Abstract
Objective
Increasing evidence supports an association between midlife cardiovascular risk factors (CVRFs) and risk of dementia, but less is known about whether CVRFs influence cognition in midlife. We examined the relationship between CVRFs and midlife cognitive decline.
Methods
In 2,675 black and white middle-aged adults (mean age 50.2 ± 3.6 years, 57% female, 45% black), we measured CVRFs at baseline: hypertension (31%), diabetes mellitus (11%), obesity (43%), high cholesterol (9%), and current cigarette smoking (15%). We administered cognitive tests of memory, executive function, and processing speed at baseline and 5 years later. Using logistic regression, we estimated the association of CVRFs with accelerated cognitive decline (race-specific decline ≥1.5 SD from the mean change) on a composite cognitive score.
Results
Five percent (n = 143) of participants had accelerated cognitive decline over 5 years. Smoking, hypertension, and diabetes mellitus were associated with an increased likelihood of accelerated decline after multivariable adjustment (adjusted odds ratio [AOR] 1.65, 95% confidence interval [CI] 1.00–2.71; AOR 1.87, 95% CI 1.26–2.75; AOR 2.45, 95% CI 1.54–3.88, respectively), while obesity and high cholesterol were not associated with risk of decline. These results were similar when stratified by race. The likelihood of accelerated decline also increased with greater number of CVRFs (1–2 CVRFs: AOR 1.77, 95% CI 1.02–3.05; ≥3 CVRFs: AOR 2.94, 95% CI 1.64–5.28) and with Framingham Coronary Heart Disease Risk Score ≥10 (AOR 2.29, 95% CI 1.21–4.34).
Conclusions
Midlife CVRFs, especially hypertension, diabetes mellitus, and smoking, are common and associated with accelerated cognitive decline at midlife. These results identify potential modifiable targets to prevent midlife cognitive decline and highlight the need for a life course approach to cognitive function and aging.
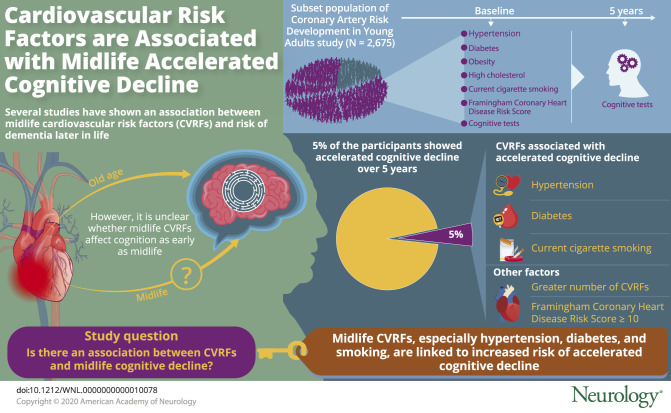
Hypertension, dyslipidemia, and diabetes mellitus, as well as smoking and obesity, are recognized as key cardiovascular risk factors (CVRFs) for cognitive impairment and decline.1–3 Some previous studies have focused primarily on late-life cognitive outcomes, with the most consistent evidence supporting a relationship between midlife CVRF exposure and greater rates of cognitive decline and dementia in late life.4–6 However, the process of cognitive aging takes place over decades,7 and data are sparse regarding CVRF effects on cognitive outcomes earlier in the life course, including in midlife.
Longitudinal studies indicate that declines on several cognitive domains, including processing speed and executive function, may begin to emerge as early as midlife.8 Midlife is also a critical time period for CVRFs with greater acceleration in the prevalence of hypertension, diabetes mellitus, and dyslipidemia compared to young adulthood,9–11 but it is unclear whether midlife CVRFs contribute to early changes in cognition during midlife. An expanded focus on the influence of CVRFs on cognitive outcomes across the life course could contribute to a more comprehensive understanding of cognitive decline, improve stratification of those at high risk for dementia, and inform the development and design of targeted interventions for healthy cognition.12–14 Furthermore, rigorous evidence on modifiable risk factors for cognitive decline across the life course is particularly limited in racially and ethnically diverse cohorts.15
As part of the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study, we sought to determine whether CVRFs are associated with increased risk of accelerated cognitive decline in middle-aged black and white adults. We hypothesized that CVRFs such as hypertension, diabetes mellitus, high cholesterol, obesity, and current smoking will be associated with increased likelihood of midlife accelerated cognitive decline.
