PURPOSE
Brigatinib, a next-generation anaplastic lymphoma kinase (ALK) inhibitor, demonstrated superior progression-free survival (PFS) and improved health-related quality of life (QoL) versus crizotinib in advanced ALK inhibitor–naive ALK-positive non–small cell lung cancer (NSCLC) at first interim analysis (99 events; median brigatinib follow-up, 11.0 months) in the open-label, phase III ALTA-1L trial (ClinicalTrials.gov identifier: NCT02737501). We report results of the second prespecified interim analysis (150 events).
METHODS
Patients with ALK inhibitor–naive advanced ALK-positive NSCLC were randomly assigned 1:1 to brigatinib 180 mg once daily (7-day lead-in at 90 mg once daily) or crizotinib 250 mg twice daily. The primary end point was PFS as assessed by blinded independent review committee (BIRC). Investigator-assessed efficacy, blood samples for pharmacokinetic assessments, and patient-reported outcomes were also collected.
RESULTS
Two hundred seventy-five patients were randomly assigned (brigatinib, n = 137; crizotinib, n = 138). With median follow-up of 24.9 months for brigatinib (150 PFS events), brigatinib showed consistent superiority in BIRC-assessed PFS versus crizotinib (hazard ratio [HR], 0.49 [95% CI, 0.35 to 0.68]; log-rank P < .0001; median, 24.0 v 11.0 months). Investigator-assessed PFS HR was 0.43 (95% CI, 0.31 to 0.61; median, 29.4 v 9.2 months). No new safety concerns emerged. Brigatinib delayed median time to worsening of global health status/QoL scores compared with crizotinib (HR, 0.70 [95% CI, 0.49 to 1.00]; log-rank P = .049). Brigatinib daily area under the plasma concentration–time curve was not a predictor of PFS (HR, 1.005 [95% CI, 0.98 to 1.031]; P = .69).
CONCLUSION
Brigatinib represents a once-daily ALK inhibitor with superior efficacy, tolerability, and QoL over crizotinib, making it a promising first-line treatment of ALK-positive NSCLC.
INTRODUCTION
Oncogenic rearrangements in the anaplastic lymphoma kinase gene (ALK) drive 3%-5% of non–small cell lung cancer (NSCLC).1-3 Patients treated with first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib experience disease progression either in the CNS, likely owing to poor drug penetration,4-6 or, predominantly extracranially, through emergence of secondary ALK mutations or secondary signaling pathways.7-10
CONTEXT
Key Objective
This study reports the results of the second interim analysis (150 events) of the phase III ALTA-1L study of first-line treatment with brigatinib versus crizotinib in patients with anaplastic lymphoma kinase–positive (ALK+) non–small cell lung cancer (NSCLC).
Knowledge Generated
Brigatinib has superior overall and intracranial efficacy compared with crizotinib in patients with ALK tyrosine kinase inhibitor–naive ALK+ NSCLC. Brigatinib is well tolerated with long-term use, and no new safety concerns were identified.
Relevance
An additional 14 months of follow-up relative to the first interim analysis better contextualizes the role of brigatinib in the treatment of ALK+ NSCLC compared with other next-generation ALK inhibitors and provides further support for brigatinib as a first-line treatment option for patients with ALK+ NSCLC.
Brigatinib (ARIAD Pharmaceuticals, Cambridge, MA) is a next-generation ALK inhibitor with broad activity against ALK resistance mutations.11-13 In crizotinib-refractory patients, brigatinib demonstrated high systemic and CNS response rates and median progression-free survival (PFS) of 16.3 and 16.7 months, respectively, in phase I/II and II trials.14-16
The phase III ALK in Lung Cancer Trial of brigAtinib in 1st Line (ALTA-1L) compared brigatinib versus crizotinib in patients with ALK-positive NSCLC not previously treated with an ALK TKI.17 The primary end point was met in the first prespecified interim analysis (performed after 50% [99/198] of expected PFS events; median follow-up: brigatinib, 11.0 months; crizotinib, 9.3 months), with brigatinib demonstrating superior PFS, as assessed by a blinded independent review committee (BIRC; hazard ratio [HR], 0.49; P < .001; 12-month event-free rate: 67%, brigatinib; 43%, crizotinib).17
Improved health-related quality of life (HRQoL) outcomes have been reported with ALK inhibitors (eg, crizotinib, alectinib, ceritinib) compared with chemotherapy in first- and second-line settings.18-21 However, the only previous head-to-head trial assessing global HRQoL between ALK inhibitors (alectinib v crizotinib in treatment-naive NSCLC) showed no statistically significant differences.22 In contrast, at the first ALTA-1L interim analysis, patients treated with brigatinib reported greater improvements from baseline in scores for global health status (GHS)/quality of life (QoL), function (e.g., physical, emotional, cognitive), and symptoms (e.g., fatigue, nausea/vomiting, appetite loss, constipation) than those treated with crizotinib (P < .05).23
This report provides updated efficacy, safety, exposure-PFS relationships, and QoL results from the second ALTA-1L prespecified interim analysis, conducted after 150 (75% of the expected 198) PFS events occurred.
METHODS
Study Design and Patients
ALTA-1L is a phase III, open-label, randomized study (ClinicalTrials.gov identifier: NCT02737501) conducted at 124 centers in 20 countries. Detailed methods have been published.17 Briefly, enrolled patients were adults with locally advanced/metastatic NSCLC and ≥ 1 measurable lesion per RECIST version 1.1 who had not received prior ALK-targeted therapy (Data Supplement). Asymptomatic or stable CNS metastases (defined as neurologically stable, without increasing doses of corticosteroids or anticonvulsant use for 7 days before randomization) were permitted. Patients were stratified by presence/absence of brain metastases and completion of ≥ 1 cycle of chemotherapy for locally advanced/metastatic disease (yes/no) and then randomly assigned (1:1) to brigatinib 180 mg once daily (with 7-day lead-in at 90 mg once daily) or crizotinib 250 mg twice daily. Patients continued treatment until progression, intolerable toxicity, or another discontinuation criterion. Dose-reduction criteria were protocol mandated, as previously described.17 Crossover from crizotinib to brigatinib was offered after BIRC-assessed progression (following ≥ 10-day washout from crizotinib).24
All patients provided written informed consent. Protocol and consent documents were approved by local institutional review boards or ethics committees. The trial was conducted in accordance with the ethical standards of the Declaration of Helsinki and International Council for Harmonization guidelines for good clinical practice.
Assessments
Chest and abdomen (computed tomography or magnetic resonance imaging [MRI] with contrast) and brain (MRI with contrast) imaging was performed at screening, every 8 weeks through cycle 14 (28 d/cycle), and then every 12 weeks through treatment discontinuation. Two BIRCs performed disease assessments: one evaluated all disease on the basis of RECIST version 1.1,25 and one evaluated intracranial CNS disease. Confirmation of response occurred ≥ 4 weeks after initial response. Adverse events (AEs) were categorized according to National Cancer Institute Common Terminology Criteria for AEs, version 4.03.
Patients completed the validated European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire (QLQ)-C30 (version 3.0)26 and its lung cancer–specific module (QLQ-LC13 version 3.0)27 at baseline, day 1 of every 4-week cycle until end of treatment, end of treatment, and 30 days after last dose.
