Abstract
Objectives
To investigate the relationships of serum albumin with in vivo Alzheimer disease (AD) pathologies, including cerebral β-amyloid (Aβ) protein deposition, neurodegeneration of AD-signature regions, and cerebral white matter hyperintensities (WMH), in the human brain.
Methods
A total of 396 older adults without dementia underwent comprehensive clinical assessments, measurement of serum albumin level, and multimodal brain imaging, including [11C] Pittsburgh compound B-PET, 18F-fluorodeoxyglucose-PET, and MRI. Serum albumin was categorized as follows: <4.4 g/dL (low albumin), 4.4 to 4.5 g/dL (middle albumin), and >4.5 g/dL (high albumin; used as a reference category). Aβ positivity, AD-signature region cerebral glucose metabolism (AD-CM), AD-signature region cortical thickness (AD-CT), and WMH volume were used as outcome measures.
Results
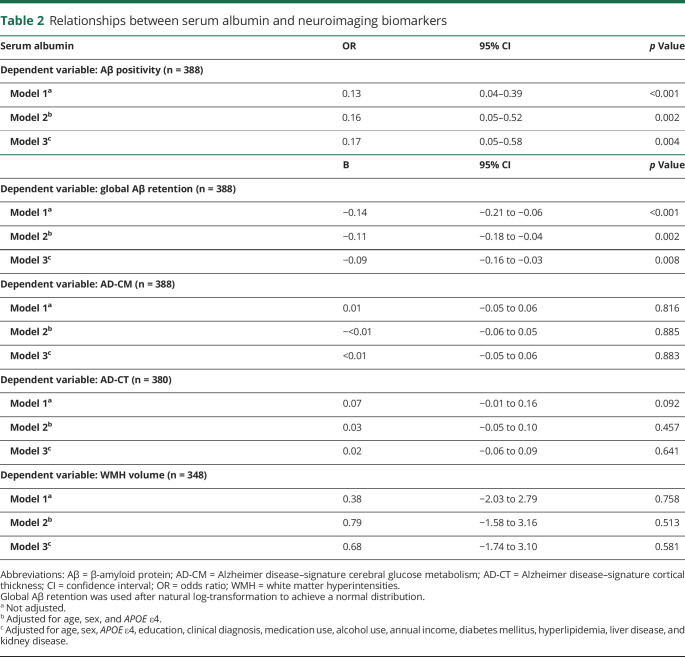
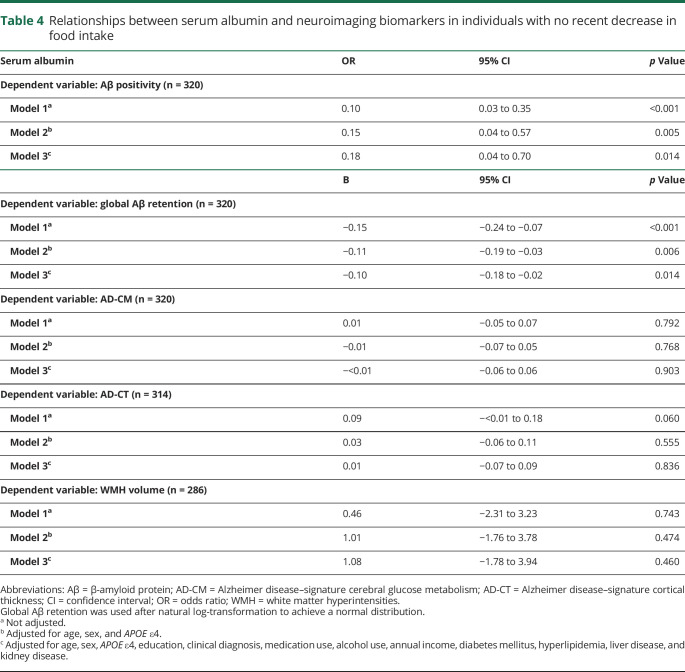
Serum albumin level (as a continuous variable) was inversely associated with Aβ deposition and Aβ positivity. The low albumin group showed a significantly higher Aβ positivity rate compared to the high albumin group (odds ratio 3.40, 95% confidence interval 1.67–6.92, p = 0.001), while the middle albumin group showed no difference (odds ratio 1.74, 95% confidence interval 0.80–3.77, p = 0.162). Neither serum albumin level (as a continuous variable) nor albumin categories were related to AD-CM, AD-CT, or WMH volume.
