Abstract
Objective
To determine time trends and distinguishing autopsy findings of sudden unexpected death in epilepsy (SUDEP) in the United States.
Methods
We identified decedents where epilepsy/seizure was listed as cause/contributor to death or comorbid condition on the death certificate among all decedents who underwent medico-legal investigation at 3 medical examiner (ME) offices across the country: New York City (2009–2016), San Diego County (2008–2016), and Maryland (2000–2016). After reviewing all available reports, deaths classified as definite/probable/near SUDEP or SUDEP plus were included for analysis. Mann-Kendall trend test was used to analyze temporal trends in SUDEP rate for 2009–2016. Definite SUDEPs were compared to sex- and age ±2 years–matched non-SUDEP deaths with a history of epilepsy regarding autopsy findings, circumstances, and comorbidities.
Results
A total of 1,086 SUDEP cases were identified. There was a decreasing trend in ME-investigated SUDEP incidence between 2009 and 2016 (z = −2.2, S = −42, p = 0.028) among 3 regions. There was a 28% reduction in ME-investigated SUDEP incidence from 2009 to 2012 to 2013–2016 (confidence interval, 17%–38%, p < 0.0001). We found no correlation between SUDEP rates and the month of year or day of week. There was no difference between SUDEP and non-SUDEP deaths regarding neurodevelopmental abnormalities, pulmonary congestion/edema, and myocardial fibrosis.
Conclusions
There was a decreasing monotonic trend in ME-investigated SUDEP incidence over 8 years, with a 28% reduction in incidence from 2009–2012 to 2013–2016. Unlike SIDS and sudden cardiac death, we found no correlation between SUDEP and the season of year or day of week. No autopsy findings distinguished SUDEP from non-SUDEP deaths.
Sudden unexpected death in epilepsy (SUDEP) is likely the most common cause of epilepsy-related mortality.1 The incidence of SUDEP is estimated to be 1.2 per 1,000 patient-years in adults with epilepsy. Early studies found the incidence in children was 0.22 per 1,000 patient-years,2,3 but recent studies found a similar incidence as adults with epilepsy.4 In the past decade, multiple efforts sought to educate patients, families, and clinicians about the risks of SUDEP and potential prevention strategies. Contemporaneously, the Affordable Care Act5 improved access to care for many underinsured and uninsured patients in the United States. It is unknown if either of these changes in patient and provider awareness or patient access to epilepsy care has affected SUDEP incidence. In addition, understanding the trends of the SUDEP rate in people with epilepsy (PWE) is important in order to design studies that assess public health campaigns targeting SUDEP risk reduction.3,6
Events in episodic disorders may follow a circaseptan (∼7 days) rhythm. In epilepsy, studies of seizure diaries have suggested that seizures are more likely to occur on Tuesdays and Wednesdays,7 while cardiovascular events are more frequent on Mondays.8 It is unknown if SUDEP follows a weekly or monthly pattern.
This study assessed trends in SUDEP incidence based on medico-legal investigation (MLI) in 3 geographically and demographically diverse regions of the United States: New York City (NYC), the state of Maryland (MD), and San Diego County (SDC) between 2009 and 2016. In addition, since most SUDEPs follow a seizure, we examined if SUDEP has a midweek pattern similar to seizures. Finally, we compared autopsy, toxicology, and clinical characteristics of SUDEP cases compared to age- and sex-matched non-SUDEP cases to examine if any features could reliably help distinguish SUDEP from non-SUDEP deaths.
Methods
Selection of cases
We retrospectively queried all decedents who presented for MLI at 3 medical examiner (ME) offices across the country: NYC (January 1, 2009–December 31, 2016), MD (January 1, 2000–December 31, 2016), and SDC (January 1, 2008–December 31, 2016). Each region has a single authority responsible for all MLIs in their catchment area with MEs with clinical research experience. We identified decedents in whom epilepsy/seizure was listed as cause/contributor to death or comorbid condition on the death certificate. All investigator notes, autopsy reports (when performed), toxicology, and medical records were reviewed independently by 2 epileptologists with experience in forensic adjudication (D.F. and O.D.). Definite SUDEP, definite SUDEP plus, probable SUDEP, probable SUDEP plus, possible SUDEP, and near SUDEP cases were classified using Nashef et al.9 criteria. A third epileptologist (E.J.D.) reviewed the cases where there was a disagreement on the cause of death, and a consensus was reached.