Pharmacokinetic Analysis
Blood for brigatinib levels was collected predose on day 1 of cycles 1-5; postdose on cycle 2, day 1 at 1, 4, and 6-8 hours; and on day 1 of cycles 3-5 any time between 1-8 hours postdose. Individual pharmacokinetic (PK) parameters were derived for each patient using a population PK model.28 The daily area under the plasma concentration–time curve (AUC) was calculated until PFS event or censoring. The exposure-PFS relationship was evaluated by a time-dependent Cox proportional hazard model relating brigatinib exposure (AUC) to PFS: λ(t) = λ0 (t) e β1×AUCDt+βTXi, where λ0(t) is the baseline hazard function at time t, AUCDt is the daily AUC up to time t, and Xi is a vector of predictor variables.
Outcomes
The primary end point was BIRC-assessed PFS. Secondary end points included BIRC-assessed confirmed objective response rate (ORR), confirmed intracranial ORR, intracranial PFS, overall survival (OS), duration of response, safety, and change from baseline in GHS/QoL (per EORTC QLQ-C30). Exploratory end points included BIRC-assessed PFS and confirmed ORR on brigatinib in patients who crossed over after BIRC-confirmed disease progression on crizotinib, and relationship between PFS and AUC. Investigator assessments of PFS were also analyzed.
Statistical Analysis
Sample size calculations assuming median PFS of 10 months for crizotinib29 estimated 198 events (progression or death) among approximately 270 randomly assigned patients would achieve approximately 90% power to detect a 6-month improvement in PFS (HR, 0.625) at final primary end point analysis. An O’Brien-Fleming Lan-DeMets30 α spending function was used to control the overall 2-sided α level at .05. Prespecified interim analyses were planned after 99 and 149 events had occurred. The primary end point (PFS) was planned to be tested at a 2-sided α level of .0031 for the first and .0183 for the second interim analyses. As the primary end point met the preplanned α level at first interim analysis, there was no inferential test for it for the second one.
Efficacy was evaluated in the intention-to-treat (ITT) population. The primary end point was compared between arms using a 2-sided stratified log-rank test. Time-to-event efficacy analyses estimated median values and 2-sided 95% CIs using Kaplan-Meier methods. To adjust for potential time-dependent confounding effects of crossover after patients discontinue crizotinib, an additional OS sensitivity analysis was conducted using marginal structural models (MSMs).31,32
Time to worsening (≥ 10-point decrease from baseline EORTC QLQ-C30 GHS/QoL score) and duration of improvement (from first ≥ 10-point improvement from baseline score to first ≥ 10-point deterioration from baseline) in all randomly assigned patients with baseline and any postbaseline assessment were compared between groups using a 2-sided stratified log-rank test. HRs and 95% CIs were estimated using a Cox proportional hazard model with baseline brain metastases and prior chemotherapy as covariates.
The safety population included patients who received ≥ 1 dose of study drug. Statistical analyses were performed using Base 9.4 SAS/STAT 13.1 software (SAS Institute, Cary, NC). Data are reported as of the June 28, 2019 data cutoff date.
Data Sharing
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
RESULTS
Patients
Between April 2016 and August 2017, 275 patients were enrolled and randomly assigned to brigatinib (n = 137) or crizotinib (n = 138). Baseline factors, including age, sex, ECOG performance status, presence/absence of brain metastases, prior radiotherapy/chemotherapy, and best response to chemotherapy, were balanced between arms.17 As of June 28, 2019, 75 patients (55%) in the brigatinib arm and 23 (17%) in the crizotinib arm remained on study treatment (Fig 1), with median (range) follow-ups of 24.9 (0-34.1) and 15.2 (0.1-36.0) months, respectively. Median (range) duration of treatment was 24.3 (0.1-34.6) months with brigatinib and 8.4 (0.1-36.0) months with crizotinib.
FIG 1.
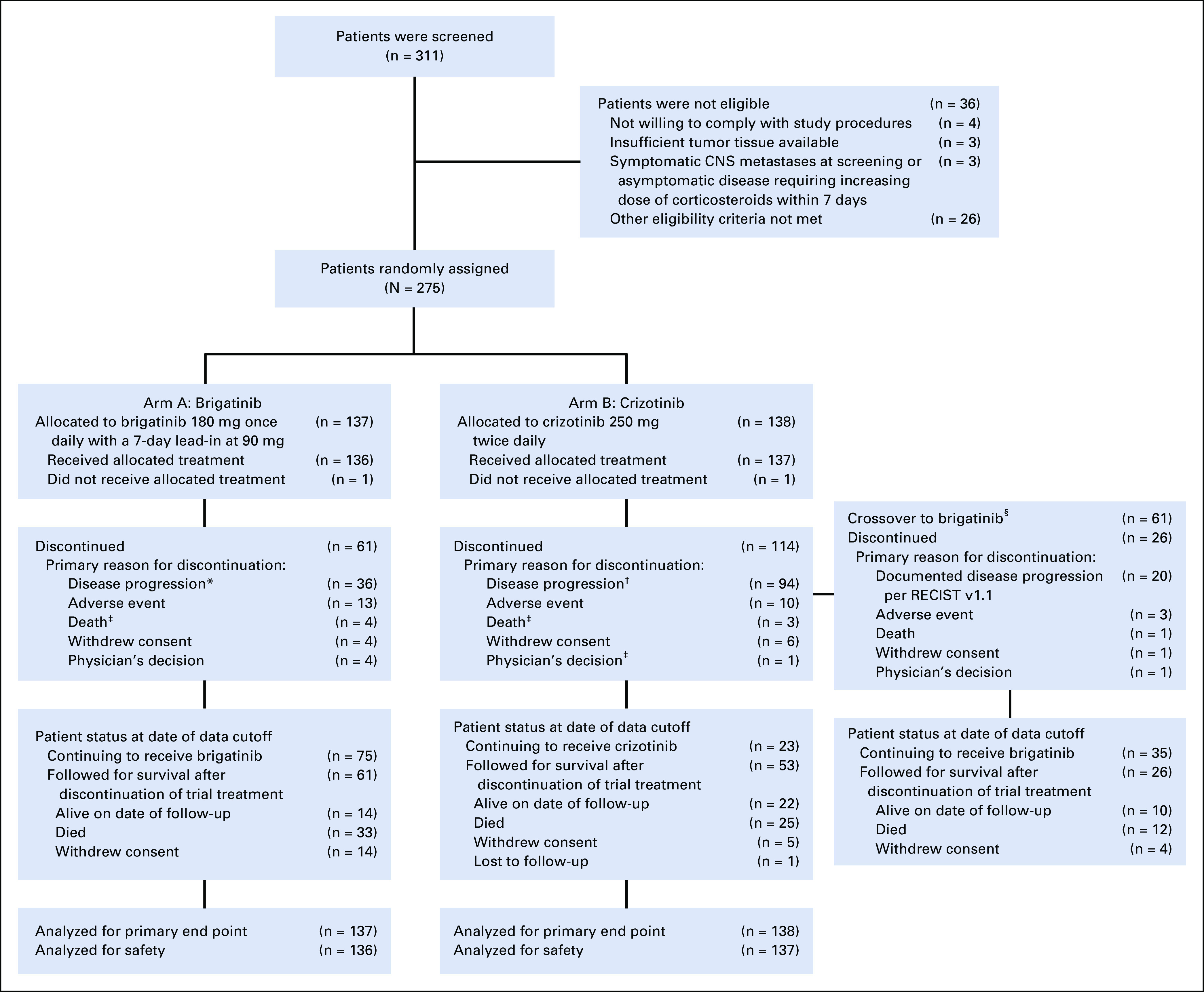
CONSORT diagram for the ALTA-1L trial. Data reported as of the cutoff date for the second interim analysis (June 28, 2019) are shown. Two patients (1 in each treatment arm) never received study treatment but are included in the intention-to-treat analyses. (*) Thirty-one patients had documented disease progression per RECIST v1.1; 5 had clinical disease progression. (†) Ninety patients had documented disease progression per RECIST v1.1; 4 had clinical disease progression. (‡) Minor differences in patient disposition from that reported in the first interim analysis17 are due to reclassification of reasons for discontinuation during data cleaning for the second interim analysis. (§) Crossover from crizotinib to brigatinib was permitted after objective progression was assessed by the blinded independent review committee. Patients who discontinued crizotinib for other reasons (eg, progression per investigator assessments) and then initiated brigatinib are not included in the number of crossover patients. CONSORT, Consolidated Standards of Reporting Trials.