Conclusions
Low serum albumin may increase the risk of AD dementia by elevating amyloid accumulation. In terms of AD prevention, more attention needs to be paid to avoid a low serum albumin level, even within the clinical normal range, by clinicians.
Alzheimer disease (AD) is characterized by extracellular deposition of β-amyloid protein (Aβ) and intraneuronal neurofibrillary tangles in the brain.1 Because Aβ deposition is the earliest pathology of AD and begins ≈10 to 15 years before the onset of the cognitive symptoms,2–4 identification of factors affecting cerebral Aβ deposition may facilitate the development of strategies for preventing AD dementia.
Serum albumin, which is the most abundant protein in human plasma,5 is regarded as one of the most potent Aβ sequestering systems in that it binds 90% to 95% of the Aβ in blood plasma.6,7 The dynamic equilibrium of Aβ between brain and blood plasma may be shifted toward the bloodstream by peripheral serum albumin that binds the Aβ.8 Therefore, a reduction in Aβ binding to serum albumin in the blood may lead to a decrease in the capacity for Aβ excretion from the brain to the blood, resulting in Aβ deposition in the brain.9
Preclinical studies suggested that serum albumin may inhibit Aβ fibril formation by binding Aβ monomers7 or oligomers.10 Several human studies have indicated that a low serum albumin level is associated with cognitive impairment11–13 and AD dementia.14,15 However, little information is available on whether serum albumin is related to Aβ deposition in the living human brain. Therefore, the present study was performed to examine the relationships of serum albumin and in vivo cerebral Aβ deposition in older adults without dementia. We additionally investigated the associations of serum albumin with AD-signature neurodegeneration and white matter hyperintensities (WMH) as a measure of cerebrovascular injury. While some human studies have reported an inverse association of serum albumin and cerebrovascular disease,16,17 few studies have investigated the relationships of serum albumin with neurodegenerative changes in the brain.
Methods
Participants
The present study was performed as part of the Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer's Disease (KBASE), which is an ongoing prospective cohort study.18 As of November 2016, a total of 396 older adults without dementia (284 cognitively normal [CN] individuals and 112 individuals with mild cognitive impairment [MCI]) between 55 and 90 years of age were enrolled in the study. The CN group consisted of participants with a Clinical Dementia Rating19 score of 0 and no diagnosis of MCI or dementia. All individuals with MCI met the current consensus criteria for amnestic MCI, which are as follows: (1) memory complaints confirmed by an informant, (2) objective memory impairments, (3) preserved global cognitive function, (4) independence in functional activities, and (5) no dementia. With regard to criterion 2, the age-, education-, and sex-adjusted z scores for at least 1 of 4 episodic memory tests were <−1.0. The 4 memory tests were the Word List Memory, Word List Recall, Word List Recognition, and Constructional Recall tests, which are included in the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery.20 All individuals with MCI had a Clinical Dementia Rating score of 0.5. The exclusion criteria were as follows: (1) presence of a major psychiatric illness; (2) significant neurologic (e.g., cerebrovascular disease) or medical conditions that could affect mental function; (3) contraindications for MRI (e.g., pacemaker or claustrophobia); (4) illiteracy; (5) presence of significant visual/hearing difficulties or severe communication or behavioral problems that would make clinical examinations or brain scans difficult; (6) taking an investigational drug; and (7) pregnant or breastfeeding. The presence of any item included in the exclusion criteria was determined by research clinicians referring to the results of laboratory examinations and MRI and the clinical data collected by trained nurses during systematic interviews of participants and their reliable informants during the screening period. More detailed information on the recruitment of the KBASE cohort is presented in a previous report from our research group.18
Standard protocol approvals, registrations, and patient consents
This study protocol was approved by the Institutional Review boards of the Seoul National University Hospital (C-1401-027-547) and SMG-SNU Boramae Medical Center (26-2015-60), Seoul, South Korea, and the study was conducted in accordance with the recommendations of the current version of the Declaration of Helsinki. The subjects or their legal representatives gave written informed consent.