Estimation of epilepsy prevalence and SUDEP rate per region and year
Region-, age-, and sex-adjusted epilepsy population was calculated by using epilepsy prevalence estimates (source: the National Health Interview Survey 2011 and 2015, National Center for Health Statistics, Centers for Disease Control and Prevention)10 and general population (source: United States Census Bureau, 201011 and American Community Survey 2005–201612). Linear interpolation was applied to calculate epilepsy prevalence for the remaining years.
Comparison of SUDEP and non-SUDEP decedents
We compared autopsy findings and comorbid conditions among adult definite SUDEPs (SUDEP group) and patients with epilepsy who did not die from SUDEP (non-SUDEP group). Non-SUDEP cases met the following criteria: (1) ≥18 years of age at death with a history of epilepsy that presented to the same ME offices within the same years; (2) decedents were residents in corresponding regions; (3) the cause of death was not SUDEP; (4) an autopsy was performed with no decomposition; (5) sex- and age ±2 years–matched control for 2 definite SUDEP cases.
Statistical analysis
The demographics, clinical data, and autopsy findings of the decedents were analyzed by SPSS Statistics (IBM, Armonk, NY, version 23). Chi-square and Fisher exact test were used for comparison of autopsy findings and comorbidities, and the Mann-Whitney U test was used for comparison of organ weights among SUDEP and non-SUDEP groups. Holm-Bonferroni correction was applied to adjust p values in comparison of multiple comorbidities between the 2 groups. SUDEP rate trends were calculated both for each region and for all regions combined from 2009 to 2016, using Mann-Kendall and region-adjusted multivariate Mann-Kendall tests. Incidence rate ratio (SUDEP rate ratio in 2013–2016 compared to 2009–2012), Mann-Kendall test, confidence intervals (CIs) of rates, and Holm Bonferroni correction were calculated using fmsb, Kendall, trend, epitools, and stats packages in R (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria). The Rayleigh test can be applied to measure the nonuniformity of circular data including cyclical time series if the data follow a von Mises distribution.13 After using the Stephens modified Watson test14 to determine whether the data followed a von Mises distribution, we performed the Rayleigh test to determine if SUDEP deaths occurred with a uniform distribution across days of the week or months of the year using the circular package in R. In order to be certain regarding the date of death, only the cases where the decedent was last seen alive on the same calendar day were included in the analysis.
The probabilistic bias analysis was performed to account for the bias due to possible incomplete SUDEP case ascertainment.15,16 The expected SUDEP incidence was calculated based on literature where epilepsy was not mentioned on the death certificate on 25%–36% of identified SUDEPs.2,17 To determine the contribution of information bias on observed trends in SUDEP rates, we performed Monte Carlo simulation (1,000,000 iterations) where the true SUDEP count for each ME region and time period was randomly sampled from a uniform distribution of 1.33–1.56 × the ME-investigated SUDEP count at each year between 2009 and 2016. A multivariate Mann-Kendall test was applied for each iteration. Simulations for bias analysis were performed in R.
Standard protocol approvals, registrations, and patient consents
This study was determined to be exempt by the New York University Institutional Review Board because the decedents do not qualify as human subjects. The research protocol was approved by ME offices.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
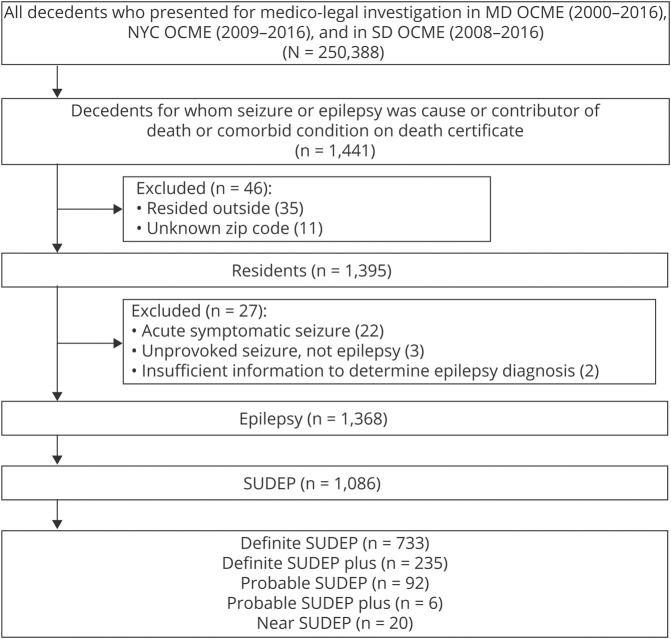
Seizure or epilepsy was listed in the death certificate of 1,441 decedents. Seventy-three cases were excluded from further analysis (34 cases with zip code of residence outside the ME office jurisdiction, 11 cases with unknown zip code of residence, and 27 cases that were determined not to have epilepsy on consensus review). A total of 1,086 out of 1,368 decedents with epilepsy where the cause of death was determined as SUDEP (definite SUDEP, probable SUDEP, or near SUDEP including SUDEP plus) were included in further analysis (figure 1). Among 1,086 SUDEP cases, 487 (44.8%) were black and 686 (63.2%) were male. The median age at death was 39 years (range, 3 months–81 years) (table 1). A total of 83.2% of deaths (904/1,086) were unwitnessed; 77.1% (837/1,086) occurred at home. Almost half of the decedents were found in bed, and 42.4% were in the prone position (table 1).