Methods
Study design and sample
CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a cohort of black and white women and men (n = 5,115) initially 18 to 30 years of age and healthy at enrollment in 1985 to 1986. CARDIA participants were recruited from 4 US cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). Community-based sampling was performed in Birmingham, Chicago, and Minneapolis, while Oakland participants were sampled from the membership of a large integrated health care system. The participant composition of each site was approximately balanced by sex, age, race, and education.
To assess the effect of CVRF exposure on cognitive changes during midlife, we included participants who completed cognitive testing at year 25 (2010–2011), our study baseline, and 5 years later (follow-up mean [SD] 5.02 (0.34) years, range 3.90–6.11 years). Participants with self-reported stroke (n = 69) at baseline were excluded. The final analytic cohort consisted of 2,675 participants. Compared to those in the final analytic cohort, participants who did not have complete cognitive testing at both baseline and follow-up were less likely to be white, had a lower level of education, and drank less alcohol (p < 0.05), but they did not differ on age, sex, or physical activity.
Standard protocol approvals, registrations, and patient consents
At each visit, participants provided written informed consent, and study protocols were reviewed by institutional review boards from each study site, the CARDIA Coordinating Center at the University of Alabama, Birmingham and the University of California, San Francisco; further details of the study are available elsewhere.16,17
CVRF measurement
At baseline, we defined measures for current cigarette smoking, obesity, diabetes mellitus, hypertension, and high cholesterol. Current cigarette smoking was based on self-reported definition of smoking at least 5 cigarettes per week almost every week. We used examination data on height and weight to calculate body mass index, and defined obesity as a body mass index ≥30 kg/m2. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL, oral glucose tolerance test ≥200 mg/dL, glycosylated hemoglobin ≥6.5%, or use of medications. We defined high cholesterol as total cholesterol >240 mg/dL or use of medications. We used examination data on blood pressure measurements and defined hypertension as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of medications.
To estimate the extent of CVRF exposure at baseline, the number of risk factors present was summed for a score of 0 to 5. Because few participants (<10%) had >3 CVRFs, the number of CVRFs was categorized as 0, 1 or 2, and ≥3. In addition, we calculated the Framingham Coronary Heart Disease Risk Score, a sex-specific algorithm based on age, total cholesterol, high-density lipoprotein cholesterol, systolic and diastolic blood pressures, smoking, and diabetes mellitus,18 and defined intermediate or higher 10-year heart disease risk as having a Framingham Coronary Heart Disease Risk Score ≥10.
Cognitive function assessment
At baseline and the 5-year follow up, interviewers who underwent a centralized training administered a battery of 3 cognitive tests: the Digit Symbol Substitution Test (DSST) to assess processing speed and executive function, with higher scores for digits correctly substituted indicating better function19; the Stroop Test to assess executive function, with lower interference scores indicating better function (the inverse of this score was used such that higher scores indicate better performance)20,21; and the Rey Auditory Verbal Learning Test (RAVLT) to assess verbal memory, with higher scores after a 10-minute delay indicating better function.22 We computed composite cognitive function scores at baseline and 5 years later. We estimated 5-year change in the composite cognitive z scores, and given that there were significant differences in baseline cognitive scores and change in score by race, we defined accelerated cognitive decline as a race-specific decline ≥1.5 SD from the mean change on the composite cognitive score derived from the 3 tests.23,24