Clinical assessments
All participants underwent comprehensive clinical and neuropsychological assessments administered by trained psychiatrists and neuropsychologists according to the KBASE assessment protocol,18 which incorporates the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease.21,22 Medication use within 4 weeks and alcohol intake status (never/former/drinker) were evaluated by nurse interviews and by a review of medical records. Participants were divided into 3 groups according to annual income: below the minimum cost of living (MCL), above the MCL but below twice the MCL, and twice the MCL or more (law.go.kr). The MCL was determined according to the administrative rules published by the Ministry of Health and Welfare, Republic of Korea, in November 2012. The MCL was 572,168 Korea Won for single-person households and added 286,840 Korea Won for each additional housemate. The presence of comorbid diabetes mellitus, dyslipidemia, liver disease, and kidney disease was assessed from data collected by trained nurses during systematic interviews of participants and their reliable informants. Information about nutritional state, including the change in food intake over the past 3 months due to loss of appetite, digestive problems, or chewing or swallowing difficulties, was systematically obtained by using the Mini Nutritional Assessment tool.23 To acquire accurate information, reliable informants were interviewed and medical records were reviewed.
Laboratory tests of blood samples
Blood samples were obtained by venipuncture after an overnight fast. The concentration of albumin was determined by bromocresol green dye binding assay (ADVIA 1800; Siemens, Washington, DC). The normal range for albumin level is 3.5 to 5.5 g/dL. Participants were categorized on the basis of serum albumin level using tertiles referring to previous studies24,25 as follows: low albumin, <4.4 g/dL; middle albumin, 4.4 to 4.5 g/dL; and high albumin, >4.5 g/dL (used as a reference category). In addition, genomic DNA was extracted from whole blood, and APOE genotyping was performed as described previously.26 APOE ε4 positivity was defined as the presence of at least 1 ε4 allele.
Measurement of cerebral Aβ deposition
All participants underwent simultaneous 3D [11C] Pittsburgh compound B (PiB)-PET and 3D T1-weighted MRI scan with a 3.0T Biograph mMR (PET-MR) scanner (Siemens) according to the manufacturer's guidelines. Details of the PiB-PET imaging acquisition and preprocessing were described previously.27 An automatic anatomic labeling algorithm and a region-combining method28 were applied to determine regions of interest (ROIs) to characterize the PiB retention levels in the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal regions. The standardized uptake value ratio (SUVR) value for each ROI was calculated by dividing the mean value for all voxels within each ROI by the mean cerebellar uptake value in the same image. A global cortical ROI consisting of the 4 ROIs was also defined, and a global Aβ retention value was generated by dividing the mean value for all voxels of the global cortical ROI by the mean cerebellar uptake value in the same image.28,29 Given the characteristic skewed distribution of cerebral Aβ deposition values, global Aβ retention data were used after natural log-transformation and removal of extreme outliers to achieve a normal distribution with reference to previous reports.30,31 Amyloid positivity was also used as an outcome variable. Each participant was classified as Aβ-positive (Aβ+) if the SUVR value was >1.4 in at least 1 of the 4 ROIs or as Aβ-negative (Aβ−) if the SUVR values were ≤1.4 for all 4 ROIs.28,32
Measurement of AD-signature neurodegeneration
All participants underwent 18F-fluorodeoxyglucose (FDG)-PET imaging with the abovementioned PET-MR machine. Details of the FDG-PET image acquisition and preprocessing were described previously.27 AD-signature FDG ROIs such as the angular gyri, posterior cingulate cortex, and inferior temporal gyri, which are sensitive to the changes associated with AD,32 were determined. AD-signature cerebral glucose metabolism (AD-CM) was defined as the voxel-weighted mean SUVR extracted from the AD-signature FDG ROIs. Details of MRI acquisition and preprocessing were described previously.27 AD-signature cortical thickness (AD-CT) was defined as the mean cortical thickness values obtained from AD-signature regions, including the entorhinal, inferior temporal, middle temporal, and fusiform gyrus, as described previously.32
Measurement of WMH
All participants underwent MRI scans with fluid-attenuated inversion recovery (FLAIR) using the abovementioned 3.0T PET-MR scanner. We followed the validated automatic procedure reported previously.33 Briefly, the procedure consisted of 11 steps: spatial coregistration of T1 and FLAIR images; fusion of T1 and FLAIR images; segmentation of T1; attainment of transformation parameters; deformation and obtainment of the white matter mask; obtainment of FLAIR within the white matter mask; intensity normalization of the masked FLAIR; nomination of candidate WMH with a designated threshold; creation of a junction map; and elimination of the junction. There were 2 modifications in the current processing procedure compared to the original study: (1) an optimal threshold of 70 was applied because it was more suitable for our data compared to the threshold of 65 used in the original study; and, (2) given that individuals with acute cerebral infarcts were not enrolled in our sample, we did not use diffusion-weighted imaging in the current automated procedure. With the use of the final WMH candidate image, the WMH volume was extracted in the native space in each subject.