Figure 1. Flowchart of selection of sudden unexpected death in epilepsy (SUDEP) cases.
MD = Maryland; NYC = New York City; OCME = Office of Chief Medical Examiner; SD = San Diego.
Table 1.
Demographics, circumstances, and medical details of sudden unexpected death in epilepsy cases (n = 1,086)
Temporal trends in SUDEP over 8 years
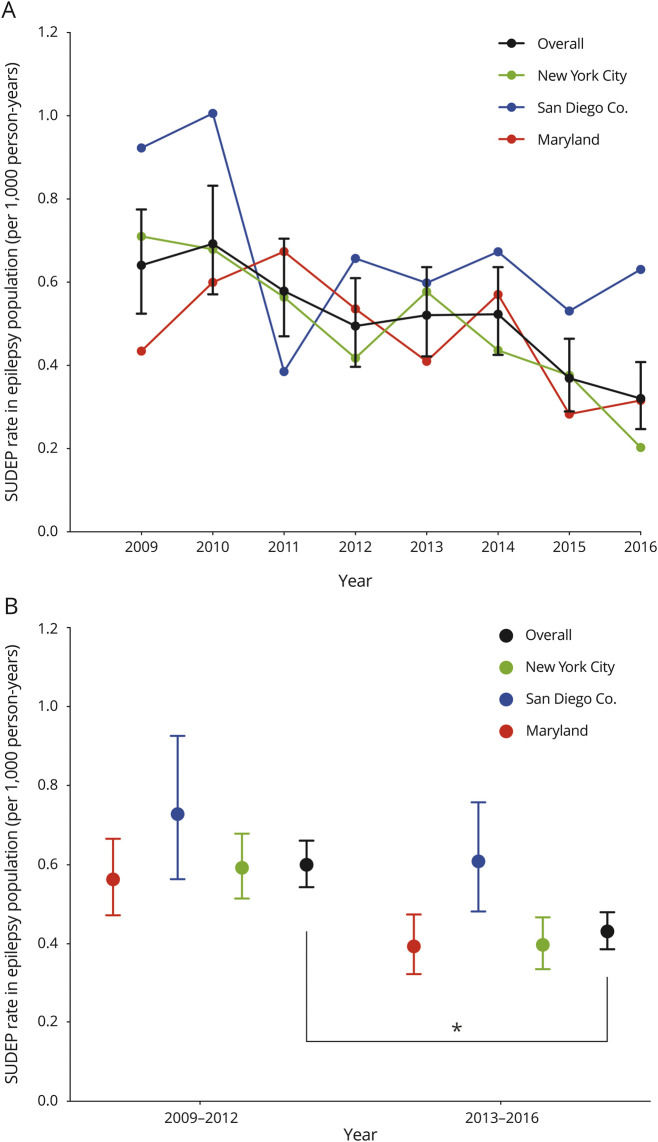
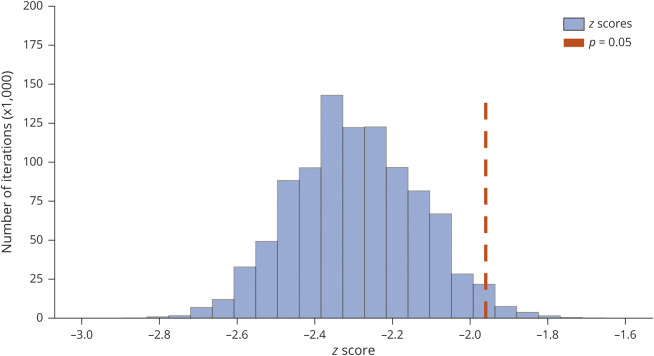
There was a decreasing trend in the ME-investigated SUDEP rate in the epilepsy population (z = −2.2, S = −42, p = 0.028) among 3 regions combined in 2009–2016 (figure 2A). When the 3 ME offices were examined individually, the decreasing trend was significant in NYC (τ = −0.79, S = −22, p = 0.009), but not MD (τ = −0.43, S = −12, p = 0.17) or SDC (τ = −0.29, S = −8, p = 0.39). There was 28% reduction in ME-investigated SUDEP incidence in 2013–2016 compared to 2009–2012 (CI, 17%–38%, p < 0.0001) (figure 2B). The decrease in SUDEP rate from 2009 to 2012 to 2013–2016 was 33% (CI, 17%–46%, p < 0.001) in NYC, 30% (CI, 10%–46%, p = 0.005) in MD, and 17% (CI, −16%–40%, p = 0.28) in SDC. We performed a sensitivity analysis examining all categories of SUDEP to account for potential trends in coexisting conditions that could lead the adjudicators to be less certain about the classification of SUDEP, such as the increasing prevalence of opioids and other drugs of abuse in toxicology reports. The decreasing trends persisted when possible SUDEP cases were included in the analysis (z = −2.2, S = −40, p = 0.025). The incidence of definite/probable SUDEP among children based on MLI was 0.22 per 1,000 patient-years (95% CI, 0.16–0.3). Observed ME-investigated SUDEP rate of 0.74–0.32 per 1,000 patient-years was lower than reported SUDEP incidence of ∼1 per 1,000 patient-years.3 According to probabilistic bias analysis performed to account for possible incomplete case ascertainment, the systematic error rate was 2.1%, and z ranged from −2.6 to −1.96, and S ranged from −48 to −38 in the 95% simulation interval (figure 3).