Other covariates
Covariates were used from baseline unless otherwise noted. Demographic characteristics, including age, sex, race, and education, were based on self-report. We measured physical activity with the CARDIA Physical Activity History questionnaire, which queries time spent in physical activities in the past 12 months25 and produces a total physical activity score in exercise units, which we used to compare participants with scores ≥300 and <300 exercise units, approximating the cutoff for recommended levels of physical activity.26 We assessed depressive symptoms with the Center for Epidemiologic Studies Depression scale.27 Alcohol use (standardized for different types of alcohol as drinks per week) was assessed by self-report, and APOE ε4 phenotype was determined by CARDIA year 7 blood samples using standard techniques.28,29
Data analysis
Participants with and without accelerated cognitive decline were compared with descriptive statistics. We ran unadjusted and adjusted logistic regression models to examine the odds associated with accelerated cognitive decline 5 years after CVRF exposure. Adjusted models included demographics (age, race, sex, and education), depressive symptoms, APOE ε4 status, and alcohol use, and we also stratified by race and examined possible interactions between race and the effects of each CVRF on accelerated decline on the cognitive score. We determined the odds associated with accelerated cognitive decline for those with 1 or 2 and ≥3 CVRFs (compared to those with 0 exposures) and the odds of decline for participants with Framingham Coronary Heart Disease Risk Score ≥10 compared to those with lower risk (Framingham Coronary Heart Disease Risk Score <10). We also conducted several sensitivity analyses, including accelerated decline on the 3 individual cognitive tests, accelerated decline based on the whole cohort (not race-specific cutoffs), and accelerated decline defined with a different cutoff of ≥1.0 SD from the mean change on the composite cognitive score. In a final sensitivity analysis, we examined the effects of CVRFs on accelerated decline, excluding participants who developed adjudicated clinical vascular events such as stroke, myocardial infarction, heart failure, or atrial fibrillation by the end of the follow-up period. Tests of statistical significance were 2 tailed, with values of p < 0.05 considered significant. Analyses were conducted with R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).30
Data availability
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
Results
Of the 2,675 participants, the mean (SD) age at baseline was 50.2 ± 3.6 years, 57.1% (n = 1,528) were female, 55.3% (n = 1,479) were white, and the mean education was 15.2 (2.6) years. Prevalence of CVRFs at baseline was 30.9% (n = 826) for hypertension, 10.9% (n = 290) for diabetes mellitus, 42.5% (n = 1,133) for obesity, 9.3% (n = 248) for high cholesterol, and 15.0% (n = 701) for current smoking.
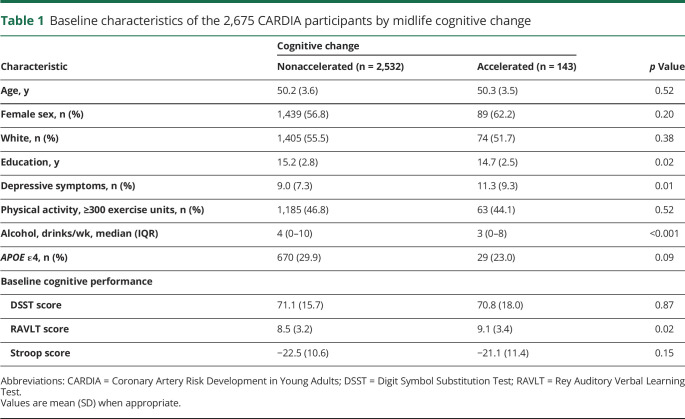
At follow-up, mean (SD) cognitive change on each test for black and white participants, respectively, was as follows: for DSST, −3.00 (9.40) and −2.09 (9.17), p = 0.01; for Stroop, −0.09 (11.26) and −0.12 (6.88), p > 0.05; and for RAVLT, 0.01 (2.65) and 0.31 (2.51), p = 0.001. Five percent (n = 75 black and 69 white) of participants met the criteria for accelerated cognitive decline. Table 1 shows the baseline characteristics of CARDIA participants by accelerated cognitive decline. Compared to participants with nonaccelerated cognitive decline, those with accelerated cognitive decline had lower education, had greater mean Center for Epidemiologic Studies Depression scale score, and drank less alcohol (p < 0.001). Participants did not differ in age, sex, physical activity, or APOE ε4 status.
Table 1.