Statistical analysis
Multiple linear regression analysis with serum albumin (continuous variable) as the independent variable and AD-neuroimaging parameters (Aβ deposition, AD-CM, AD-CT, and WMH) as the dependent variables was performed. Multiple logistic regression analysis with serum albumin (continuous variables or categorical variables, i.e., low albumin, middle albumin, and high albumin) as the independent variable and Aβ positivity as the dependent variable was also conducted. Three models were tested for stepwise control of potential confounders that could affect the relationships between albumin and AD biomarkers. The first model did not include any covariates. The second model included age (<70 vs ≥70 years), sex (female vs male), and APOE ε4 positivity (+/−) as covariates. The third model included all potential covariates: age, sex, APOE ε4, education, clinical diagnosis (CN vs MCI), medication use within 4 weeks (+/−), alcohol use (no/former/drinker), annual income, diabetes mellitus (+/−), hyperlipidemia (+/−), liver disease (+/−), and kidney disease (+/−), which have been considered possible confounders in previous studies.25,34–38 In the analysis, high albumin was used as a reference (i.e., high albumin vs middle albumin, or high albumin vs low albumin). As sensitivity analyses, the same analyses were performed for the subjects with no decrease in food intake over the past 3 months due to loss of appetite, digestive problems, or chewing or swallowing difficulties to eliminate any influence of physical or mental conditions that can potentially relate to both serum albumin level and brain status. Statistical analyses were performed with IBM SPSS Statistics 24 (IBM Corp, Armonk, NY). In all analyses, values of p < 0.05 were taken to indicate statistical significance.
Data availability
The data for this analysis are owned by the KBASE research group. Requests for data access can be submitted to the administrative coordinator of the group by e-mail (kbasecohort@gmail.com).
Results
Participant characteristics
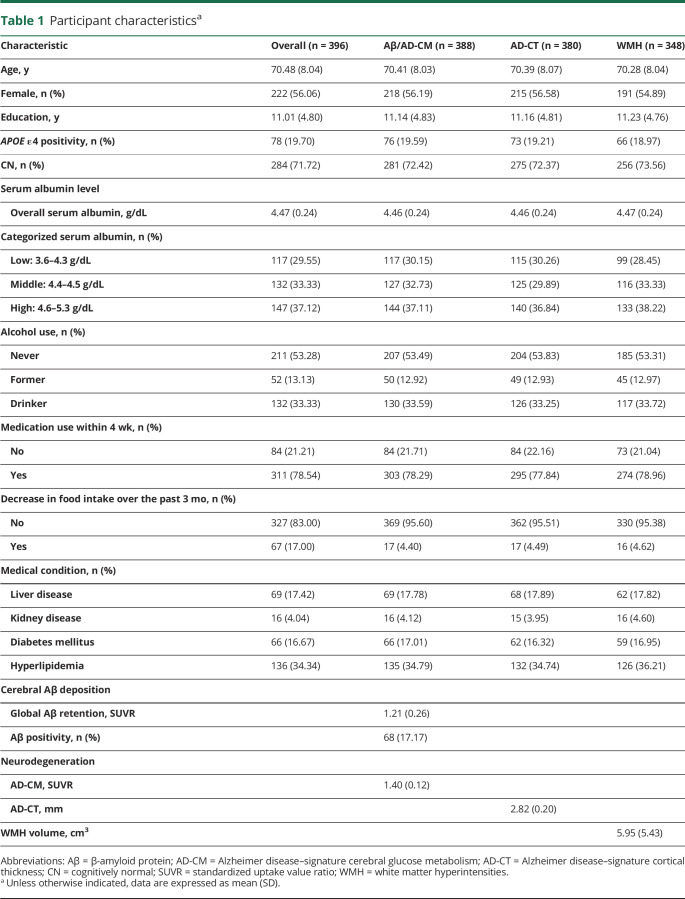
The demographic and clinical characteristics of the participants are presented in table 1. All participants had serum albumin levels within normal range. Among them, 117 were categorized as having low albumin, 132 as having middle albumin, and 147 as having high albumin group.
Table 1.