Figure 2. Temporal trends in medical examiner (ME)–investigated sudden unexpected death in epilepsy (SUDEP) incidence in 3 regions in 2009–2016.
(A) There was a decreasing monotonic trend in ME-investigated SUDEP incidence (z = −2.2, S = −42, p = 0.028) in 3 regions in 2009–2016. (B) There was a 28% reduction in ME-investigated SUDEP incidence in 3 regions in 2013–2016 compared to 2009–2012 (confidence interval, 17%–38%; p < 0.0001).
Figure 3. The distribution of z values in probabilistic bias analysis for the bias due to possible incomplete sudden unexpected death in epilepsy (SUDEP) case ascertainment.
Z ranged from −2.6 to −1.96, and S ranged from −48 to −38 in 95% simulation interval according to probabilistic bias analysis with 1,000,000 simulations. The systematic error rate was 2.1%.
Monthly and daily variations in SUDEP
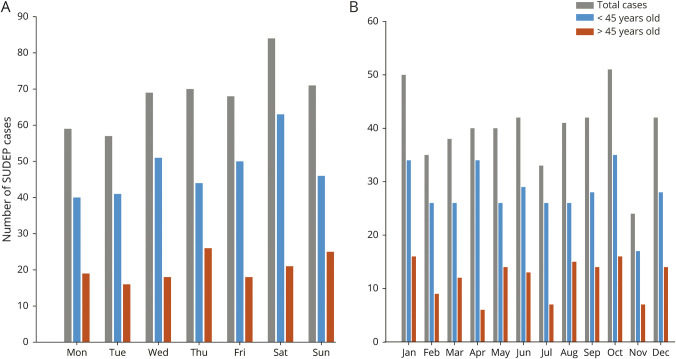
The exact date of death could be determined with certainty for 478 SUDEP cases. The distribution of SUDEP across the days of the week or months of the year followed a von Mises distribution and it did not differ significantly from an expected uniform distribution across days of the week (Rayleigh z = 1.09, p = 0.35) or months of the year (Rayleigh z = 1.33, p = 0.27). No daily or monthly patterns were observed in specific age groups (<45 year old and ≥45 year old) (p > 0.05) (figure 4, A and B). While there was a higher rate of SUDEP on weekends compared to weekdays in our sample (relative risk, 1.2; 95% CI, 0.98–1.46), the difference was not statistically significant.
Figure 4. Plot of the number of sudden unexpected death in epilepsy (SUDEP) cases across the days of the week and the months of the year for the subset of decedents who were last seen alive on the same day (n = 478).
(A) Days of the week. (B) Months of the year. There was no significant monthly or daily pattern in total SUDEPs (gray), younger decedents (<45 years) (blue), and older decedents (>45 years) (orange) (p > 0.05).