Efficacy
PFS.
At data cutoff, 150 PFS events had occurred in the ITT population (brigatinib, 63 of 137 patients [46%]; crizotinib, 87 of 138 patients [63%]). Brigatinib demonstrated superior BIRC-assessed PFS versus crizotinib, with 2-year probability (95% CI) of no progression of 48% (39% to 57%) in the brigatinib arm and 26% (18% to 35%) in the crizotinib arm (HR, 0.49 [95% CI, 0.35 to 0.68]; log-rank P < .0001; Fig 2A). Investigator-assessed PFS demonstrated improvements with brigatinib (2-year PFS probability [95% CI], 56% [46% to 64%]) v crizotinib (24% [16% to 32%]; HR, 0.43 [95% CI, 0.31 to 0.61]; log-rank P < .0001; Fig 2B). BIRC-assessed PFS improvements were consistent across subgroups (Fig 2C). Cox regression analysis on the basis of dynamic daily AUC demonstrated no effect of brigatinib exposure on BIRC-assessed PFS (regression coefficient [SE], 0.005 [0.013]; HR, 1.005 [95% CI, 0.98 to 1.031]; P = .69).
FIG 2.
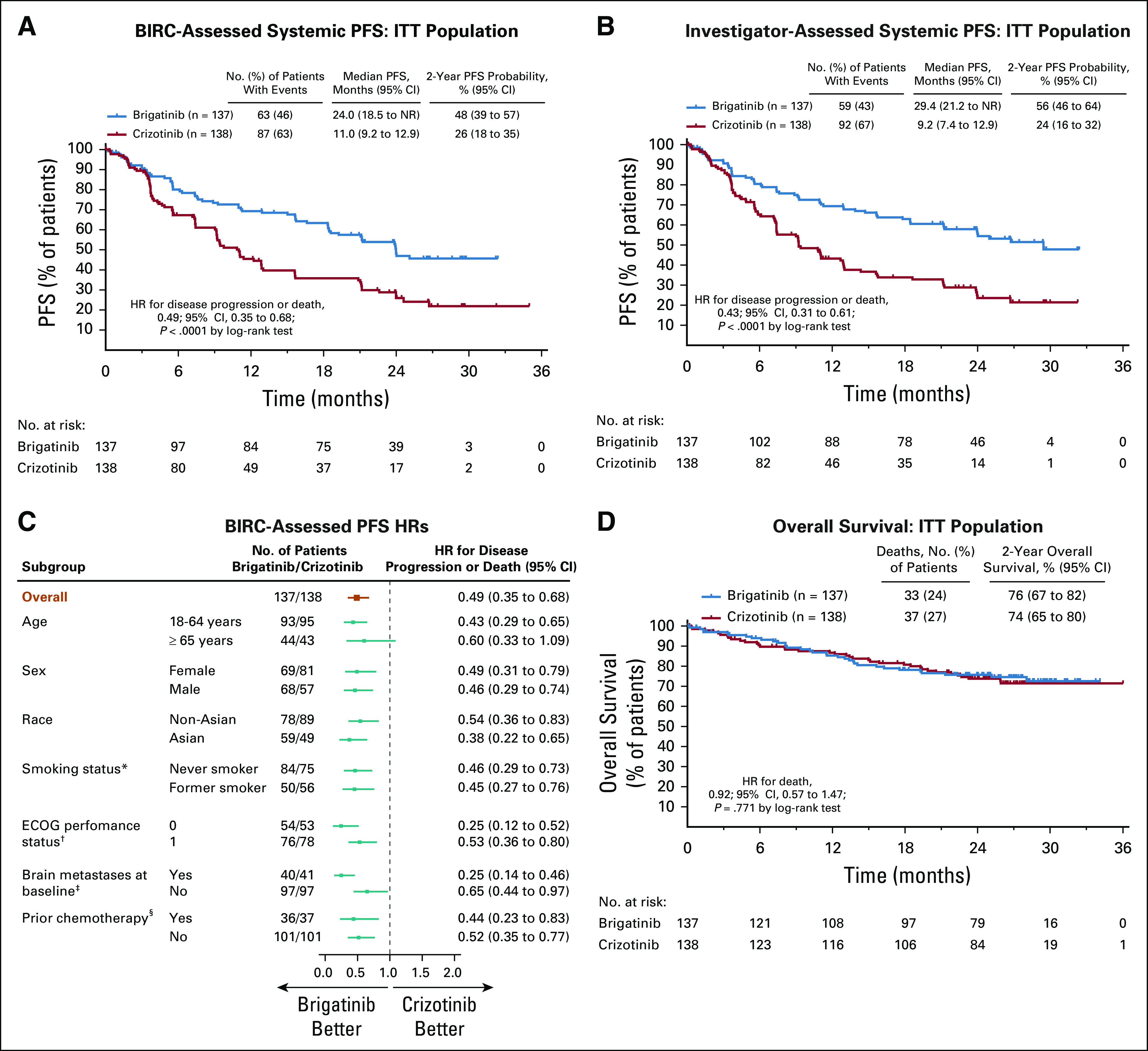
Efficacy of brigatinib and crizotinib in tyrosine kinase inhibitor (TKI)–naive anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer. Kaplan-Meier–estimated (A) blinded independent review committee (BIRC)–assessed progression-free survival (PFS), and (B) investigator-assessed PFS for the intention-to-treat (ITT) population. (C) Hazard ratios (HRs) for BIRC-assessed PFS across predefined patient subgroups. (D) Overall survival in the ITT population. (*) HR was not calculated for patients who were current smokers because of insufficient patient numbers as dictated by the Statistical Analysis Plan (brigatinib, n = 3; crizotinib, n = 7). (†) HR was not calculated for patients who had ECOG performance status of 2 because of insufficient patient numbers as dictated by the Statistical Analysis Plan (brigatinib, n = 7; crizotinib, n = 7). (‡) Brain metastases at baseline as assessed by the investigator. (§) Prior chemotherapy in a locally advanced or metastatic setting. ECOG, Eastern Cooperative Oncology Group; NR, not reached.
Response rate and durability of response.
