 most frequent clones are shown in descending order (mean percentage over all patients). Each bar represents an individual clonotype.
Immunoscore predefined cut points29 were applied to cohorts 1 and 2. The intratumoral densities and densities quantified at the IM were not influenced by the age of the patients (Fig 1B). Immunoscore was not associated with clinical parameters except T stage (Data Supplement). Similarly, the proportion of patients with high, intermediate, and low Immunoscore was independent of the age interval (Fig 1C). The mean number of total LN found was slightly higher in younger patients, with 21.4 (95% CI, 18.9 to 23.9), 20 (95% CI, 17.9 to 22), 16.9 (95% CI, 15.7 to 18.2), and 17.6 (95% CI, 14.7 to 20.6) LN found in < 60, ≥ 60-70, ≥ 70-85, and ≥ 85-year-old categories. No age-related difference was observed in the T-cell receptor (TCR) diversity, intensity, inhomogeneity, and rearrangement intensity (Fig 1D). Analyses of the clonal configuration of intratumoral TCR transcripts showed that the diversity of the top 100 most frequent clones does not differ in young and elderly patients (Fig 1E).
Immunoscore and the Outcome of Patients With Stage III CC
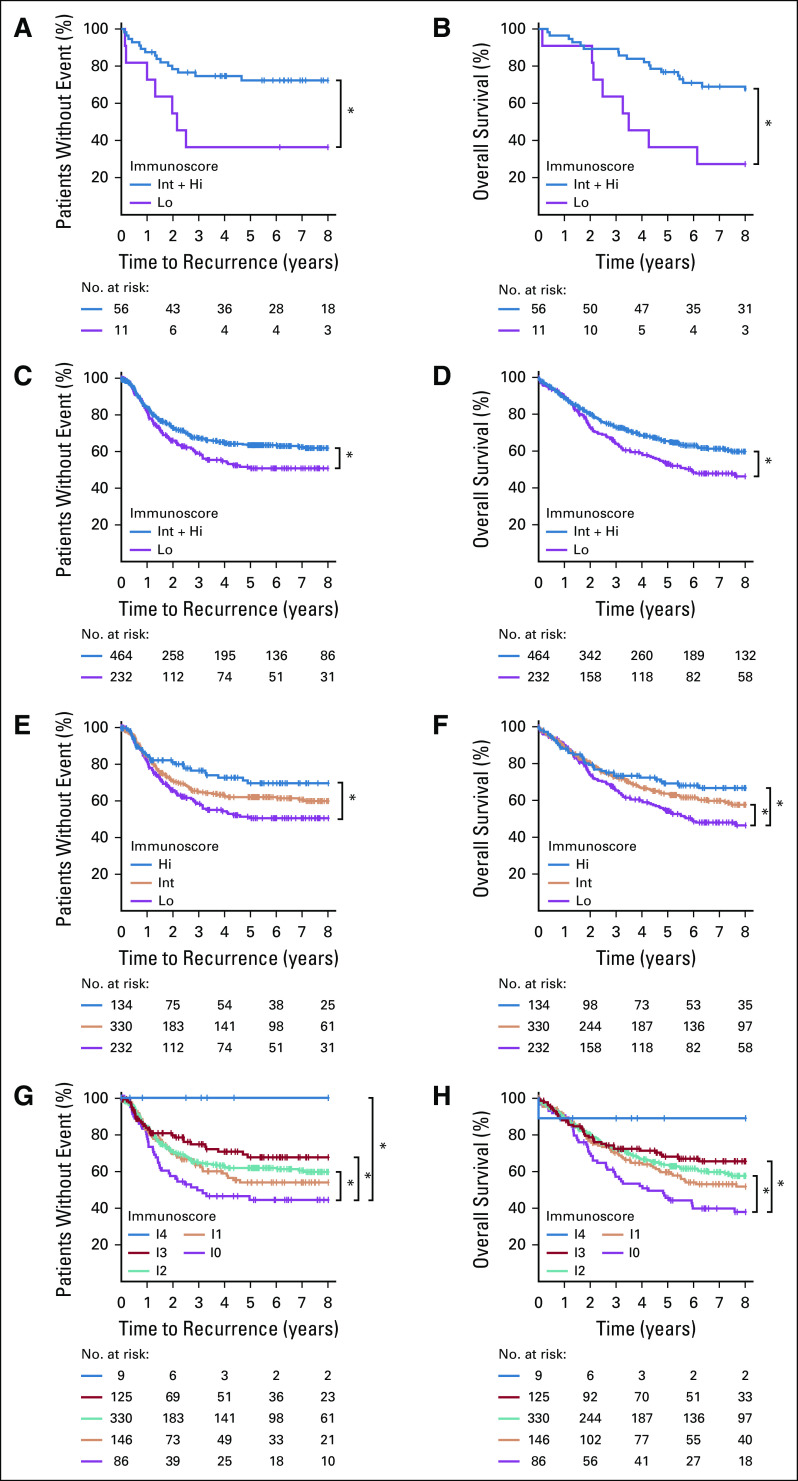
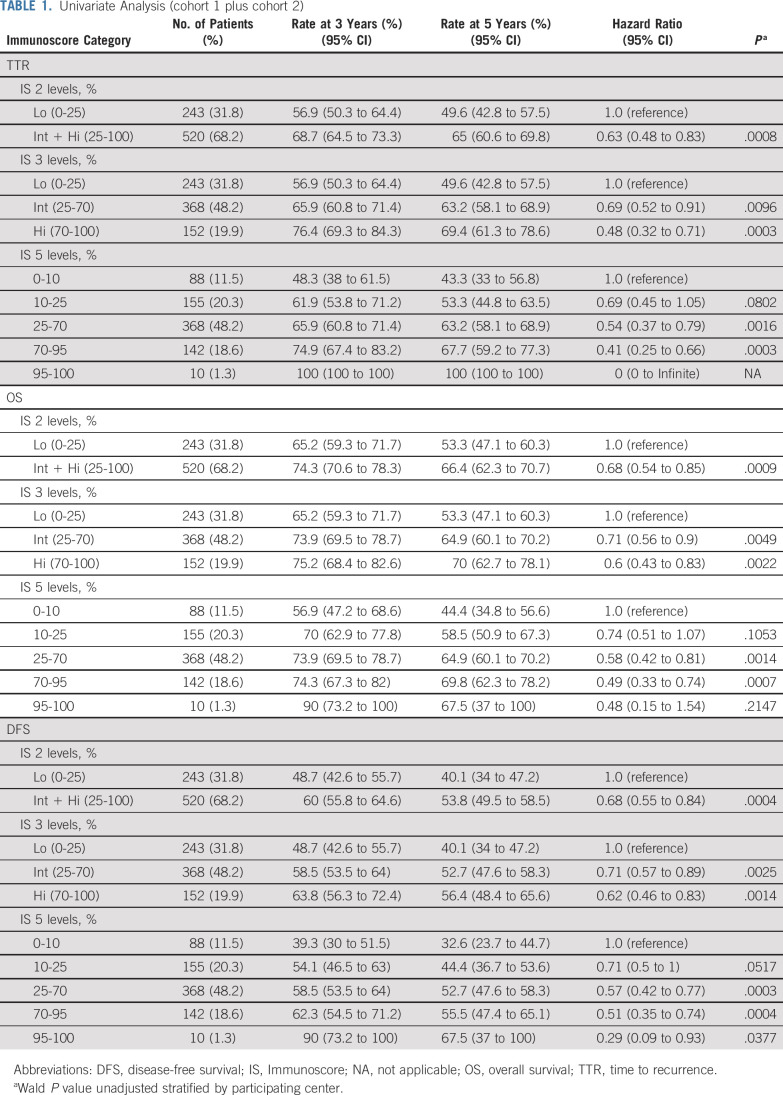
The prognostic value of Immunoscore for TTR, OS, and DFS of patients with stage III CC was further evaluated in cohorts 1 and 2 (Fig 2; Table 1). The 2-category Immunoscore identified patients with distinct clinical outcome for TTR and OS (Figs 2A-2D). Patients with a high Immunoscore from cohort 1 (83.6% [n = 67]; Figs 2A and 2B; Data Supplement) had a significantly longer survival for TTR (5-year recurrence rate, Int + Hi: 27.7%, Lo: 63.6%; unadjusted hazard ratio [HR; Int + Hi v Lo], 0.34; 95% CI, 0.14 to 0.83; P = .0176) and OS (unadjusted HR [Int + Hi v Lo], 0.32; 95% CI, 0.15 to 0.7; P = .0045). The 2-category Immunoscores were also analyzed in cohort 2 (n = 696; Figs 2C and 2D; Data Supplement) and among patients from both cohorts (Table 1; Data Supplement). DFS rates at 3 years were 48.7% and 60.0% for Immunoscore-Lo and Immunoscore–Int + Hi, respectively (unadjusted HR [Int + Hi v Lo], 0.68; 95% CI, 0.55 to 0.84; P = .0004; Table 1; Data Supplement). In