Comparison of SUDEP to non-SUDEP deaths
The demographics of the 190 sex- and age ±2 years–matched non-SUDEP cases and 380 definite SUDEPs are summarized in table 2. There was no difference between 2 groups regarding remote neurologic injury including brain tumor, stroke, head trauma, encephalitis/meningitis, developmental delay, and autism (p > 0.05). There was also no difference in the proportion of patients who had undergone epilepsy surgery or vagus nerve stimulation placement (p > 0.05). Depression, anxiety, bipolar disorder, and suicide attempt were significantly more common in the non-SUDEP group compared to the SUDEP group (p = 0.002 to p = 0.01; table 2). Chronic diseases such as hypertension, diabetes, and chronic obstructive pulmonary disease were significantly more prevalent in the non-SUDEP than SUDEP group (p = 0.01 to p = 0.02; table 2). The significant difference between groups persisted among psychiatric comorbidities and these chronic diseases after Holm-Bonferroni correction. There was no difference in the 2 groups regarding alcohol (p = 0.38) and cannabis abuse (p = 0.08); the non-SUDEP group had more cocaine, heroin, and amphetamine abusers (p < 0.001). There was no difference between the 2 groups in next-of-kin reported nonadherence to medicines (p = 0.69, table 2).
Table 2.
Demographics, comorbidities, and autopsy findings of sudden unexpected death in epilepsy (SUDEP) and non-SUDEP groups
There was no difference in the frequency of epileptogenic lesions found on autopsies such as hippocampal atrophy/sclerosis, acute hypoxic–ischemic changes, and neurodevelopmental abnormalities including focal cortical dysplasia, heterotopia, polymicrogyria, and hippocampal abnormality between SUDEP and non-SUDEP groups. Cerebral infarcts were more common in the non-SUDEP group (p = 0.004) and vascular malformation was more common in the SUDEP group (p = 0.02). Myocyte hypertrophy (p = 0.02) and coronary atherosclerosis (p < 0.001) were more commonly found in the non-SUDEP group; there were no differences in interstitial or perivascular myocardial fibrosis. Pulmonary congestion/edema was present in 48.7% of SUDEPs and 41.1% of the non-SUDEP group (p = 0.09). There was no difference in brain and heart weights between the groups. Combined lung weight was higher in the SUDEP group (p = 0.004) (table 2).
Discussion
We examined clinical and pathologic findings and temporal incidence trends among 1,086 SUDEP cases from 3 ME offices, with jurisdiction over 139 million people in urban, suburban, and rural communities, over an 8-year period. Most cases had typical SUDEP presentation: unwitnessed, with the decedent found prone in bed. We observed an overall decreasing trend in the ME-investigated SUDEP rate in the 3 ME jurisdictions, but mainly in NYC over an 8-year period using the estimated age, sex, and geographic region–adjusted prevalent epilepsy population. There was a 28% decrease in SUDEP rate in 2013–2016 compared to 2009–2012.
Our study suggests that the SUDEP rate has decreased over the past decade in the United States. The SUDEP rate also declined in patients treated with vagus nerve stimulation therapy during a 10-year follow-up in the United States.18 However, this study was limited to a specific epilepsy population and lacked a control group. The decrease could be the result of treatment or a natural consequence of decreased SUDEP incidence in the general population. A prior study reported that within a cohort of people with prevalent epilepsy in 2006, there was a 7% decline in SUDEP rate per year over the next 5 years,19 but this may reflect early deaths of the highest risk patients in the cohort. However, in our study we include both prevalent and incident epilepsy cases, thereby replenishing the population at risk. The reasons for this observed decline are unknown. PWE face many barriers to epilepsy care, including access to specialists and medications.20 The Affordable Care Act5 was passed by the US Congress in 2010 and included several methods for expanding health insurance coverage, including expansion of Medicaid eligibility in many states, including those in our study. The full consequences of this expansion of insurance coverage for PWE are not known but some evidence supports that it has been associated with reductions in disparities in epilepsy surgery.21 The decrease in SUDEP rate is mainly driven by NYC in our study. The presence of 9 level 3 or 4 epilepsy centers in NYC—most of which expanded the number of epilepsy physicians and nurse practitioners during this period—may have contributed to this reduction.22 Improved access to medications and quality epilepsy care may have led to improved seizure control, including through epilepsy surgery, in the studied populations that resulted in lower rates of seizure-related death including SUDEP. Another possibility to explain the trend could be that the increased availability of better tolerated or once-daily antiepileptic drugs over the observation period was associated with improved adherence. Finally, the decade under study also corresponded to a significant increase of SUDEP awareness among patients, caregivers, and clinicians as a result of efforts by advocacy groups.23,24 Relatedly, there was a ∼10,000-fold increase in NIH funding for SUDEP research between 2009 and 2016.25 In the 8 years of our study, 441 SUDEP-related journal articles were indexed in PubMed, compared to 77 during the preceding 8 years26 Greater awareness of SUDEP and SUDEP risk factors among patients, caregivers, and clinicians may have translated into interventions to reduce SUDEP risk (e.g., improved medication adherence and sleep hygiene, willingness to undergo curative and palliative surgical procedures, and improved nocturnal supervision). Further studies are needed to determine the mechanisms that underlie our observation of declining SUDEP rates including comparisons with ME offices located in states that did not expand Medicare eligibility. Our observation also has implications for future studies of public health interventions of SUDEP prevention such as educational campaigns.6 Our study suggests that SUDEP rates are dynamic and such studies will need to employ well-matched control arms.