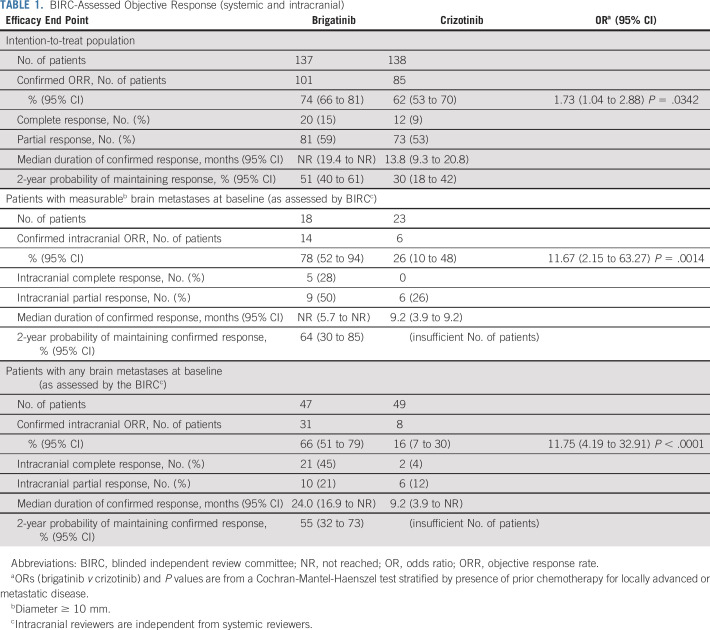
The BIRC-assessed confirmed ORR (95% CI) was 74% (66% to 81%) with brigatinib and 62% (53% to 70%) with crizotinib (Table 1). Median duration of response in confirmed responders (95% CI) was not reached (19.4 months to not reached) with brigatinib and 13.8 (9.3 to 20.8) months with crizotinib.
TABLE 1.
BIRC-Assessed Objective Response (systemic and intracranial)
Overall survival.
As of the data cutoff date, 70 patients had died (brigatinib, 33 [24%]; crizotinib, 37 [27%]). The OS probability at 2 years (95% CI) was 76% (67% to 82%) with brigatinib and 74% (65% to 80%) with crizotinib (HR, 0.92 [95% CI, 0.57 to 1.47]; log-rank P = .771; Fig 2D).
Efficacy in patients with and without baseline brain metastases.
Among 81 patients with baseline brain metastases per medical history (brigatinib, n = 40; crizotinib, n = 41), BIRC-assessed nonprogression probability at 2 years (95% CI) was 43% (25% to 59%) with brigatinib and 10% (2% to 25%) with crizotinib (HR, 0.25 [95% CI, 0.14 to 0.46]; log-rank P < .0001; Fig 3A). Among patients without baseline brain metastases (brigatinib, n = 97; crizotinib, n = 97), PFS was less mature by percentage with events but still favored brigatinib (HR, 0.65 [95% CI, 0.44 to 0.97]; log-rank P = .030; Fig 3B). Investigator assessments were consistent with BIRC assessments (Figs 3C and 3D).
FIG 3.
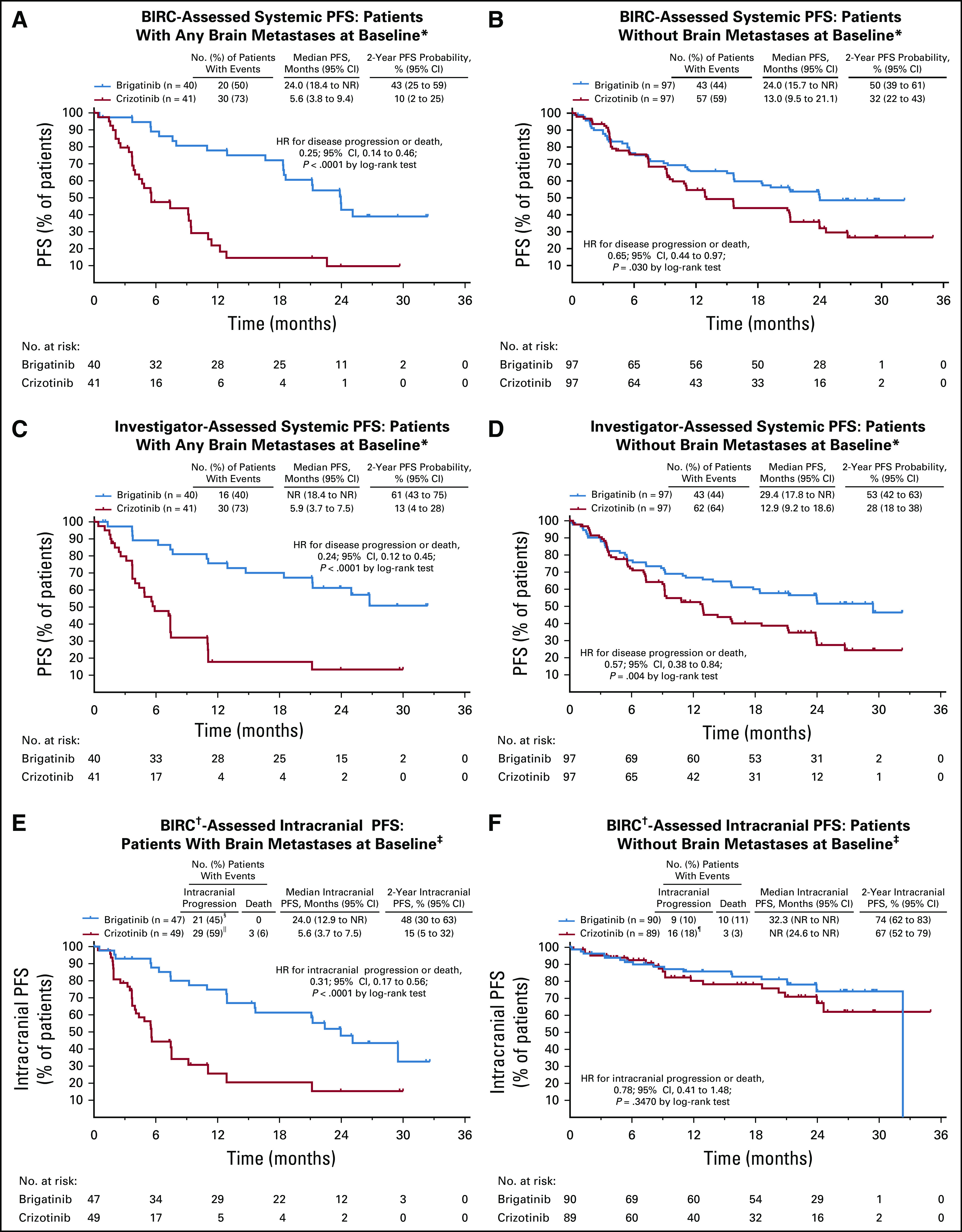
Efficacy of brigatinib and crizotinib in patients with and without baseline brain metastases: Kaplan-Meier–estimated blinded independent review committee (BIRC)–assessed progression-free survival (PFS) in patients (A) with, and (B) without brain metastases at baseline and investigator-assessed PFS in patients (C) with, and (D) without brain metastases at baseline. Kaplan-Meier estimated intracranial PFS by BIRC in patients (E) with, and (F) without any brain metastases at baseline. HR, hazard ratio; NR, not reached. (*) Per investigator assessment. (†) Intracranial reviewers were independent from systemic reviewers. Only brain lesions were reviewed. Patients were counted as having an event if there was radiologic progression, radiotherapy to the brain, or death. (‡) Per BIRC assessment. (§) Includes 1 patient with radiotherapy to the brain. (||) Includes 2 patients with radiotherapy to the brain. (¶) Includes 3 patients with radiotherapy to the brain.