Baseline characteristics of the 2,675 CARDIA participants by midlife cognitive change
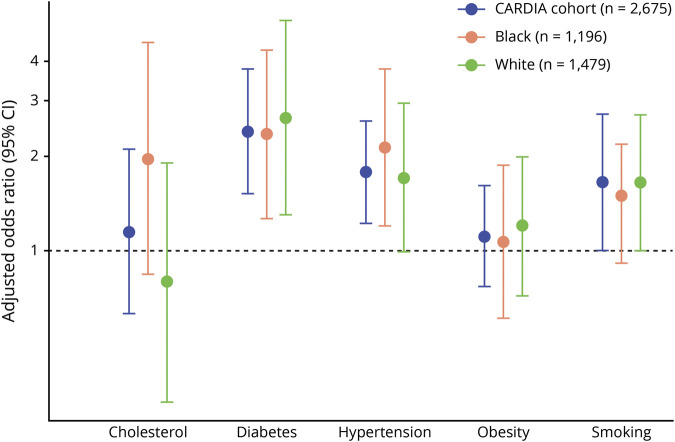
In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs 4.3%, odds ratio [OR] 1.79, 95% confidence interval [CI] 1.27–2.52), diabetes mellitus (10.3% vs 4.7%, OR 2.33, 95% CI 1.53–3.56), and smoking (7.7% current smokers vs 4.3% never smokers, OR 1.87, 95% CI 1.21–2.90). For high cholesterol and obesity, no significant effect was observed (high cholesterol: 6.9% vs 5.2%, OR 1.35, 95% CI 0.80–2.28; obesity: 6.1% vs 4.8%, OR 1.29, 95% CI 0.92–1.82). The figure shows the results of these associations (for the cohort as a whole and stratified by race) after multivariable adjustment for age, sex, race (in models for the total cohort), education, depressive symptoms, APOE, and alcohol use. In the overall cohort, the associations for hypertension (adjusted OR [AOR] 1.87, 95% CI 1.26–2.75) and diabetes mellitus (AOR 2.45, 95% CI 1.54–3.88) remained significant and for smoking (AOR 1.65, 95% CI 1.00–2.71) remained borderline significant. The pattern of CVRF associations with accelerated decline was similar when stratified by race (figure), and there was no evidence of an interaction between CVRFs, race, and associations with accelerated cognitive decline for the composite cognitive score (p for interaction > 0.05 for all).
Figure. Multivariable-adjusteda odds of developing accelerated cognitive decline 5 years after cardiovascular risk factor exposure in midlife among the 2,675 CARDIA participants.
CARDIA = Coronary Artery Risk Development in Young Adults; CI, confidence interval. aAdjusted for age, race (in models of the total cohort), sex, education, depressive symptoms, APOE ε4, and alcohol use.
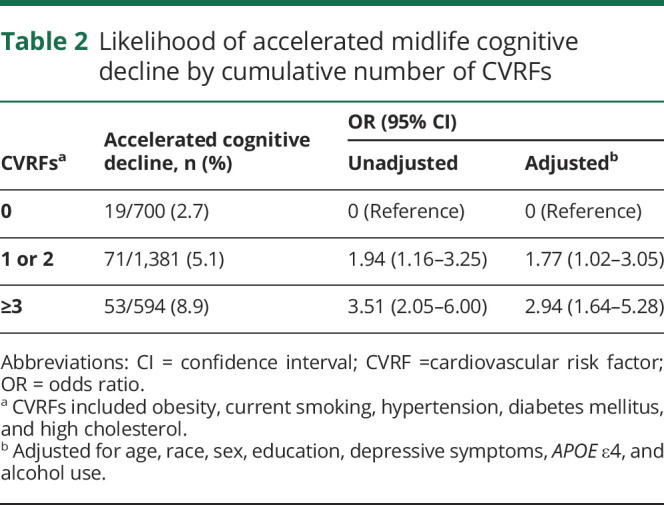
In the overall sample, 26.2% (n = 700) of participants had 0 CVRF exposures, 25.9% (n = 692) had 1, 25.8% (n = 689) had 2, and 22.0% (n = 594) had ≥3. Table 2 presents the association between cumulative number of CVRF exposures and the likelihood of accelerated decline. The odds of accelerated cognitive decline increased with a greater number of CVRFs. Compared with 0 CVRF exposures, the OR was greater with 1 to 2 CVRFs (OR 1.94, 95% CI 1.16–3.25) and even greater with ≥3 CVRFs (OR 3.51, 95% CI 2.05–6.00). After multivariable adjustment, the association of having 1 to 2 and ≥3 CVRFs with accelerated cognitive decline remained significant. We also evaluated associations categorized by Framingham Coronary Heart Disease Risk Score. Overall, 153 (5.7%) participants had Framingham Coronary Heart Disease Risk Score ≥10, the cutoff for intermediate risk, and compared to those with lower risk, the odds of accelerated cognitive decline was 2 times higher in those with at least an intermediate risk score (OR 2.29, 95% CI 1.21–4.34).