We did not observe a relationship between the months of the year and SUDEP occurrence as reported for sudden infant death syndrome and sudden cardiac death.27,28 A prior population-based study in Sweden suggested a nonsignificant trend for higher SUDEP incidence on weekends/holidays compared to weekdays.29 Similarly, we observed a 20% increased rate of SUDEP on weekends compared to weekdays though wide CIs surrounding this point estimate preclude any conclusions on whether there is a predilection to SUDEP on weekends, perhaps related to seizures due to sleep deprivation or alcohol use.
SUDEP is more common in young adults without serious comorbidities that could explain the cause of death; as compared to non-SUDEP epilepsy decedents, SUDEP decedents are less likely to have psychiatric diseases (e.g., depression, anxiety, and bipolar disorder) and chronic medical diseases (e.g., diabetes, hypertension, and chronic obstructive pulmonary disease). We found no difference between next-of-kin–reported antiepileptic drug adherence between SUDEP and non-SUDEP groups, although the reliability of this retrospectively acquired data is uncertain.
The most common pathologic finding in autopsies was pulmonary congestion/edema (∼50%) in the SUDEP group. There was no difference in the 2 groups in neurodevelopmental abnormalities, which are often the etiology of epilepsy. The SUDEP group had heavier lungs compared to the non-SUDEP group. Atherosclerosis and cardiac hypertrophy were significantly higher in the non-SUDEP group, whereas there was no difference regarding interstitial or perivascular fibrosis in the 2 groups. We may conclude that interstitial or perivascular fibrosis is a relatively common nonspecific finding (∼20%) in the SUDEP and non-SUDEP epilepsy population that supports a previous study that the frequency and degree of cardiac fibrosis in SUDEP and trauma cases are similar.30 The higher rate of medical comorbidities and pathologies in the non-SUDEP group likely reflects a bias inherent in SUDEP classification. The greater the severity of medical comorbidities and related autopsy findings, the more likely a case will be classified as possible or not SUDEP.
Study limitations
Limitations of our study result primarily from data derived from MLIs. First, for deaths to be considered for SUDEP adjudication, epilepsy or seizure must have been listed in the death certificate as the cause or contributor of the death or a comorbid condition. However, MEs may underestimate the role of epilepsy as the cause of the death and may not list it in the death certificate.17,31 Further, access to medical records is limited, contributing to uncertainty regarding the diagnosis of epilepsy among decedents. We reviewed medico-legal cases with a seizure or epilepsy history from the SDC ME Office for 2014–2016 and from the MD ME Office for 2016. Seizure/epilepsy was not mentioned in the death certificate in 17.9% of SUDEP cases in MD, while seizure/epilepsy was listed in all SUDEP cases in SDC. Even though all 3 Office of Chief ME (OCME) offices mandate investigation of all unexpected deaths occurring outside the hospital or within 24 hours of hospital admission, interpretation and enforcement of this policy may not be uniform for deaths in patients with epilepsy. We reviewed deaths among patients with epilepsy that were “waived” in SDC; that is, the OCME investigators declined MLI and none was SUDEP. We attempted to account for these sources of information bias in ascertaining SUDEP using probabilistic bias analysis and found that accounting for this uncertainty in determining SUDEP counts from MLI, decreasing trends persisted with a 2% error rate. Another potential confound is the rising opioid epidemic in this time period. Concomitant positive toxicology may cause more cases to be classified as possible SUDEP or not SUDEP instead of definite SUDEP over the study period. Therefore, we performed sensitivity analysis by examining trends on all SUDEP classifications including possible SUDEP and we still observed a decreasing trend in SUDEP rate over 8 years. Another limitation is the inherent imprecision in estimates of epilepsy prevalence that was extrapolated from the Centers for Disease Control and Prevention telephone survey. There could be changes in overall case volume and staffing in ME offices over time; however, there were no substantive changes in MLI criteria. By contrast, we expected an increase in SUDEP ascertainment over the study period because our active collaboration with these ME offices began in 2011–2012, including the design of screening forms and educational programs to aid the investigation of deaths in PWE and seizures, and should have contributed to increased awareness of the contribution of epilepsy to sudden death among MEs.