cohort 2, 66.7% of the patients had Int + Hi Immunoscore (Figs 2C and 2D; Data Supplement) and had a significantly lower risk to relapse (5-year recurrence rate, Int + Hi: 36.1%, Lo: 49.6%; unadjusted HR [Int + Hi v Lo], 0.67; 95% CI, 0.5 to 0.88; P = .0041) and death (unadjusted HR [(Int + Hi v Lo], 0.72; 95% CI, 0.57 to 0.91; P = .0061).
FIG 2.
The impact of Immunoscore on patient outcome. Kaplan-Meier curves of Immunoscore are shown for (A, C, E, G) time to recurrence and (B, D, F, H) overall survival (OS) for patients from cohort 1 (A, B) and cohort 2 (C-H). (A-D) Immunoscore 2 categories: Lo (0%-25%, purple), Int + Hi (> 25%-100%, blue). (E-F) Immunoscore three categories: Lo (0%-25%, purple), Int (> 25%-70%, orange), and Hi (> 70%-100%, blue). (G-H) Immunoscore 5 categories: I0 (0%-10%, purple), I1 (> 10%-25%, orange), I2 (> 25%-70%, green), I3 (> 70%-95%, red), and I4 (> 95%-100%, blue). (*) Significant log-rank P value .01 ≥ P < .05.
TABLE 1.
Univariate Analysis (cohort 1 plus cohort 2)
Overall for patients with stage III disease (n = 763), recurrence rates at 5 years were 30.6%, 36.8%, and 50.4% for Immunoscore Hi, Int, and Lo, respectively (unadjusted HR [Hi v Lo], 0.48; 95% CI, 0.32 to 0.71; P = .003, Table 1; Data Supplement). This positive association with survival of a high Immunoscore was also confirmed for OS and for cohort 2 (5-year survival rate, Hi: 69.1%, Int: 63.5%, Lo: 54.2%; unadjusted HR [Hi v Lo], 0.63; 95% CI, 0.45 to 0.9; P = .0099; Fig 2F; Data Supplement). Similar significant results were found for DFS (Table 1; Data Supplement). Expanding Immunoscore into five categories (I0, I1, I2, I3, I4) further discriminated patients into higher risk (I0), and lower risk (I4), with significant differences in clinical outcome for TTR (5-year recurrence rate, I4: 0%, I0: 56.7%), OS and DFS (Figs 2E-2H, Table 1; Data Supplement). High-immune densities quantified within the tumor nest and stromal tissue had a similar, positive association with the TTR of the patients (Data Supplement). Such positive association of Hi- and Int + Hi–Immunoscore with TTR can be observed within multiple age-defined categories (Data Supplement), including elderly patients (≥ 68 years; P = .0258; Data Supplement).