BIRC assessment identified 96 patients with baseline brain metastases, of whom 41 had measurable (diameter ≥ 10 mm) lesions. In patients with measurable brain metastases, confirmed intracranial ORR was 78% (14 of 18 patients; 95% CI, 52% to 94%) with brigatinib and 26% (6 of 23 patients; 95% CI, 10% to 48%) with crizotinib (Table 1). Intracranial PFS rate at 2 years by BIRC assessment in patients with baseline brain metastases was 48% (95% CI, 30% to 63%) with brigatinib and 15% (5% to 32%) with crizotinib (Fig 3E), and in patients without baseline brain metastases it was 74% (62% to 83%) and 67% (52% to 79%), respectively (Fig 3F).
Crossover treatment.
Among 61 patients in the crizotinib arm who crossed over to brigatinib (44% of total crizotinib arm, 65% of those after BIRC progression), median BIRC-assessed PFS was 15.6 (95% CI, 9.4 to not reached) months, with median follow-up of 14.4 (range, 0.2 to 26.5) months. The BIRC-assessed confirmed ORR was 54% (95% CI, 41% to 67%).
OS after adjustment for crossover.
In the MSM sensitivity analysis, OS HR after adjusting for treatment crossover effect was 0.70 (95% CI, 0.39 to 1.26) in favor of brigatinib, suggesting the crossover did affect the ability to detect OS improvement in this trial.
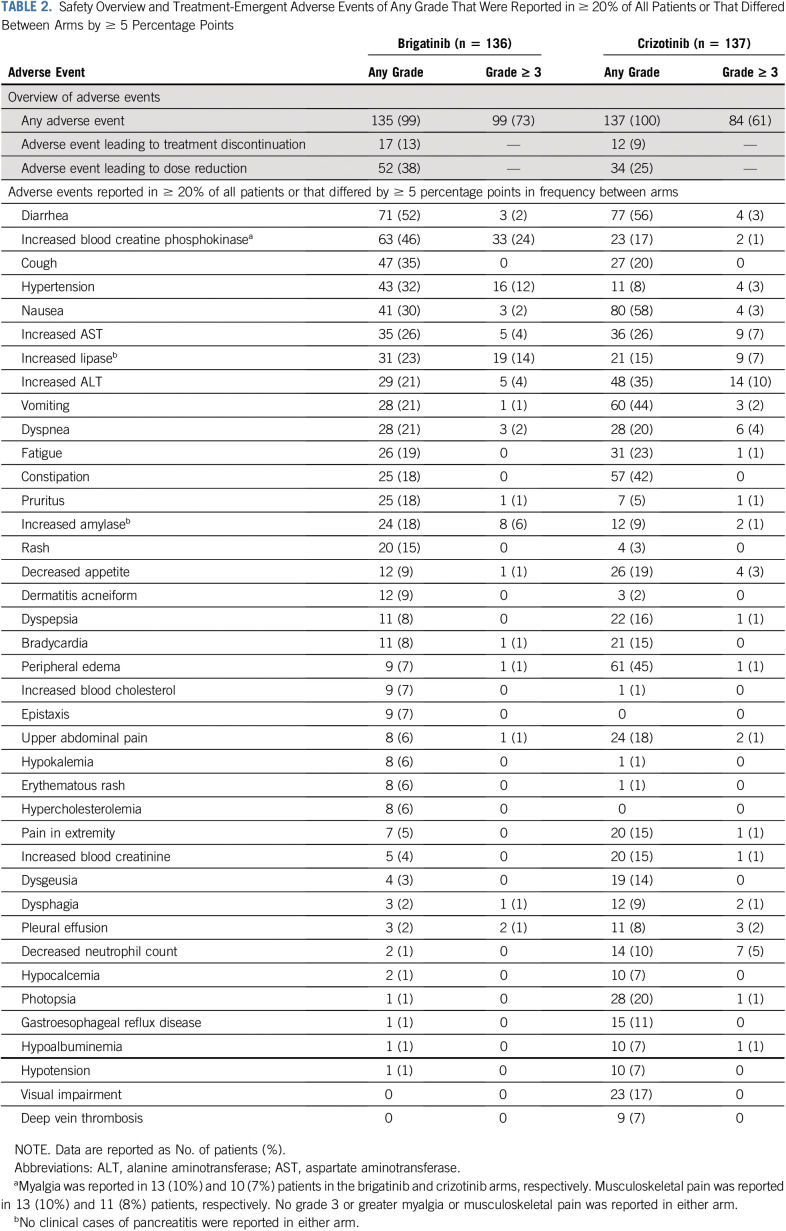
Safety
The most common (> 25% of patients overall) any-grade treatment-emergent AEs (TEAEs) were GI events, increased blood creatine phosphokinase (CPK), cough, and increased aminotransferases (Table 2). Grade 3-5 TEAEs occurred in 73% versus 61% of patients on brigatinib and crizotinib, respectively. No clinically diagnosed pancreatitis or rhabdomyolysis cases were reported. Symptoms possibly related to increased blood CPK (eg, myalgia, muscle pain) did not differ between treatment arms or appear related to grade of increased CPK (Table 2). Twenty patients had AEs leading to death within 30 days of the last brigatinib (9 [7%]) or crizotinib (11 [8%]) dose; none were deemed related to study treatment. Interstitial lung disease (ILD) or pneumonitis at any time occurred in 5% (7 of 136) and 2% (3 of 137) of patients in the brigatinib and crizotinib arms, respectively; grade 3 or 4 ILD/pneumonitis occurred in 3% (4 of 136) and < 1% (1 of 137) of patients. Any-grade early-onset ILD/pneumonitis occurred in 3% (4 of 136) of patients in the brigatinib arm (onset, days 3 to 8), 2% (1 of 61) of patients crossing from crizotinib to brigatinib and no patients in the crizotinib arm.17
TABLE 2.
Safety Overview and Treatment-Emergent Adverse Events of Any Grade That Were Reported in ≥ 20% of All Patients or That Differed Between Arms by ≥ 5 Percentage Points
Dose escalation of brigatinib 90 mg to 180 mg daily occurred as planned in 94% of patients; however, approximately 40% of those who escalated subsequently had dose reduction because of AEs. Dose reduction due to AEs was mandated by investigator or protocol in 38% and 25% of treated patients in the brigatinib and crizotinib arms, respectively. AEs leading to dose reduction in ≥ 1 patient in the brigatinib arm were increased blood CPK (15%), increased lipase (7%), increased amylase (4%), hypertension (2%), increased AST (2%), increased alanine aminotransferase (1%), pneumonitis (1%), and pruritic rash (1%). Thirteen percent of patients treated with brigatinib and 9% treated with crizotinib discontinued due to AEs.
HRQoL
Median time to worsening of GHS/QoL score (95% CI) across all patients was 26.7 (8.3 to not reached) months for brigatinib and 8.3 (5.7 to 13.5) months for crizotinib (HR, 0.70 [95% CI, 0.49 to 1.00]; log-rank P = .049; Fig 4A). In the subset of patients without prior chemotherapy, median time to worsening was not reached (13.9 months to not reached) for brigatinib and 8.3 (6.4 to 18.5) months for crizotinib (HR, 0.68 [0.44 to 1.04]; log-rank P = .0695; Fig 4B).
FIG 4.