In this large cohort study, we found a significant decreasing trend in SUDEP incidence based on MLI over 8 years from 3 diverse US regions. Over this same time period, there has been a significant increase in SUDEP awareness among patients and clinicians, improved understanding of individual SUDEP risk factors, and greater health insurance coverage in the United States. Our findings suggest that there may be social, economic, and health system factors beyond the individual patient that can significantly influence epilepsy mortality. Future studies to focus on identifying these population-level influences will be important to guide public health strategies to reduce SUDEP.
Acknowledgment
The authors thank Rosemarie Kobau, PhD, and Mathew Zack, PhD, for providing the National Health Interview Survey data.
Glossary
- CI
confidence interval
- MD
Maryland
- ME
medical examiner
- MLI
medico-legal investigation
- NYC
New York City
- OCME
Office of Chief Medical Examiner
- PWE
people with epilepsy
- SDC
San Diego County
- SUDEP
sudden unexpected death in epilepsy
Appendix. Authors
Study funding
This study was funded by Finding a Cure for Epilepsy and Seizures (FACES). D. Fowler was supported in part by the NYU CTSA grant UL1 TR001445 from the National Center for Advancing Translational Sciences, NIH.
Disclosure
E. Cihan reports no disclosures. O. Devinsky has received grant support, paid to his institutions, from GW Pharmaceuticals Ltd., Novartis, PTC Pharmaceuticals, Marinus, and Zogenix, and has equity interest in Qstate Biosciences, Tilray/Privateer Holdings, Tevard, Rettco, Empatica, Engage, Receptor Life Sciences, Silver Spike, and Egg Rock/Papa & Barkley. He has received consulting fees from Cavion and GW Pharma. He is the Principal Investigator of NASR and receives funding from the NINDS Center for SUDEP Research as well as other NINDS, NIMH, CDC, DOD, and MURI agencies. D. Hesdorffer is an Associate Editor of Epilepsia, an advisor to the Mount Sinai Injury Prevention Center, has a subcontract from the Epilepsy Study Consortium, and serves as a consultant for a mindfulness study funded by PCORI. M. Brandsoy, L. Li, D.R. Fowler, J.K. Graham, M.W. Karlovich, J.E. Yang, and A.E. Keller report no disclosures. E.J. Donner received grant support from the Ontario Brain Institute. D. Friedman receives salary support for consulting and clinical trial–related activities performed on behalf of The Epilepsy Study Consortium, a nonprofit organization. D. Friedman receives no personal income for these activities. NYU receives a fixed amount from the Epilepsy Study Consortium towards Dr. Friedman's salary. Within the past year, The Epilepsy Study Consortium received payments for research services performed by D. Friedman from Biogen, Crossject, Engage Pharmaceuticals, Eisai, SK Life Science, Takeda, Xenon, and Zynerba. He has also served as a paid consultant for Eisai. He has received travel reimbursement from Medtronics and the Epilepsy Foundation. He receives research support from the CDC, NINDS, Epilepsy Foundation, Empatica, Epitel, UCB, Inc., and Neuropace not related to the current work. He serves on the scientific advisory board for Receptor Life Sciences. He holds equity interests in Neuroview Technology and Receptor Life Sciences. Go to Neurology.org/N for full disclosures.
References
- 1.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med 2010;363:2522–2529. [DOI] [PubMed] [Google Scholar]
- 2.Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population-based cohort study. Neurology 2017;89:170–177. [DOI] [PubMed] [Google Scholar]
- 3.Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2017;88:1674–1680. [DOI] [PubMed] [Google Scholar]
- 4.Keller AE, Whitney R, Li SA, Pollanen MS, Donner EJ. Incidence of sudden unexpected death in epilepsy in children is similar to adults. Neurology 2018;91:e107–e11. [DOI] [PubMed] [Google Scholar]
- 5.HealthCare.gov. Affordable Care Act. [Internet]. Baltimore: US Centers for Medicare & Medicaid services. Available at: healthcare.gov/glossary/affordable-care-act/. Accessed June 6, 2019. [Google Scholar]
- 6.Devinsky O, Ryvlin P, Friedman D. Preventing sudden unexpected death in epilepsy. JAMA Neurol 2018;75:531–532. [DOI] [PubMed] [Google Scholar]
- 7.Karoly PJ, Goldenholz DM, Freestone DR, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol 2018;17:977–985. [DOI] [PubMed] [Google Scholar]
- 8.Gallerani M, Pala M, Fedeli U. Circaseptan periodicity of cardiovascular diseases. Heart Fail Clin 2017;13:703–717. [DOI] [PubMed] [Google Scholar]
- 9.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics [Internet]. Atlanta: Centers for Disease Control and Prevention. Available at: cdc.gov/nchs/index.htm. Updated May 31, 2019. Accessed March 1, 2019. [Google Scholar]
- 11.Decennial Census of Population and Housing 2010 [Internet]. US Census Bureau. Available at: census.gov/2010census/. Accessed March 1, 2019. [Google Scholar]
- 12.American Community Survey 2011–2015 [Internet]. US Census Bureau. Available at: census.gov/programs-surveys/acs/. Accessed March 1, 2019.