Immunoscore, MSI Status, and Mutations
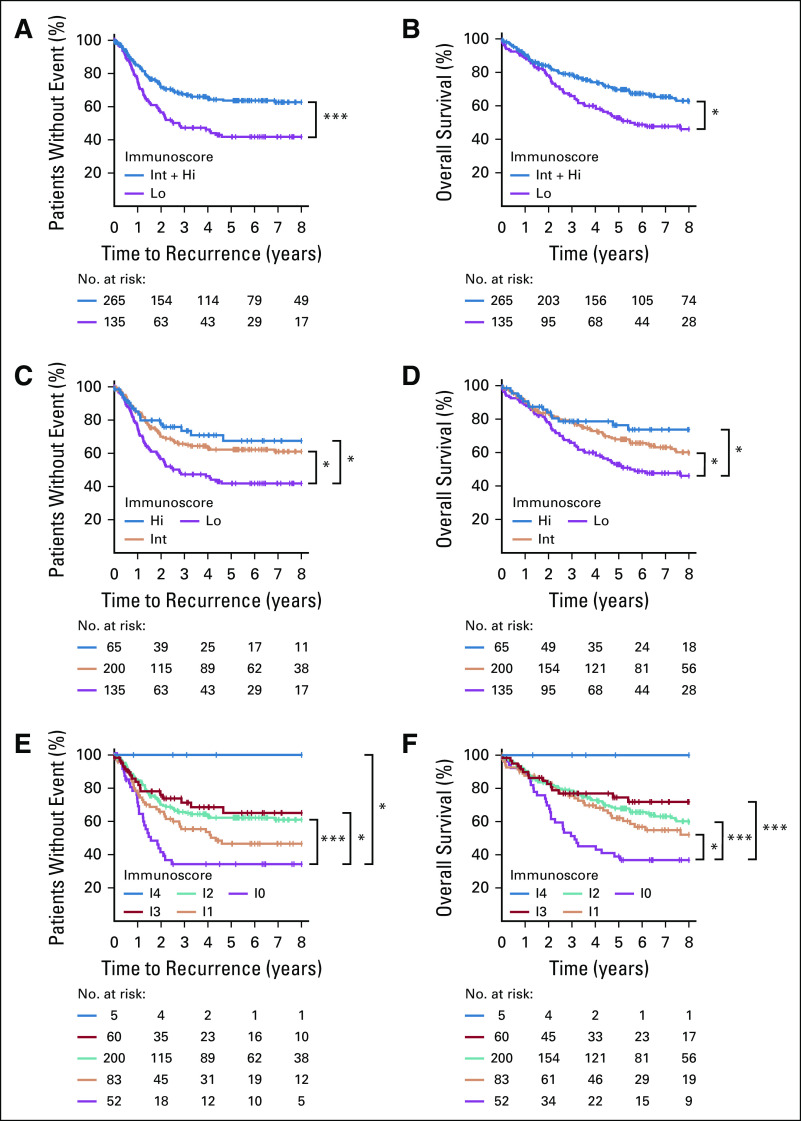
Although only 65% of the patients had MSI status information available, this yielded sufficient power given the low rate of MSI-high status. When stratified into two-category Immunoscore, MSI tumors were associated with Immunoscore-high in 73% (56/76) of cases, and an Immunoscore-high was observed in 66.25% (265/400) of MSS tumors. MSS patients with Int + Hi–Immunoscore had significantly longer TTR (Fig 3A; Data Supplement; 5-year recurrence rate, Int + Hi: 36.4%, Lo: 58.2%; unadjusted HR [Int + Hi v Lo], 0.48; 95% CI, 0.34 to 0.67; P < .0001) as well as OS (Fig 3B; Data Supplement; unadjusted HR [Int + Hi v Lo], 0.52; 95% CI, 0.37 to 0.72; P = .0001). Patients with highly infiltrated MSS tumors had a survival advantage in both TTR (Fig 3C; Data Supplement; 5-year recurrence rate, Hi: 32.5%, Int: 37.8%, Lo: 58.2%; unadjusted HR [Hi v Lo], 0.36; 95% CI, 0.21 to 0.62; P = .0003) and OS (Fig 3D; Data Supplement; unadjusted HR [Hi v Lo], 0.39; 95% CI, 0.23 to 0.67; P =.0006) compared with patients with weakly infiltrated tumors. A similar pro-file was observed when five-category Immunoscore was applied (Figs 3E and 3F). This analysis identified patients with high-risk MSS (I0) with a significantly shorter TTR (Fig 3E) and OS (Fig 3F) compared with patients with Immunoscore-I4 MSS. In a multivariable Cox model stratified by center, MSI did not remain significant for TTR or OS and depended on Immunoscore (Figs 3A-3D; Data Supplement). Mutations of BRAF, APC, TP53 and KRAS were not associated with TTR (Data Supplement) and did not remain significant for TTR and OS in a multivariable Cox model including Immunoscore, T stage, N stage, and sex (Data Supplement). The power of Immunoscore to predict TTR and OS was superior to that of tumor risk parameters, including mutations (Data Supplement).
FIG 3.
The impact of Immunoscore on the outcome of microsatellite-stable (MSS) patients with colon cancer. Kaplan-Meier curves of Immunoscore are shown for (A, C, E) time to recurrence and (B, D, F) overall survival for MSS patients from cohorts 1 and 2. (A, B) Immunoscore two categories: Low (Lo, 0%-25%, purple) and Intermediate + high (Int + Hi), > 25%-100%, blue). (C, D) Immunoscore 3 categories: Lo (0%-25%, purple), Int (> 25%-70%, orange), and Hi (> 70%-100%, blue). (E, F) Immunoscore 5 categories: I0 (0%-10%, purple), I1 (> 10%-25%, orange), I2 (> 25%-70%, green), I3 (> 70%-95%, red), I4 (> 95%-100%, blue). (*) Significant log rank P value .01 ≥ P < .05. (***) Significant log rank P value < .005.