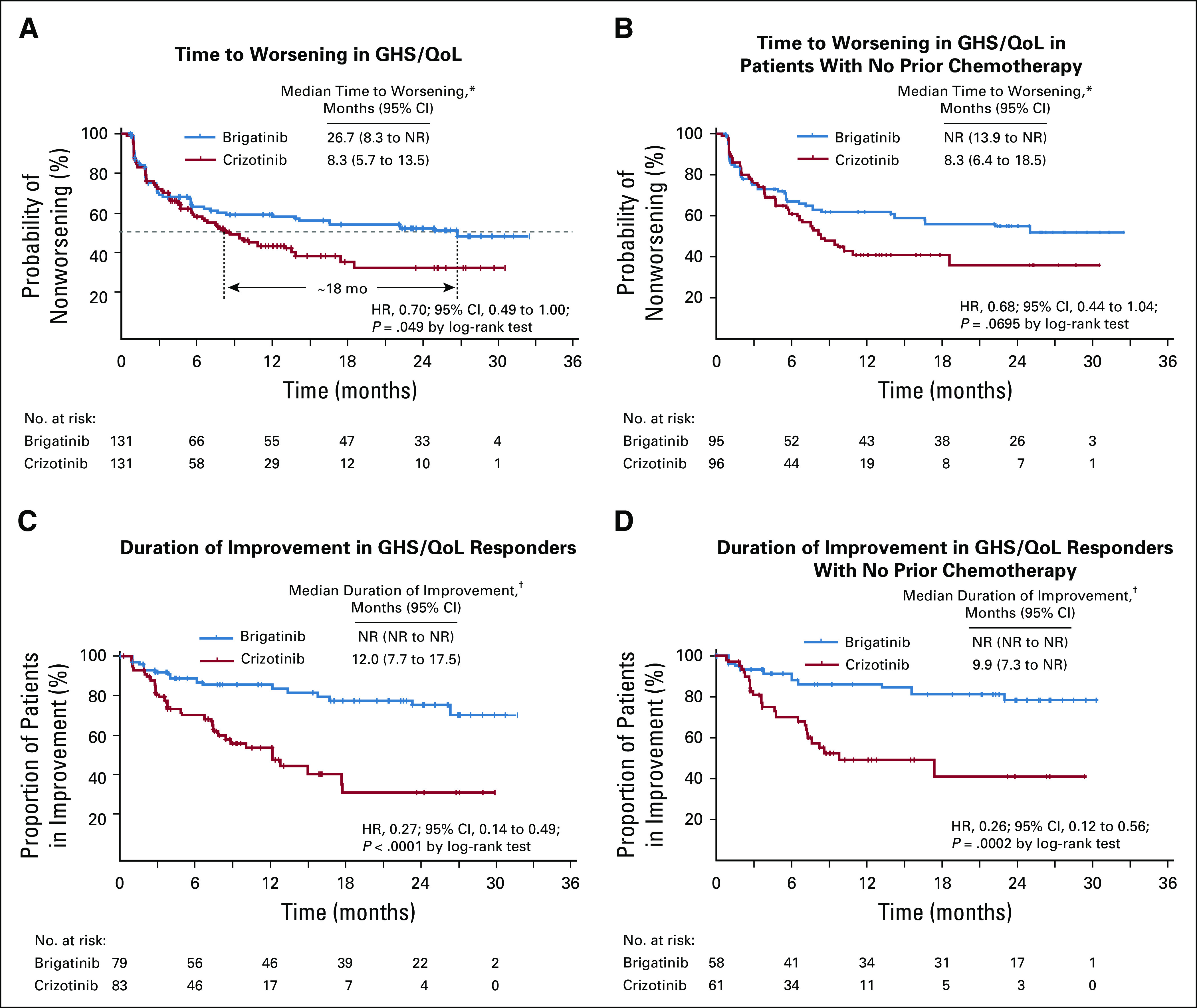
Effect of brigatinib and crizotinib on global health status/quality of life (GHS/QoL): Time to worsening in GHS/QoL score from the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire-C30 (version 3.0) in (A) all patients, and (B) patients without prior chemotherapy. Duration of improvement in GHS/QoL score from the EORTC QoL Questionnaire-C30 (version 3.0) in (C) all patients, and (D) patients without prior chemotherapy. (*) A change of ≥ 10 points was defined as the minimal clinically meaningful deterioration. Time to worsening was defined as time from the date of random assignment to the earliest date at which the patient’s score had a ≥ 10-point deterioration from baseline. (†) A change of ≥ 10 points was defined as the minimal clinically meaningful improvement. Duration of improvement was defined as time from the date of first improvement to the date of first deterioration after the improvement. The first improvement was defined as a ≥ 10-point improvement from baseline.
Among patients with improved GHS/QoL, the median duration of improvement was not reached for brigatinib versus 12.0 (7.7 to 17.5) months for crizotinib (HR, 0.27 [95% CI, 0.14 to 0.49]; log-rank P < .0001; Fig 4C). In patients without prior chemotherapy, median duration of improvement was not reached for brigatinib and 9.9 (7.3 to not reached) months for crizotinib (HR, 0.26 [0.12 to 0.56]; log-rank P = .0002; Fig 4D).
DISCUSSION
Results of this second ALTA-1L interim analysis confirmed that brigatinib has superior overall and intracranial efficacy compared with crizotinib in patients with ALK TKI–naive ALK-positive NSCLC. With median follow-up of approximately 25 months, brigatinib showed consistent BIRC-assessed PFS superiority. Notable durability was observed for delaying both overall and intracranial progression. PFS favored brigatinib over crizotinib across all subgroups, with BIRC-assessed efficacy most prominent in patients with baseline brain metastases and good ECOG performance status. Median PFS data are still maturing among patients without baseline CNS disease in the brigatinib arm (44% with events), consistent with CNS progression occurring later in such patients.33 The overall and especially the intracranial confirmed response rates were higher with brigatinib than crizotinib.
The most common causes of dose reduction due to AEs were related to laboratory changes (eg, increased CPK, lipase, amylase). The rate of dose reductions increased from the first interim analysis (29% brigatinib, 21% crizotinib),17 consistent with some events being related to increased treatment duration. Treatment discontinuation due to AEs was rare. Outside of a clinical trial, asymptomatic changes may not prompt dose reduction. The influence of these protocol-mandated dose reductions on efficacy in the brigatinib arm was considered in an exposure-PFS analysis. Dynamic daily exposure metric was considered the appropriate method for this analysis.34 Results of this analysis indicated that there was no statistically significant relationship between brigatinib daily exposure and risk of disease progression or death, suggesting that the efficacy benefit of brigatinib is consistent across the range of systemic exposures achieved with the brigatinib regimen used in the study.
Brigatinib was associated with delayed time to worsening and prolonged duration of improvement in GHS/QoL score compared with crizotinib. Further analyses showed that brigatinib delayed worsening and prolonged duration of improvement of other functions and symptoms (manuscript in preparation). These differences in global QoL could reflect differences in efficacy on disease-related symptoms and in treatment-related AEs.