Table 2.
Likelihood of accelerated midlife cognitive decline by cumulative number of CVRFs
We conducted several additional analyses investigating the relationship between CVRFs and accelerated decline on individual cognitive tests. We found that the pattern of association with CVRFs and accelerated decline was similar for the DSST and Stroop as observed for the composite measure, but CVRFs were not significantly associated with decline on the RAVLT. In a sensitivity analysis, we repeated our main analysis by defining cognitive decline on the basis of the whole cohort's cognitive changes rather than those stratified by race and found almost identical results for CVRF associations with cognitive decline (table e-1, doi.org/10.7272/Q64J0C9D). Similar to our main analysis, the interactions between CVRFs, race, and accelerated cognitive decline were not significant (p for interaction > 0.05 for all). Given that there is no standard clinical cutoff for accelerated cognitive decline in middle-aged adults, we also evaluated these associations using a less stringent cutoff of race-specific decline >1.0 SD from the mean, and the results were similar in direction and pattern, but the CIs were wider (table e-2, doi.org/10.7272/Q64J0C9D) Finally, the strength of these associations was similar and remained significant when we excluded participants who developed clinical vascular events by the end of the follow-up period (n = 80 with stroke, myocardial infarction, chronic heart failure, or atrial fibrillation).
Discussion
In a diverse cohort of middle-aged adults, we found that CVRFs, including hypertension, diabetes mellitus, and smoking, were each associated with increased odds of accelerated cognitive decline 5 years later. In addition, participants who had a greater number or risk score of midlife CVRFs were more likely to develop accelerated decline.
Our results align with a substantial literature on the contribution of midlife CVRFs to risk of late-life cognitive decline and dementia1,3,12,31,32 and with a small but growing literature demonstrating the importance of CVRFs for cognitive performance in earlier stages of the life course. In a previous CARDIA analysis, we observed an association between cumulative exposure to elevated but subclinical levels of blood pressure and glucose during young to middle adulthood and cognitive performance in midlife.33 The Young Finns Study also focused on an earlier window of cumulative exposure to CVRFs, early childhood to young adulthood, and found significant associations between blood pressure, cholesterol, and smoking and cognitive function at midlife.34 In contrast, our investigation concentrated more specifically on the midlife period, evaluating midlife exposure to clinically defined CVRFs and accelerated 5-year cognitive decline. These results document an early divergence of cognitive trajectories in black and white middle-aged adults and could be especially important in that larger cognitive changes in midlife may establish and strongly influence cognitive trajectories into late life. Furthermore, the emergence of CVRFs such as diabetes mellitus and hypertension in midlife could be a critical period for education and intervention to delay the process of accelerated decline.
These findings are likewise consistent with those reported by the Atherosclerosis Risk in Communities (ARIC) study, which examined midlife CVRFs and risk of cognitive decline from late-middle age to late life2 and found that both hypertension and diabetes mellitus were associated with cognitive decline over 6 years. Additional studies in more homogeneous, slightly older cohorts such as the Whitehall II and the Doetinchem Cohort Study also support our finding of associations for high blood pressure,35 diabetes mellitus,36,37 and smoking3,38 with cognitive outcomes.