Immunoscore and Time-to-Event Analysis Among Patients With High-Risk and Low-Risk Stage III Disease
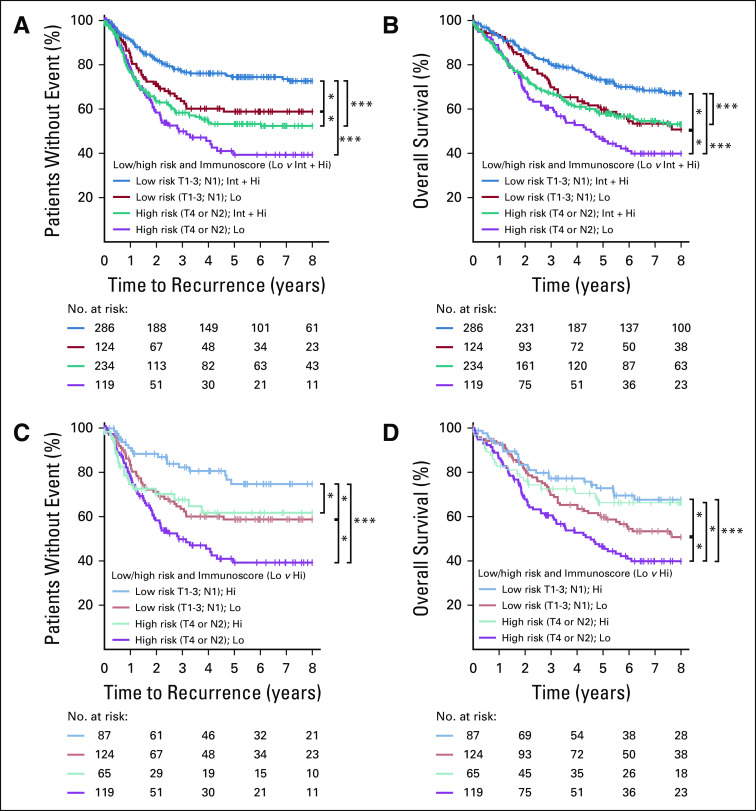
In both pathology-based low-risk and high-risk stage III subgroups, high Immunoscore was associated with a longer survival for most of the patients (Data Supplement). Low-risk patients with Int + Hi Immunoscore presented significantly better outcomes for TTR (5-year recurrence rate, Int + Hi: 25.6%, Lo: 41.2%; unadjusted HR [Int + Hi v Lo], 0.49; 95% CI, 0.32 to 0.74; P = .0007) and OS (unadjusted HR [Int + Hi v Lo], 0.63; 95% CI, 0.43 to 0.88; P = .014) compared with patients with low Immunoscore (Figs 4A and 4B). Similar results were obtained with Immunoscore-high, including patients with the highest intratumoral infiltration (Figs 4C and 4D). In high-risk patients, high Immunoscore significantly predicted lower risk for OS (5-year survival rate, Hi: 66.3%, Int: 54.9%, Lo: 46.5%; unadjusted HR [Hi v Lo], 0.55; 95% CI, 0.33 to 0.91; P = .019; Data Supplement). Similar significant results were also found for TTR (Data Supplement). Within the Immunoscore-Int subgroup, tumor risk parameters were significantly associated with TTR, OS, and DFS (Data Supplement). Thus, Immunoscore significantly predicted survival in subgroups of stage III CC.
FIG 4.
The impact of Immunoscore on the outcome of low- and high-risk patients with colon cancer. Kaplan-Meier curves of Immunoscore are shown for (A, C) time to recurrence and (B, D) overall survival for low-risk (T stage 1-3 and N stage 1) and high-risk (T stage 4 or N stage 2) patients from cohorts 1 and 2. (A, B) Immunoscore 2 categories: 0%-25% (low-risk patients in red; high-risk patients in purple) and > 25%-100% (low-risk patients in blue; high-risk patients in green). (C, D) Immunoscore 2 categories: 0%-25% (low-risk patients in orange; high-risk patients in purple) and > 70%-100% (low-risk patients in light blue; high-risk patients in green). (*) Significant log rank P value .01 ≥ P < .05. (***) Significant log rank P value < .005.
Performance of Immunoscore in Multivariable Analyses
Cox multivariable analyses adjusted for Immunoscore, sex, T stage, N stage, MSI, sidedness, and stratified by city center revealed a significant prognostic value of Immunoscore 2 categories (Data Supplement) and three categories (Figs 5A and 5B). Among tumor-related parameters, only N stage was significant for OS (all P < .01; Fig 5A). The power of Immunoscore to predict TTR and OS was superior to that of tumor-risk parameters, including venous emboli, lymphatic invasion, perineural invasion (VELIPI), mucinous-colloid type, and differentiation (Fig 5B; Data Supplement). Variables with the most important relative contribution to the risk (χ2 proportion) were: Immunoscore, T stage, N stage, and sex in TTR (Data Supplement), and in OS: Immunoscore and N stage (Fig 5B; Data Supplement). Other parameters had a small relative contribution (< 10%; Fig 5B). Immunoscore remained significant in multivariable analysis for TTR (P < .05) without discretization (continuous variable). Furthermore, adding Immunoscore to a model combining all clinical variables significantly improved the prediction for recurrence (likelihood-ratio test, P = .0026) and death (likelihood-ratio test, P = .0124).
FIG 5.
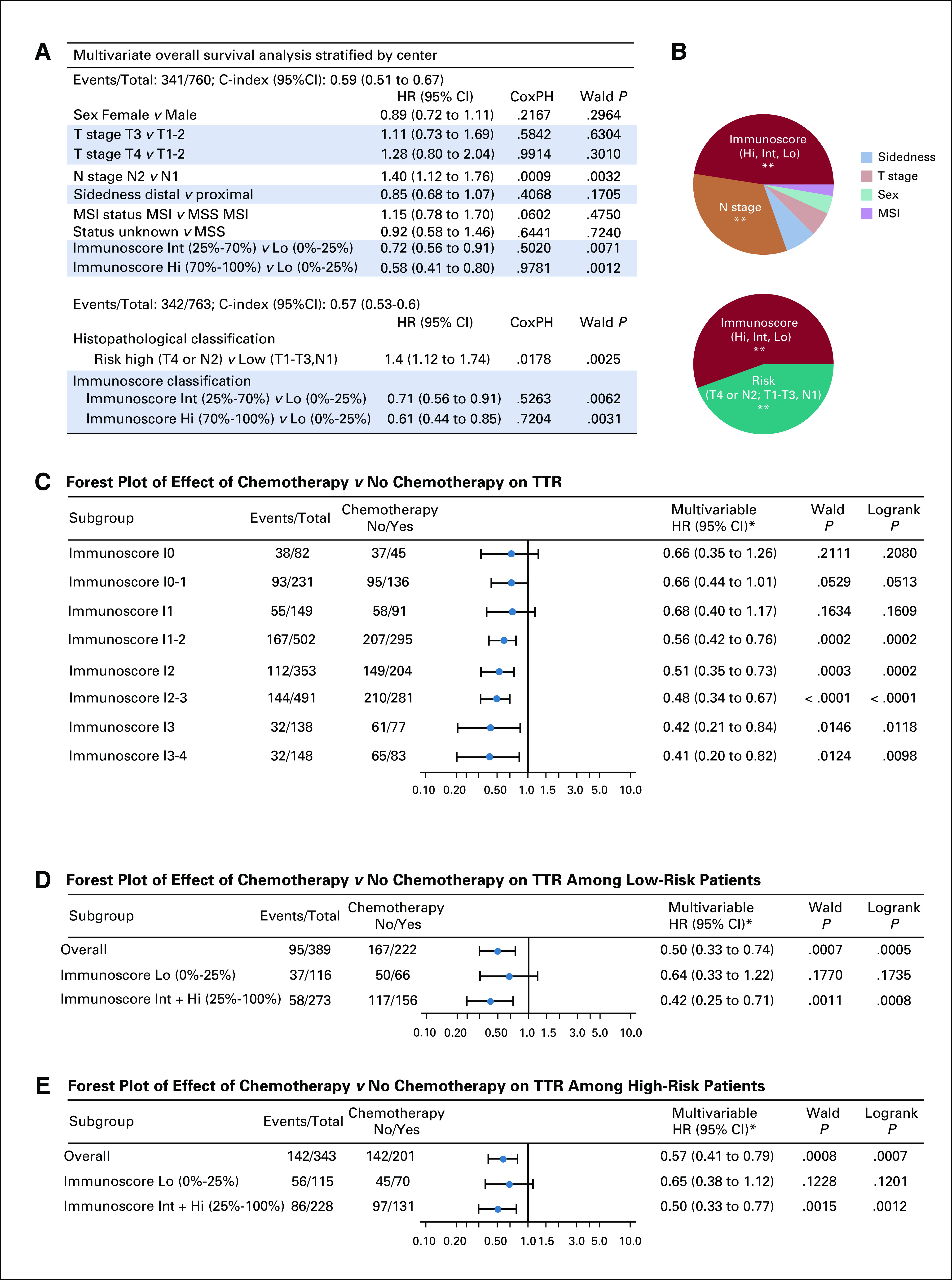
Clinical performance of tumor- and immune-related risk parameters and predictive value. (A) Cox multivariable regression analysis of overall survival combining the three-category Immunoscore: low (Lo, 0%-25%), Intermediate (Int, > 25%-70%), and high (Hi, > 70%-100%) with clinical parameters: sex, T stage, N stage, sidedness, and microsatellite instability (MSI) status. (B) Relative importance of each risk parameter to survival risk for overall survival using the χ2 proportion test for clinical parameters and Immunoscore. Significant prediction of survival using likelihood ratio test. Forest plots representing the predictive value of response to chemotherapy (time to recurrence [TTR]) in (C) Immunoscore groups: I0 (0%-10%), I0-1, I1(> 10%-25%), I1-2, I2 (> 25%-70%), I2-3, I3, (> 70%-95%), and I3-4( > 95%-100%), (D) Immunoscore Lo and Int + Hi, and (D) among high-risk (T4 or N2) and (E) low-risk (T1-T3 and N1) patient subgroups. (*) Subgroup analysis for postoperative chemotherapy with No Chemotherapy as reference. (**): Significant multivariable Wald P value < .01. CoxPH, Cox proportional hazards test P value; HR, hazard ratio, MSS, microsatellite stable.