Across first-line ALK inhibitor studies, including ALTA-1L, efficacy estimates have been consistently higher by investigator assessments than by independent review committees,17,35 important to consider when comparing data across studies. In ALEX (ClinicalTrials.gov identifier: NCT02075840), which compared alectinib36 with crizotinib in the treatment-naive setting, the IRC-assessed PFS HR was 0.50 (median, 25 months), similar to the BIRC results from ALTA-1L. Investigator-assessed PFS (ALEX primary end point), the only form of assessment made past initial data cut, demonstrated a mature HR of 0.43 and 2-year PFS rate of 57%,37,38 almost the same as in ALTA-1L. The median point estimate by investigators of 34.8 months (95% CI, 17.7 months to not estimable) exceeded that in ALTA-1L, but with broadly overlapping CIs in the 2 trials.35,37 In contrast to the ALTA-1L results, there were no statistically significant differences in patient-centered HRQoL outcomes between alectinib and crizotinib in ALEX.22 There were differences in clinical trial design and patient characteristics between ALEX and ALTA-1L; for example, prior chemotherapy was permitted in ALTA-1L but not ALEX. The impact, if any, of these differences on HRQoL or efficacy is unknown. As outcomes in the crizotinib control arms were similar in ALTA-1L and ALEX, the impact of these differences on efficacy is likely to be low. In the post hoc analysis of patients without prior chemotherapy, mean time to worsening was not reached for brigatinib and was 8.3 months for crizotinib. The duration of HRQoL also favored brigatinib treatment. Thus, brigatinib appears to have similar efficacy to alectinib in the first-line setting but potentially better HRQoL outcomes versus crizotinib.
The lack of OS benefit to date is likely due to the ALTA-1L protocol allowing patients in the crizotinib arm to switch to brigatinib after BIRC-confirmed progression in contrast to ALEX, which did not include crossover. Notably, the 2-year OS of the brigatinib and crizotinib arms in ALTA-1L (76% and 74%, respectively) resembles that of the alectinib arm in ALEX (73%), with both ALTA-1L arms appearing superior to the 2-year OS of the crizotinib arm in ALEX (65%).38 These data raise important issues in relation to the acceptability of noncrossover designs in similar populations in the future. The results from the MSM approach in ALTA-1L also showed that there appeared to be a positive trend for OS favoring brigatinib if treatment crossover had not been allowed.
In conclusion, brigatinib represents a single-tablet dose ALK inhibitor with significantly superior efficacy and tolerability and better QoL than crizotinib, making it a promising first-line treatment option for patients with ALK-positive NSCLC.
ACKNOWLEDGMENT
We thank the patients, their families, and their caregivers, and the study investigators and their team members at each site for participation in the ALTA-1L trial. We thank Teodor G. Paunescu, PhD (Millennium Pharmaceuticals, Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited), for editorial assistance.
PRIOR PRESENTATION
Presented in part at the 5th Congress of the European Society for Medical Oncology Asia, Singapore, November 22-24, 2019; British Thoracic Oncology Group 2020 (encore), Dublin, Ireland, January 29-31, 2020; and Deutscher Krebs Kongress Congress 2020 (encore), Berlin, Germany, February 19-22, 2020.
SUPPORT
Supported by ARIAD Pharmaceuticals, Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. Professional medical writing assistance was provided by Lauren Gallagher, RPh, PhD, and Lela Creutz, PhD, of Peloton Advantage, Parsippany, NJ, an OPEN Health company, and funded by Millennium Pharmaceuticals. Supported by National Health Service funding to the Royal Marsden Hospital/Institute of Cancer Research NIHR Biomedical Research Centre (S.P.).
CLINICAL TRIAL INFORMATION
See accompanying editorial on page 3581
AUTHOR CONTRIBUTIONS
Conception and design: Maximilian J. Hochmair, Scott N. Gettinger, Neeraj Gupta, Sanjay Popat
Financial support: Alexander Spira, Quanhong Ni
Administrative support: Ji-Youn Han, Enriqueta Felip
Provision of study material or patients: Myung-Ju Ahn, James C. H. Yang, Ki Hyeong Lee, Maria Rosario García Campelo, Dong-Wan Kim, Frank Griesinger, Enriqueta Felip, Alexander Spira, Marcello Tiseo, Sanjay Popat
Collection and assembly of data: Hye Ryun Kim, Ji-Youn Han, Maximilian J. Hochmair, Ki Hyeong Lee, Angelo Delmonte, Frank Griesinger, Enriqueta Felip, Alexander Spira, Scott N. Gettinger, Marcello Tiseo, Huamao M. Lin, Neeraj Gupta, Pingkuan Zhang, Sanjay Popat
Data analysis and interpretation: D. Ross Camidge, Hye Ryun Kim, Myung-Ju Ahn, James C. H. Yang, Maximilian J. Hochmair, Ki Hyeong Lee, Maria Rosario García Campelo, Dong-Wan Kim, Frank Griesinger, Enriqueta Felip, Raffaele Califano, Alexander Spira, Scott N. Gettinger, Marcello Tiseo, Huamao M. Lin, Neeraj Gupta, Michael J. Hanley, Quanhong Ni, Pingkuan Zhang, Sanjay Popat
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Brigatinib Versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
D. Ross Camidge
Honoraria: Roche, G1 Therapeutics, Takeda, AstraZeneca, Daiichi Sankyo, Hansoh, Bio-Thera, Blueprint Medicines, Regeneron, Hengrui Pharmaceutical, Ribon Therapeutics, Bristol Myers Squibb, Arrys/Kyn, Inivata, CBT Pharmaceuticals, AbbVie, Achilles Therapeutics, BeyondSpring Pharmaceuticals, Apollomics, Elevation, Archer, EMD Serono, Helsinn Therapeutics, Lilly, Medtronic
Research Funding: Takeda
Hye Ryun Kim
Speakers' Bureau: Ono Pharmaceutical, Roche/Genentech
James C. H. Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Roche/Genentech, Clovis Oncology, Lilly, MSD Oncology, Merck Serono, Celgene, Astellas Pharma, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), AstraZeneca (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Amgen, Takeda Oncology, Amgen, Incyte
Travel, Accommodations, Expenses: Pfizer
Ji-Youn Han
Honoraria: Roche, AstraZeneca, Bristol Myers Squibb, MSD, Takeda
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Takeda, Pfizer
Research Funding: Roche, Pfizer, Ono Pharmaceutical, Takeda
Maximilian J. Hochmair
Honoraria: Boehringer Ingelheim, Takeda, MSD, Bristol Myers Squibb, AstraZeneca, Roche
Ki Hyeong Lee
Honoraria: Bristol Myers Squibb, MSD, AstraZeneca
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca
Maria Rosario García Campelo
Consulting or Advisory Role: Roche/Genentech, MSD Oncology, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, Janssen Oncology
Speakers' Bureau: Roche, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, MSD Oncology
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology, Pfizer
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo, Amgen
Enriqueta Felip
Consulting or Advisory Role: Pfizer, Roche, Boehringer Ingelheim, AstraZeneca, Bristol Myers Squibb, Guardant Health, Novartis, Takeda, AbbVie, Blueprint Medicines, Lilly, Merck, Merck Sharp & Dohme, Springer, GSK, Bayer, Janssen, Medscape, Samsung, Bayer
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Novartis, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Pfizer, Lilly, Takeda, Medscape, Prime Oncology, TouchIME
Research Funding: Fundación Merck Salud (Inst), EMD Serono (Inst)
Other Relationship: Grifols
Raffaele Califano
Stock and Other Ownership Interests: The Christie Private Care
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Speakers' Bureau: AstraZeneca, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Research Funding: Roche (Inst), AstraZeneca (Inst), Pfizer (Inst), Clovis (Inst), Lilly Oncology (Inst), MSD (Inst), BMS (Inst), AbbVie (Inst), Takeda (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche, Lilly Oncology, MSD, Takeda
Alexander Spira
Stock and Other Ownership Interests: Eli Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb
Consulting or Advisory Role: Array BioPharma (Inst), Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst)