Participant characteristicsa
Association of serum albumin with cerebral amyloid deposition
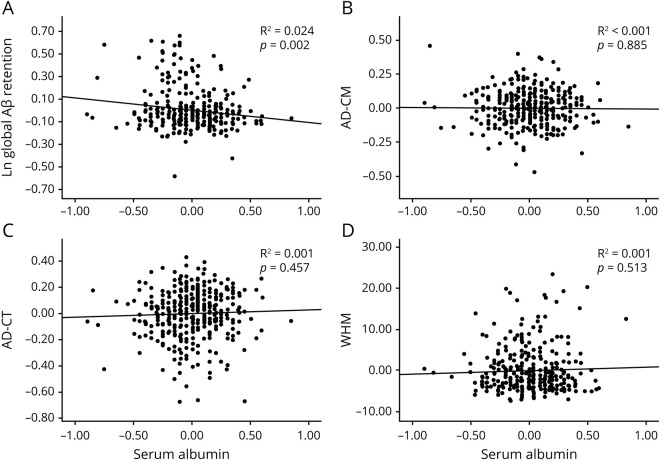
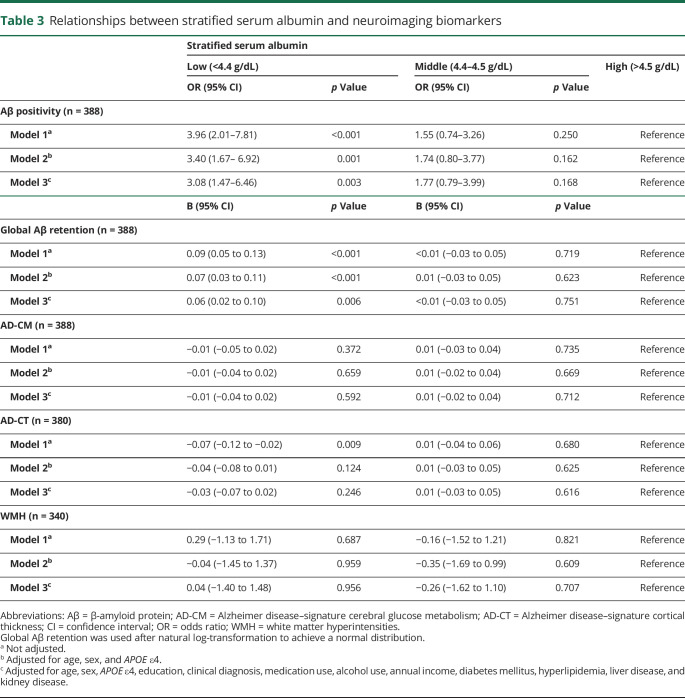
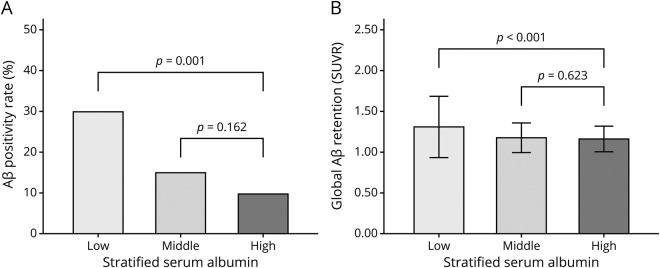
Serum albumin level (as a continuous variable) was significantly associated with global Aβ retention (table 2 and figure 1A) and Aβ positivity after controlling for the covariates (table 2). Furthermore, the low albumin category was significantly associated with higher Aβ positivity compared to the high albumin category after controlling for the covariates, while the middle albumin category showed no relation to Aβ positivity (table 3 and figure 2).
Table 2.
Relationships between serum albumin and neuroimaging biomarkers
Figure 1. Partial correlation plots for the relationships of serum albumin with neuroimaging biomarkers.
Multiple linear regression analysis was performed for (A) global Aβ retention, (B) AD-CM, (C) AD-CT, and (D) WHM after adjustment for age, sex, and APOE ε4. Global Aβ retention was used after natural log transformation to achieve a normal distribution. Aβ = beta-amyloid protein; AD-CM = Alzheimer disease–signature cerebral glucose metabolism; AD-CT = Alzheimer disease–signature cortical thickness; WMH = white matter hyperintensities.
Table 3.
Relationships between stratified serum albumin and neuroimaging biomarkers
Figure 2. Relationship of stratified serum albumin with Aβ deposition.