A model combining Immunoscore and the histopathological risk factor stratification showed the importance of these 2 classifications for the survival of the patients (OS: HR, 0.61; 95% CI, 0.44 to 0.85; P = .0031; Fig 5A). Immunoscore remains the most important parameter for OS (Fig 5D). Similar results were observed for DFS (Data Supplement).
Immunoscore and Benefit From Adjuvant Chemotherapy
To evaluate whether patients might benefit from adjuvant chemotherapy depending on their Immunoscore, we investigated the association between Immunoscore and TTR among patients who did or did not receive adjuvant chemotherapy. Untreated patients were significantly older (without chemotherapy: 73.0 ± 11.8 v with chemotherapy: 62.4 ± 12.0 years old; Data Supplement). Elderly patients were more frequently treated with FU in contrast to younger patients, who received a higher proportion of combination therapies. Treatment with adjuvant chemotherapy was associated with better TTR in stage III (5-year recurrence rate of 32.3% with chemotherapy v 50.6% with no chemotherapy; unadjusted HR, 0.54; 95% CI, 0.41 to 0.72; P < .0001; Data Supplement), low-risk (P = .0007; Fig 5D), and high risk (P = .0008; Fig 5E) stage III subgroups. Forest plot analysis of TTR (Figs 5D and 5E) revealed that Immunoscore predicted chemotherapy benefit, and RMST TTR analysis illustrated major significant differences in the survival months gained by chemotherapy treatment (Data Supplement). Analysis of the predictive value of Immunoscore for response to chemotherapy revealed that the association of chemotherapy with the survival increased within Immunoscore subgroups, from I0 to I4 (Fig 5C). In I0 and I0-1 groups, chemotherapy was not significantly associated with TTR. In contrast, in I2, I2-3, I3, I3-4, and I2-3-4 groups there was a significant association of chemotherapy with TTR (I3-4 group; HR, 0.41; 95% CI, 0.20 to 0.82; P < .009; Fig 5C; Data Supplement). When adding interaction terms in a Cox model, only Immunoscore was significant for TTR (P = .0062), whereas interactions of chemotherapy with age and with Immunoscore were not significant (Data Supplement). In OS, the interaction between Immunoscore and chemotherapy was significant (P = .0092), as well as age (P < .0001; Data Supplement), showing the predictive value of Immunoscore. In low-Immunoscore, chemotherapy was not significantly associated with prolonged TTR, either in high-risk (P = .12) or in low-risk (P = .17) stage III disease (Figs 5D and 5E). Patients with Immunoscore I0 had similar outcome regardless of chemotherapy treatment, in high-risk (P = .12) and low-risk (P = .83) stage III disease (Data Supplement). In contrast, patients with Int + Hi Immunoscore and low-risk stage III disease benefited the most (HR, 0.42; 95% CI, 0.25 to 0.71; P = .0011; Fig 5D; Data Supplement), as well as patients with Int + Hi Immunoscore and high-risk disease (HR, 0.5; 95% CI, 0.33 to 0.37; P = .0015; Fig 5E; Data Supplement). The association between Immunoscore and adjuvant chemotherapy was not significantly confounded by risk factors that are known to affect survival rates among patients with stage III disease (age, sex, differentiation, sidedness, T stage, N stage; Data Supplement). Without interaction terms, Immunoscore and chemotherapy were significant in multivariable analyses for TTR and OS. However, with interaction terms included in the model, Immunoscore was significant with and without interaction with chemotherapy in OS and TTR, respectively (Data Supplement).