Our investigation also contributes to the field by including the evaluation of CVRF composite profiles, particularly in middle age.1,39,40 In the CARDIA cohort, the odds of accelerated cognitive decline was 3 times higher in participants with ≥3 CVRFs compared to those without any CVRFs. Similarly, the odds of accelerated decline was greater in those with at least an intermediate Framingham Coronary Heart Disease risk score. These results further reinforce current approaches to prevent cognitive decline and dementia that are focused on identifying those at highest risk and targeting multiple risk factors to increase efficiency and effectiveness.12 Successes from recent trials of modifiable risk factors for dementia, including Systolic Blood Pressure Intervention Trial - Memory and Cognition in Decreased Hypertension for intensive blood pressure control, highlight the potential impact of CVRF reduction41; however, these trials have focused primarily on older adults, and the implications for earlier stages of the life course are uncertain. For middle-aged adults such as those in CARDIA, the clinical significance of accelerated cognitive decline over 5 years has yet to be defined. Such changes may be the beginning of an early trajectory leading to later-life cognitive impairment, or this decline could be a mild but mostly static change in midlife. Notably, we did not observe a difference in accelerated decline in midlife by APOE status; however, this is consistent with other studies that reported that differences in cognitive trajectories by APOE are minimal in middle age and become larger only in later life.42,43
Accumulating biomarker, genetic, and imaging data support a crucial relationship between CVRFs and decline in cognitive function.32 As expected, when we looked at individual cognitive tests, associations were most robust for executive function and processing speed and less robust for memory. CVRFs drive ischemic and atherosclerotic processes, which can accelerate brain aging, especially in subcortical frontal regions,32 and CVRF exposures have been connected to increased markers of inflammation and stress.44–46 Neuropathologic and imaging studies have suggested that CVRF exposure may also interact with amyloid pathways, disrupting amyloid clearance and production while increasing amyloid deposition and plaques.47–49
CARDIA is a well-characterized longitudinal cohort study in which we were able to evaluate the relationship of midlife CVRF exposure with change in cognition. We also used a population-based sampling method, accounted for several potential confounders, and were able to examine midlife cognitive changes in a diverse cohort of black and white adults. However, there are also limitations to consider. We were unable to assess every cognitive domain but had sensitive tests for memory, executive function, and processing speed, and there may be bias due to loss to follow-up. Furthermore, one of our aggregate measures of cardiovascular risk, the Framingham Coronary Heart Disease Risk score, was developed in a more homogeneous cohort. Finally, although the definition of clinically significant cognitive decline, a decrease of >1.5 SD from the mean, is standard in epidemiologic studies of older adults,23,24,50 characterization of cognitive change in middle-aged adults is not as well defined, and the clinical significance of such decline this early in the life course is not yet clear.
Our findings link midlife CVRFs to increased risk of accelerated cognitive decline even just 5 years later in midlife. While current public health prevention efforts focus on late life, our study provides evidence for the need to investigate the entire spectrum of cognitive performance. Middle-aged adults with CVRFs, especially hypertension, diabetes mellitus, and current smoking, or those with >1 CVRF may represent critical subgroups for early monitoring and education. While we found a similar pattern of CVRF effects in both black and white participants, more work is needed to investigate whether CVRF incidence and treatment in young to middle adulthood may underlie disparities in cognitive and brain aging outcomes. Additional research is needed to improve the early detection of those at highest risk of accelerated cognitive decline and to determine effective intervention models to prevent or delay cognitive decline across the life course.
Glossary
- AOR
adjusted OR
- ARIC
Atherosclerosis Risk in Communities
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- CVRF
cardiovascular risk factor
- DSST
Digit Symbol Substitution Test
- OR
odds ratio
- RAVLT
Rey Auditory Verbal Learning Test
Appendix. Authors
Study funding
The CARDIA study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The CARDIA Cognitive Function ancillary study is supported by NHLBI grant R01 HL122658 (multiple principal investigators: K. Yaffe and S. Sidney). This manuscript has been reviewed by CARDIA for scientific content. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the NIH; or the US Department of Health and Human Services.