Multiple regression analyses were performed for (A) β-amyloid (Aβ) positivity and (B) global Aβ retention after adjustment for age, sex, and APOE ε4. Error bars represent standard deviation.
Association of serum albumin with AD-signature neurodegeneration and WMH
Serum albumin level (as a continuous variable) was not related to AD-CM, AD-CT, or WMH (table 2 and figure 1, B–D). Moreover, no differences were observed in AD-CM, AD-CT, or WMH between the serum albumin categories (table 3).
Sensitivity analyses
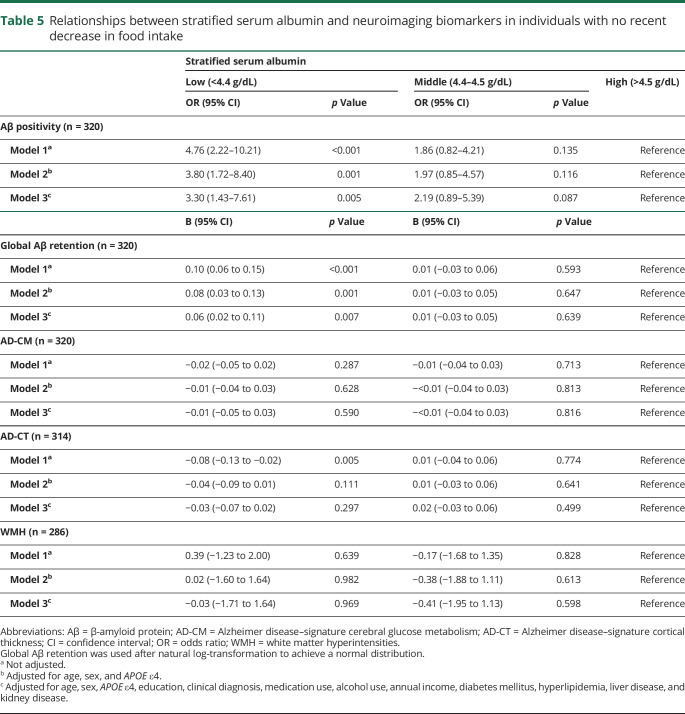
The same analyses for the individuals with no decrease in food intake over the past 3 months produced similar results for Aβ deposition (or Aβ positivity), AD-CM, AD-CT, and WMH (tables 4 and 5 and figure 3).
Table 4.
Relationships between serum albumin and neuroimaging biomarkers in individuals with no recent decrease in food intake
Table 5.
Relationships between stratified serum albumin and neuroimaging biomarkers in individuals with no recent decrease in food intake
Figure 3. Partial correlation plot for the relationship of serum albumin with global Aβ retention in individuals with no recent decrease in food intake.
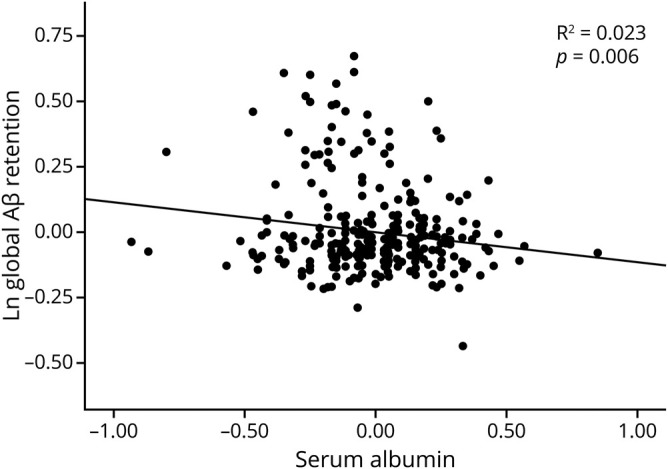
Multiple linear regression analysis was performed after adjustment for age, sex, and APOE ε4. Global β-amyloid (Aβ) retention was used after natural log-transformation to achieve a normal distribution.