DISCUSSION
The recent demonstration of the major prognostic impact of the immune contexture,8,28,31-33 together with new capabilities of image assessment software to enumerate cells in the tumor, led the recent international validation of Immunoscore in stage I/II/III CC.29 The prognostic impact of the tumor microenvironment and Immunoscore has been clearly established for precancerous lesions,34 primary tumor,8,9,13,14,18,21,23,29 and metastasis.24-28,35 Beyond these results,9,14,21,24-29,35 the relevance of Immunoscore in patients with stage III disease remained to be established. Herein, we demonstrate the ability of the consensus Immunoscore to accurately stratify high- and low-risk patients with significant differences in clinical outcome. In contrast to the peripheral immunity known to decline in time,36 we found that the local intratumoral adaptive immune reaction is not influenced by patients’ age. The MSI status, commonly used for clinical decisions, depended on Immunoscore, as demonstrated by multivariable stratified Cox model. Similarly, the mutational pattern was not associated with survival.
Apart from its prognostic ability, the Immunoscore acts as a predictive factor of response to chemotherapy. Chemotherapy is recommended for all stage III CC. However, the proportion of patients with stage III CC actually receiving adjuvant chemotherapy is only 62%-64% in France and the United States.3,4 The decision to give chemotherapy or not is based on neither scientific-, nor biomarker-, nor knowledge-based medical assessments, even though recent data support the use of predictive immune biomarkers.37 The risk of death decreases by 10%-15% when fluorouracil monotherapy follows surgical resection, and by 20% when fluoropyrimidine-oxaliplatin combination therapy is used.38-40 In stage III CC, only 20% of patients actually benefit from adjuvant chemotherapy, exposing 80% of patients to unnecessary toxicity. Indeed, 50% of patients with stage III CC would be cured by surgery alone, and even with chemotherapy 30% experience recurrence, which is generally fatal within 2-3 years.3 It has been demonstrated that chemotherapy affects the immune system negatively and positively and that the anticancer activity of chemotherapy was also mediated by the immune response.41-44 The predefined consensus Immunoscore analysis revealed that patients with low-Immunoscore stage III disease do not benefit from chemotherapy, whereas patients with a better preexisting immunity (Int + Hi Immunoscore) do benefit the most from chemotherapy, even in low-risk patients. This would suggest that effective chemotherapy at least partly relies on the presence of high densities of tumor-infiltrating T cells.
None of the few patients with the highest Immunoscore (I4) relapsed, even when untreated with chemotherapy, supporting the hypothesis that they may be spared from chemotherapy. Conversely, patients with low Immunoscore not benefiting from chemotherapy should be proposed to enter into novel clinical trials, or at least to be spared from such long and toxic treatments.
Limitations of the study might be due to the heterogeneity of the patient population in real-life clinical practice with standard-of-care treatments from 13 different countries, with 65% and 16% of the patients having MSI and mutational status, respectively; this nonrandomized approach was aimed at demonstrating the robustness of the consensus Immunoscore across multiple ethnicities and patient-care practices. Both Immunoscore and genetic biomarkers should be investigated in larger prospective studies to determine whether mutations occurring at different disease stages have a differential effect on the intratumoral immune infiltrates and on patients’ prognosis. It will now be important to further validate the Immunoscore in randomized clinical trials (such as N0147, IDEA) for prognostic purpose and prediction of chemotherapy response.45,46 We argue for the importance of revising stratification systems to include the consensus Immunoscore in cancer guidelines (such as National Comprehensive Cancer Network, College of American Pathologists, AJCC/UICC-TNM), as it has been recently done in the fifth edition of the WHO classification of colorectal cancer. In addition, a combination of several biomarkers, including parameters of cytotoxic T-cell response, T-cell exhaustion, tumor mutational burden, and immune suppression, could be considered in future immune-including classification. Nonetheless, herein we demonstrated that Immunoscore provides a new patient stratification method that could help directing the therapeutic strategy in colon cancer.
ACKNOWLEDGMENT
In memoriam of Daniel J. Sargent.
SUPPORT
Supported and led by the Society for Immunotherapy of Cancer (SITC), which provided organizational and financial support, and the steering committee that oversaw the performance of this study and managed potential conflicts of interest. This work was also supported by grants from INSERM, the LabEx Immuno-oncology, the Transcan ERAnet European Project, Association pour la Recherche contre le Cancer (ARC), CARPEM, AP-HP, INCA Translationnel, Italian Association for Cancer Research (AIRC), Japan-AMED (P-DIRECT, P-CREATE) and MEXT (Grants-in-aid for Scientific Research-S), Ministry of Health of the Czech Republic Grant No. AZV CR 15-28188A, Progres Q25-LF1, League against Cancer, Czech Republic, and by support from PathForce and Definiens. Also supported by the European Academy of Tumor Immunology (EATI), La Fondazione Melanoma Onlus, US National Cancer Institute (NCI), Biotherapy Development Association (BDA), Canadian Cancer Immunotherapy Consortium (CCIC), Cancer Immunotherapy Consortium (CIC), Cancer Research Institute (CRI), Association for Cancer Immunotherapy (CIMT), Committee for Tumor Immunology and Bio-therapy (TIBT), European Society for Cancer Immunology and Immunotherapy (ESCII), Italian Network for Tumor Biotherapy (NIBIT), Japanese Association of Cancer Immunology (JACI), Nordic Center for Development of Antitumor Vaccines (NCV-network), Progress in Vaccination Against Cancer (PIVAC), Adoptive engineered T cell Targeting to Activate Cancer Killing (ATTACK), Tumor Vaccine and Cell Therapy Working Group (TVACT), and the Institut National du Cancer, France (INCa).