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), NewLink Genetics (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), LAM Therapeutics (Inst), Trovagene (Inst), Takeda (Inst), Macrogenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Arch Therapeutics (Inst), Gritstone (Inst), Plexxikon (Inst), Amgen (Inst), Daiichi Sankyo (Inst), ADCT (Inst), Janssen Oncology (Inst), Mirati Therapeutics (Inst)
Scott N. Gettinger
Consulting or Advisory Role: Bristol Myers Squibb, Nektar
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Ariad/Takeda (Inst), Iovance Biotherapeutics (Inst)
Marcello Tiseo
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, MSD, Boehringer Ingelheim, Takeda
Research Funding: AstraZeneca, Boehringer Ingelheim
Huamao M. Lin
Employment: Takeda Pharmaceutical
Stock and Other Ownership Interests: Change Healthcare (I)
Travel, Accommodations, Expenses: Takeda Pharmaceutical
Neeraj Gupta
Employment: Takeda Pharmaceutical
Stock and Other Ownership Interests: Takeda
Michael J. Hanley
Employment: Takeda Pharmaceutical
Quanhong Ni
Employment: Takeda
Stock and Other Ownership Interests: Takeda
Pingkuan Zhang
Employment: Takeda
Stock and Other Ownership Interests: GlaxoSmithKline, Novartis, Amgen
Travel, Accommodations, Expenses: Takeda
Sanjay Popat
Honoraria: Boehringer Ingelheim, AstraZeneca, Roche, Takeda, Chugai Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche, Novartis, Pfizer, AstraZeneca, Bristol Myers Squibb, MSD, Guardant Health, AbbVie, EMD Serono, Takeda, Paredox Therapeutics, Incyte
Research Funding: Boehringer Ingelheim (Inst), Epizyme (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Roche (Inst), Lilly (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Merck Sharp & Dohme, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gainor JF Varghese AM Ou SH, et al. : ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 19:4273-4281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong DW Leung EL So KK, et al. : The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115:1723-1733, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Koivunen JP Mermel C Zejnullahu K, et al. : EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14:4275-4283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa DB Kobayashi S Pandya SS, et al. : CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 29:e443-e445, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Costa DB Shaw AT Ou SHI, et al. : Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 33:1881-1888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang I Zaorsky NG Palmer JD, et al. : Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 16:e510-e521, 2015 [DOI] [PubMed] [Google Scholar]
- 7.McCoach CE Le AT Gowan K, et al. : Resistance mechanisms to targeted therapies in ROS1(+) and ALK(+) non-small cell lung cancer. Clin Cancer Res 24:3334-3347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor JF Dardaei L Yoda S, et al. : Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 6:1118-1133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doebele RC Pilling AB Aisner DL, et al. : Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 18:1472-1482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama R Shaw AT Khan TM, et al. : Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med 4:120ra17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama R Khan TM Benes C, et al. : Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci USA 108:7535-7540, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang WS Liu S Zou D, et al. : Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem 59:4948-4964, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Gettinger SN Bazhenova LA Langer CJ, et al. : Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol 17:1683-1696, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Huber RM Hansen KH Paz-Ares Rodríguez L, et al. : Brigatinib in crizotinib-refractory ALK+ NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J Thorac Oncol 15:404-415, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Camidge DR Kim DW Tiseo M, et al. : Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol 36:2693-2701, 2018 [DOI] [PubMed] [Google Scholar]
- 16. Bazhenova LA, Gettinger SN, Langer CJ, et al: Brigatinib (BRG) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC): Long-term efficacy and safety results from a phase 1/2 trial. Ann Oncol 28:479-480, 2017 (suppl 5; abstr 1344P) [Google Scholar]
- 17.Camidge DR Kim HR Ahn MJ, et al. : Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med 379:2027-2039, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Blackhall F Kim DW Besse B, et al. : Patient-reported outcomes and quality of life in PROFILE 1007: A randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol 9:1625-1633, 2014 [DOI] [PubMed] [Google Scholar]
- 19. Mazieres J, Novello S, De Castro J, et al: P1.01-013. Patient-reported outcomes and safety from the phase III ALUR study of alectinib versus chemotherapy in pre-treated ALK + NSCLC. J Thorac Oncol 12:S1897, 2017 (suppl 2; abstr P.1.01-013) [Google Scholar]
- 20.Shaw AT Kim DW Nakagawa K, et al. : Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368:2385-2394, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Tan DS, Soria J, De Castro G Jr, et al: P3.02a-025. PROs with ceritinib versus chemotherapy in patients with previously untreated ALK-rearranged nonsquamous NSCLC (ASCEND-4). J Thorac Oncol 12:S1176-S1177, 2017 (suppl; abstr P3.02a-025) [Google Scholar]
- 22.Pérol M Pavlakis N Levchenko E, et al. : Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer 138:79-87, 2019 [DOI] [PubMed] [Google Scholar]
- 23. Garcia Campelo R, Lin HM, Perol M, et al: Health-related quality of life (HRQoL) results from ALTA-1L: Phase 3 study of brigatinib vs crizotinib as first-line (1L) ALK therapy in advanced ALK+ non-small cell lung cancer (NSCLC). J Clin Oncol 37 2019 (suppl 15, abstr 9084) [Google Scholar]
- 24.Camidge DR Pabani A Miller RM, et al. : Management strategies for early-onset pulmonary events associated with brigatinib. J Thorac Oncol 14:1547-1555, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Aaronson NK Ahmedzai S Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Bergman B Aaronson NK Ahmedzai S, et al. : The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer 30A:635-642, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Gupta N, Wang X, Prohn M, et al: Population pharmacokinetic (PK) analysis of the ALK inhibitor brigatinib: Model-informed posology decisions and global drug development. Clin Pharmacol Drug Dev 7:61-62, 2018 (abstr 076) [Google Scholar]
- 29.Solomon BJ Mok T Kim DW, et al. : First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167-2177, 2014 [DOI] [PubMed] [Google Scholar]
- 30. doi: 10.1002/sim.4780131308. DeMets DL, Lan KK: Interim analysis: The alpha spending function approach. Stat Med 13:1341-1352, 1994; discussion 1353-1356. [DOI] [PubMed] [Google Scholar]
- 31.Cole SR, Hernán MA: Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168:656-664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fewell Z Hernan MA Wolfe F, et al. : Controlling for time-dependent confounding using marginal structural models. Stata J 4:402-420, 2004 [Google Scholar]
- 33.Gadgeel S Peters S Mok T, et al. : Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 29:2214-2222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau YY Gu W Ho YY, et al. : Application of time-dependent modeling for the exposure-efficacy analysis of ceritinib in untreated ALK-rearranged advanced NSCLC patients. Cancer Chemother Pharmacol 84:501-511, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Peters S Camidge DR Shaw AT, et al. : Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829-838, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Alecensa [package insert]. South San Francisco, CA, Genentech USA, 2018
- 37. doi: 10.1016/j.jtho.2019.03.007. Camidge DR, Dziadziuszko R, Peters S, et al: Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol 14:1233-1243, 2019 [Erratum: J Thorac Oncol 14:2023, 2019] [DOI] [PubMed] [Google Scholar]
- 38. Mok TSK, Shaw AT, Camidge RD, et al: Final PFS, updated OS and safety data from the randomised, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC. Ann Oncol 30:v607, 2019 (abstr 1484PD) [Google Scholar]