- 13.Fisher NI. Statistical Analysis of Circular Data. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 14.Stephens MA. Use of the Kolmogorov-Smirnov, Cramer-von Mises and related Statistics without Extensive tables. J R Stat Soc Ser B Stat Methodol 1970;32:115–122. [Google Scholar]
- 15.Lash TL, Fox MP.. A Guide to Implementing Quantitative Bias Analysis. Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Statistics for Biology and Health: Springer; 2009. [Google Scholar]
- 16.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol 2014;43:1969–1985. [DOI] [PubMed] [Google Scholar]
- 17.Devinsky O, Friedman D, Cheng JY, Moffatt E, Kim A, Tseng ZH. Underestimation of sudden deaths among patients with seizures and epilepsy. Neurology 2017;89:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 2018;59:562–572. [DOI] [PubMed] [Google Scholar]
- 19.Tomson T, Sveinsson O, Carlsson S, Andersson T. Evolution over time of SUDEP incidence: a nationwide population-based cohort study. Epilepsia 2018;59:e120–e124. [DOI] [PubMed] [Google Scholar]
- 20.Thurman DJ, Kobau R, Luo YH, Helmers SL, Zack MM. Health-care access among adults with epilepsy: the U.S. National Health Interview Survey, 2010 and 2013. Epilepsy Behav 2016;55:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma K, Kalakoti P, Henry M, et al. Revisiting racial disparities in access to surgical management of drug-resistant temporal lobe epilepsy post implementation of Affordable Care Act. Clin Neurol Neurosurg 2017;158:82–89. [DOI] [PubMed] [Google Scholar]
- 22.National Association of Epilepsy Centers [Internet]. Washington, DC. Available at: naec-epilepsy.org/. Updated 2019. Accessed March 1, 2019. [Google Scholar]
- 23.Kroner BL, Wright C, Friedman D, et al. Characteristics of epilepsy patients and caregivers who either have or have not heard of SUDEP. Epilepsia 2014;55:1486–1494. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine Committee. On the public health dimensions of the epilepsies: the National Academies Collection: reports funded by National Institutes of Health. In: England MJ, Liverman CT, Schultz AM, Strawbridge LM, eds. Epilepsy across the spectrum: promoting health and understanding. Washington, DC: National Academies Press, National Academy of Sciences; 2012. [Google Scholar]
- 25.Research portfolio online reporting Tools [Internet]. US Department of Health & Human Services. Available at: projectreporter.nih.gov. Accessed March 1, 2019.
- 26.Sudden unexpected death in epilepsy. In: Pubmed.gov [Internet]. US National Library of Medicine National Institutes of Health. Available at: ncbi.nlm.nih.gov/pubmed/?term=%22sudden+unexpected+death+in+epilepsy%22. Accessed March 1, 2019.
- 27.Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death: population-based analysis of 24,061 consecutive cases. Eur Heart J 2000;21:315–320. [DOI] [PubMed] [Google Scholar]
- 28.Douglas AS, Helms PJ, Jolliffe IT. Seasonality of sudden infant death syndrome (SIDS) by age at death. Acta Paediatr 1998;87:1033–1038. [DOI] [PubMed] [Google Scholar]
- 29.Sveinsson O, Andersson T, Carlsson S, Tomson T. Circumstances of SUDEP: a nationwide population-based case series. Epilepsia 2018;59:1074–1082. [DOI] [PubMed] [Google Scholar]
- 30.Devinsky O, Kim A, Friedman D, Bedigian A, Moffatt E, Tseng ZH. Incidence of cardiac fibrosis in SUDEP and control cases. Neurology 2018;91:e55–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schraeder PL, Delin K, McClelland RL, So EL. Coroner and medical examiner documentation of sudden unexplained deaths in epilepsy. Epilepsy Res 2006;68:137–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.