AUTHOR CONTRIBUTIONS
Conception and design: Francesco M. Marincola, Paolo A. Ascierto, Bernard A. Fox, Franck Pagès, Jérôme Galon
Financial support: Tomonobu Fujita, Yutaka Kawakami, Franck Pagès, Bernard A. Fox, Jérôme Galon
Administrative support: Carol Geppert, Bradly G. Wouters, Daniela Bruni, Anastasia Lanzi, Tomonobu Fujita, Franck Pagès
Provision of study material or patients: Florence Marliot, Tilman T. Rau, Iris D. Nagtegaal, Arndt Hartmann, Carol Geppert, Susanne Merkel, Marc Van den Eynde, Anne Jouret-Mourin, Julia Y. Wang, Michael H. A. Roehrl, Emilia K. Andersson, Eva Zavadova, Lubos Petruzelka, Bohuslav Konopasek, Gerardo Botti, Paolo Delrio, Nacilla Haicheur, Shannon van Lent–van Vliet, Tomonobu Fujita, Shoichi Hazama, Nobuaki Suzuki, Hiroaki Nagano, Kiyotaka Okuno, Toshihiko Torigoe, Noriyuki Sato, Tomohisa Furuhata, Prabhu S. Patel, Hemangini H. Vora, Birva Shah, Jayendrakumar B. Patel, Kruti N. Rajvik, Shashank J. Pandya, Shilin N. Shukla, Yili Wang, Yutaka Kawakami, Franck Pagès
Collection and assembly of data: Bernhard Mlecnik, Carlo Bifulco, Gabriela Bindea, Florence Marliot, Alessandro Lugli, Inti Zlobec, Tilman T. Rau, Martin D. Berger, Iris D. Nagtegaal, Elisa Vink-Börger, Arndt Hartmann, Carol Geppert, Julie Kolwelter, Anne Jouret-Mourin, Susanne Merkel, Robert Grützmann, Marc Van den Eynde, Alex Kartheuser, Daniel Léonard, Christophe Remue, Julia Y. Wang, Prashant Bavi, Michael H. A. Roehrl, Pamela S. Ohashi, Linh T. Nguyen, SeongJun Han, Heather L. MacGregor, Sara Hafezi-Bakhtiari, Bradly G. Wouters, Giuseppe V. Masucci, Emilia K. Andersson, Eva Zavadova, Michal Vocka, Jan Spacek, Lubos Petruzelka, Bohuslav Konopasek, Pavel Dundr, Helena Skalova, Kristyna Nemejcova, Gerardo Botti, Fabiana Tatangelo, Paolo Delrio, Gennaro Ciliberto, Michele Maio, Luigi Laghi, Fabio Grizzi, Tessa Fredriksen, Bénédicte Buttard, Lucie Lafontaine, Carine El Sissy, Nacilla Haicheur, Amos Kirilovsky, Anne Berger, Christine Lagorce, Christopher Paustian, Carmen Ballesteros-Merino, Jeroen Dijkstra, Carlijn van de Water, Shannon van Lent–van Vliet, Nikki Knijn, Ana-Maria Muşină, Dragos-Viorel Scripcariu, Boryana Popivanova, Mingli Xu, Tomonobu Fujita, Shoichi Hazama, Nobuaki Suzuki, Hiroaki Nagano, Kiyotaka Okuno, Toshihiko Torigoe, Noriyuki Sato, Tomohisa Furuhata, Ichiro Takemasa, Kyogo Itoh, Prabhu S. Patel, Hemangini H. Vora, Birva Shah, Jayendrakumar B. Patel, Kruti N. Rajvik, Shashank J. Pandya, Shilin N. Shukla, Yili Wang, Guanjun Zhang, Yutaka Kawakami, Bernard A. Fox, Franck Pagès, Jérôme Galon
Data analysis and interpretation: Bernhard Mlecnik, Gabriela Bindea, J. Jack Lee, Jérôme Galon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bernhard Mlecnik
Patents, Royalties, Other Intellectual Property: INSERM
Carlo Bifulco
Stock and Other Ownership Interests: Roche, PrimeVax
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: 32898 US2 - U.S. Patent Application No. 15/910972 filed March 2, 2018
Uncompensated Relationships: PrimeVax
Alessandro Lugli
Honoraria: Sysmex, 3DHISTECH, Sakura
Consulting or Advisory Role: Amgen
J. Jack Lee
Consulting or Advisory Role: AbbVie
Martin D. Berger
Travel, Accommodations, Expenses: Merck, Astellas Pharma
Arndt Hartmann
Honoraria: BMS, MSD, Roche, AstraZeneca, Boehringer Ingelheim, AbbVie, Janssen-Cilag, Ipsen
Consulting or Advisory Role: Bristol-Myers Squibb, MSD, Roche, Cepheid, Qiagen, Janssen-Cilag, AstraZeneca, Ipsen, NanoString Technologies, Illumina, 3DHISTECH, Diaceutics
Research Funding: Cepheid, BioNTech AG, Roche, Janssen-Cilag, NanoString Technologies, AstraZeneca
Expert Testimony: NanoString Technologies
Carol Geppert
Honoraria: Sysmex Deutschland
Travel, Accommodations, Expenses: Sysmex Deutschland
Marc Van den Eynde
Consulting or Advisory Role: Merck (Inst), Amgen (Inst), SERVIER (Inst), Lilly (Inst)
Research Funding: Merck (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche (Inst), Amgen (Inst)
Julia Y. Wang
Employment: Curandis
Leadership: Curandis
Consulting or Advisory Role: Proscia (I), Trans-Hit (I), UDX (I)
Research Funding: Curandis
Travel, Accommodations, Expenses: Curandis
Michael H. A. Roehrl
Honoraria: Gerson Lehrman Group
Consulting or Advisory Role: Trans-Hit, Proscia, UDX
Pamela S. Ohashi
Consulting or Advisory Role: Symphogen, Providence Therapeutics
Travel, Accommodations, Expenses: Symphogen, Providence Therapeutics
Bradly G. Wouters
Stock and Other Ownership Interests: Northern Biologics
Patents, Royalties, Other Intellectual Property: 2014 to present, founder and shareholder, Northern Biologics
Emilia K. Andersson
Employment: Unilabs, Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: AI-algorithm for distance-based tissue state determination
Travel, Accommodations, Expenses: Unilabs
Fabiana Tatangelo
Travel, Accommodations, Expenses: Ipsen
Michele Maio
Stock and Other Ownership Interests: Theravance
Honoraria: Bristol-Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, AstraZeneca, MSD, Merck, Pierre Fabre, Alfasigma
Patents, Royalties, Other Intellectual Property: DNA hypomethylating agents for cancer therapy
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Roche, MSD, Merck, Amgen, Pierre Fabre, Alfasigma
Luigi Laghi
Patents, Royalties, Other Intellectual Property: I share a European patent for colorectal and pancreatic cancer diagnosis with Humanitas Clinical and Research Centre (Inst)
Bénédicte Buttard
Employment: ImCheck Therapeutics
Research Funding: ImCheck Therapeutics
Travel, Accommodations, Expenses: ImCheck Therapeutics
Daniela Bruni
Employment: Roche (I)
Christine Lagorce
Patents, Royalties, Other Intellectual Property: my husband, F. Pagès, has patents associated with the immune prognostic biomarkers filled by INSERM and licensed to HalioDx company (I)
Christopher Paustian
Employment: UbiVac
Shoichi Hazama
Research Funding: Toyo Kohan, NEC, CYTLIMIC
Ichiro Takemasa
Research Funding: Johnson & Johnson (Inst), Medtronic (Inst), Stryker (Inst)
Kyogo Itoh
Consulting or Advisory Role: Taiho Pharmaceutical
Patents, Royalties, Other Intellectual Property: BrightPath Biotherapeutics
Yutaka Kawakami
Honoraria: Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD, AstraZeneca, Chugai Pharma
Consulting or Advisory Role: Taiho Pharmaceutical
Research Funding: Ono Pharmaceutical, Carna Biosciences
Francesco M. Marincola
Employment: Refuge Biotechnologies
Honoraria: Biomed Central
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol-Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai
Research Funding: Bristol-Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Bernard A. Fox
Employment: UbiVac, UbiVac (I)
Leadership: UbiVac
Stock and Other Ownership Interests: UbiVac, PrimeVax
Honoraria: Janssen Biotech, Nodality
Consulting or Advisory Role: Bayer, MedImmune, Celldex, Definiens, Bristol-Myers Squibb, OncoSec, Macrogenics, Ultivue, AstraZeneca, Turnstone Bio, Incyte
Research Funding: Janssen Biotech, Bristol-Myers Squibb, MedImmune (Inst), Viralytics, Perkin Elmer, Definiens, Macrogenics (Inst), NanoString Technologies, OncoSec, Shimadzu, Merck, Akoya Biosciences
Patents, Royalties, Other Intellectual Property: Patent around the DPV-001 cancer vaccine. This patent is owned by the Providence Health System and licensed to UbiVac
Travel, Accommodations, Expenses: Bristol-Myers Squibb, NanoString Technologies, Definiens
Franck Pagès
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Janssen, Merck, Gilead
Speakers' Bureau: Gilead
Research Funding: HalioDx, Bristol-Meyers Squibb
Patents, Royalties, Other Intellectual Property: Patents associated with the immune prognostic markers filled by INSERM
Travel, Accommodations, Expenses: HalioDX
Jérôme Galon
Stock and Other Ownership Interests: HalioDx
Consulting or Advisory Role: BMS, Roche, GSK, Gilead, Sanofi, BMS, Northwest Biotherapeutics, Amgen, Gilead, CatalYm, IO Biotech
Research Funding: Perkin-Elmer, MedImmune, AstraZeneca, Janssen, ImCheck Therapeutics
Patents, Royalties, Other Intellectual Property: Patent holder: INSERM
No other potential conflicts of interest were reported.
REFERENCES
- 1.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auclin E, Zaanan A, Vernerey D, et al. Subgroups and prognostication in stage III colon cancer: Future perspectives for adjuvant therapy. Ann Oncol. 2017;28:958–968. doi: 10.1093/annonc/mdx030. [DOI] [PubMed] [Google Scholar]
- 4.Upadhyay S, Dahal S, Bhatt VR, et al. Chemotherapy use in stage III colon cancer: A National Cancer Database analysis. Ther Adv Med Oncol. 2015;7:244–251. doi: 10.1177/1758834015587867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grothey A, Venook AP. Optimizing adjuvant therapy for localized colon cancer and treatment selection in advanced colorectal cancer. J Natl Compr Canc Netw. 2018;16:611–615. doi: 10.6004/jnccn.2018.0038. [DOI] [PubMed] [Google Scholar]
- 6. Shi Q, Sombrero AF, Shields AF, et al: Prospective pooled analysis of six phase III trials investigating duration of adjuvant oxaliplatin-based therapy (3 vs 6 months) for patients with stage III colon cancer. J Clin Oncol 35, 2017 (suppl 18, abstr LBA1) [Google Scholar]
- 7.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Angell HK, Bedognetti D, et al. The continuum of cancer immunosurveillance: Prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Koelzer VH, Dawson H, Andersson E, et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res. 2015;166:207–217. doi: 10.1016/j.trsl.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee WS, Park S, Lee WY, et al. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116:5188–5199. doi: 10.1002/cncr.25293. [DOI] [PubMed] [Google Scholar]
- 13.Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 15.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mlecnik B, Bindea G, Angell HK, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med. 2014;6:228ra37. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- 21.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 22.Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–603. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 23.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Angelova M, Mlecnik B, Vasaturo A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765.e16. doi: 10.1016/j.cell.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. OncoImmunology. 2015;5:e1057388. doi: 10.1080/2162402X.2015.1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 27.Mlecnik B, Van den Eynde M, Bindea G, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110:438. doi: 10.1093/jnci/djx123. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1016/j.ccell.2018.11.003. Van den Eynde M, Mlecnik B, Bindea G, et al: The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 34:1012-1026.e3, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 30.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–2385. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angell HK, Bruni D, Barrett JC, et al. The Immunoscore: Colon cancer and beyond. Clin Cancer Res. 2020;26:332–339. doi: 10.1158/1078-0432.CCR-18-1851. [DOI] [PubMed] [Google Scholar]
- 32.Galon J, Bruni D. Tumor immunology and tumor evolution: Intertwined histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 34.Mascaux C, Angelova M, Vasaturo A, et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571:570–575. doi: 10.1038/s41586-019-1330-0. [DOI] [PubMed] [Google Scholar]
- 35.Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 36.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malesci A, Bianchi P, Celesti G, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. OncoImmunology. 2017;6:e1342918. doi: 10.1080/2162402X.2017.1342918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 39.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 40.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheema AR, Hersh EM. Patient survival after chemotherapy and its relationship to in vitro lymphocyte blastogenesis. Cancer. 1971;28:851–855. doi: 10.1002/1097-0142(1971)28:4<851::aid-cncr2820280408>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Emens LA, Machiels JP, Reilly RT, et al. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- 43.Mathé G. Chemotherapy, a double agent in respect of immune functions. Cancer Chemother Pharmacol. 1978;1:65–68. doi: 10.1007/BF00254037. [DOI] [PubMed] [Google Scholar]
- 44.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: Chemotherapy with immunogenic cell death inducers. OncoImmunology. 2014;3:e27878. doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benson AB, III, Hamilton SR. Path toward prognostication and prediction: An evolving matrix. J Clin Oncol. 2011;29:4599–4601. doi: 10.1200/JCO.2011.37.8646. [DOI] [PubMed] [Google Scholar]
- 46.Emens LA. It’s TIME for a biomarker-driven approach to cancer immunotherapy. J Immunother Cancer. 2016;4:43. doi: 10.1186/s40425-016-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]