Disclosure
K. Yaffe serves on Data Safety Monitoring boards for Eli Lilly and several National Institute on Aging–sponsored studies, is a board member of Alector, and is also a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. A. Bahorik, T. Hoang, S. Forrester, D. Jacobs, C. Lewis, D. Lloyd-Jones, S. Sidney, and J. Reis report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 2.Knopman D, Boland L, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48. [DOI] [PubMed] [Google Scholar]
- 3.Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II study. Am J Epidemiol 2009;170:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 2007;166:367–378. [DOI] [PubMed] [Google Scholar]
- 5.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev 2008;9:204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851. [PubMed] [Google Scholar]
- 7.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park DC, Festini SB. The middle-aged brain: a cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2016. [Google Scholar]
- 9.Hardy R, Lawlor DA, Kuh D. A life course approach to cardiovascular aging. Future Cardiol 2015;11:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA 2005;294:1773–1781. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Mishra GD, Dobson AJ, Jones M. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med 2018;15:e1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington: National Academies Press; 2015. [PubMed] [Google Scholar]
- 13.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med 2013;369:2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewster P, Barnes L, Haan M, et al. Progress and future challenges in aging and diversity research in the United States. Alzheimers Demen 2019;5:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Controlled Clin Trials 1987;8:68–73. [DOI] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D, Coalson DL, Raiford SE. WAIS-III: Wechsler Adult Intelligence Scale. San Antonio: Psychological Corp; 1997. [Google Scholar]
- 20.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662. [Google Scholar]
- 21.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991;109:163–203. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 23.Petersen. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:760. [DOI] [PubMed] [Google Scholar]
- 24.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc 2010;58:1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulmonary Rehabil Prev 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabriel KP, Sidney S, Jacobs DR Jr, et al. Convergent validity of a brief self-reported physical activity questionnaire. Med Sci Sports Exerc 2014;46:1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 28.Kamboh M, Ferrell R, Kottke B. Genetic studies of human apolipoproteins, V: a novel rapid procedure to screen apolipoprotein E polymorphism. J Lipid Res 1988;29:1535–1543. [PubMed] [Google Scholar]
- 29.Howard BV, Gidding SS, Liu K. Association of apolipoprotein E phenotype with plasma lipoproteins in African-American and white young adults: the CARDIA study. Am J Epidemiol 1998;148:859–868. [DOI] [PubMed] [Google Scholar]
- 30.R version 3.4.3 [computer program]. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 31.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015;12:267–277. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rovio SP, Pahkala K, Nevalainen J, et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns Study. J Am Coll Cardiol 2017;69:2279–2289. [DOI] [PubMed] [Google Scholar]
- 35.Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. J Clin Epidemiol 2005;58:1308–1315. [DOI] [PubMed] [Google Scholar]
- 36.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol 2014;2:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nooyens AC, Baan CA, Spijkerman AM, Verschuren WM. Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care 2010;33:1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nooyens AC, van Gelder BM, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health 2008;98:2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anstey KJ, Sargent-Cox K, Garde E, Cherbuin N, Butterworth P. Cognitive development over 8 years in midlife and its association with cardiovascular risk factors. Neuropsychology 2014;28:653–665. [DOI] [PubMed] [Google Scholar]
- 40.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dementia risk score vs the Framingham vascular risk scores. Neurology 2013;80:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl Psychiatry 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 2011;32:63–74. [DOI] [PubMed] [Google Scholar]
- 44.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004;292:2237–2242. [DOI] [PubMed] [Google Scholar]
- 45.Marsland AL, Gianaros PJ, Kuan DCH, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun 2015;48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaffe K, Lindquist K, Schwartz A, et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011;77:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010;120:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Norden AG, van Dijk EJ, de Laat KF, Scheltens P, OldeRikkert MG, De Leeuw F. Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochim Biophys Acta 2012;1822:340–349. [DOI] [PubMed] [Google Scholar]
- 49.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrzak RH, Maruff P, Woodward M, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. Am J Geriatr Psychiatry 2012;20:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).