Discussion
The results of the present study showed that low serum albumin was associated with an increased cerebral Aβ positivity rate compared to high serum albumin in older adults without dementia. The findings presented here were consistent with results of previous human studies on the relationships between serum albumin level and cognitive decline or AD dementia. One population-based longitudinal study showed that low albumin within the normal range was an independent risk marker for cognitive decline in community-living older adults.34 Previous clinical studies in hospital inpatients also showed that low serum albumin was associated with cognitive impairment11,39 and late-onset AD dementia compared with controls.14,40,41
The inverse association of serum albumin with Aβ deposition observed in the present study supports the so-called sink hypothesis,42,43 which suggests that Aβ shifts from the brain into the blood plasma, forming a decrease in the Aβ concentration gradient between the brain and blood plasma. In this process, serum albumin binds most of the Aβ in the blood plasma and, in turn, lowers the blood plasma Aβ concentration. Therefore, serum albumin plays an important role in the Aβ shift from the brain to the blood plasma for balancing the dynamic equilibrium of Aβ between the brain and blood plasma. Low serum albumin results in less binding to Aβ in blood, which increases blood plasma Aβ concentration, resulting in increased Aβ deposition in the brain by blocking the Aβ shift due to its lower concentration difference. An alternative hypothesis suggests that serum albumin is an endogenous inhibitor of Aβ fibril formation.7,10 Serum albumin may act on Aβ in 3 ways—monomer stabilizer, dissociation catalyst, or monomer competitor—and thus may prevent fibril formation. However, the possibility of reverse causality should also be considered. Poor nutrition in patients with dementia can reduce serum albumin level. This may explain the relationships between low serum albumin and AD dementia reported previously.14,15 Because all of our subjects were without dementia, however, reverse causality could not explain our observation of the relationship between albumin and Aβ deposition.
In terms of Aβ positivity risk, the odds ratio for low albumin category was 3.40 (95% confidence interval 1.67–6.92, p = 0.001) compared with the high albumin category. This means that, even within the normal range of serum albumin, individuals with serum albumin <4.4 g/dL have an ≈3 times higher risk of pathologic Aβ deposition. This finding also indicates that nutritional interventions such as high dietary protein or albumin replacement may be helpful for those with low serum albumin in terms of AD prevention or treatment. Recently, the Alzheimer's Management by Albumin Replacement (AMBAR) trial was conducted to demonstrate the clinical efficacy of therapeutic albumin replacement with plasma exchange as a new treatment of AD.44,45 Our result may provide an additional rationale for this kind of trial.
Unlike the relationship with Aβ, serum albumin was not related to either AD-signature neurodegeneration or WMH. While the relationships between low serum albumin and AD dementia have been reported as mentioned above, very little information is available on the relationships of serum albumin with degenerative changes in the brain.14,15 The lack of association between serum albumin and AD-CM or AD-CT in the present study indicated that serum albumin does not directly affect metabolism or structural changes in AD-related brain regions in older adults. We did not find any relationship between serum albumin and WMH. In addition, some human studies have reported an inverse association between serum albumin and cerebrovascular disease.16,17 The discrepancy may be related to the difference in range of serum albumin concentrations. While our subjects had serum albumin levels within the normal range, the previous studies mentioned above included subjects with serum albumin levels below the normal range (total levels 3.0–5.7 g/dL, low levels 3.0–3.9 g/dL in 1 study16; total levels 2.7–5.5 g/dL, low levels 2.7–4.2 g/dL in the other17; and total levels 3.6–5.3 g/dL, low levels 3.6–4.3 g/dL in our study). Therefore, serum albumin level below the normal range may increase cerebrovascular injury, as shown in the previous studies, while low serum albumin within the normal range may have no such effect.
The present study had several limitations that should be considered. First, because this was a cross-sectional study, we could not confirm a causal relationship between chronic albumin status in blood and brain Aβ deposition. Some findings support the long-term stability of serum albumin level in healthy older adults.46–49 We also excluded individuals with severe medical conditions that could affect mental function. In addition, the results were not changed even when the individuals with decreased food intake were excluded. Together, our results may support a long-term causal effect of serum albumin on brain Aβ deposition. Nevertheless, further long-term follow-up studies are required to clarify the causal relationships. In addition, the lack of repeated assessments of serum albumin level may have resulted in measurement errors because of diurnal variation.50 However, to minimize such errors, all blood samples for serum albumin measurement were obtained at the same time of the day (8–9 am) in all participants.
The findings of present study suggest that low serum albumin may increase the risk of AD dementia by elevating amyloid accumulation. In terms of AD prevention, more attention needs to be paid to avoid low serum albumin level, even within the clinical normal range, by clinicians.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- AD-CM
AD-signature cerebral glucose metabolism
- AD-CT
AD-signature cortical thickness
- AMBAR
Alzheimer's Management by Albumin Replacement
- CN
cognitively normal
- FDG
18F-fluorodeoxyglucose
- FLAIR
fluid-attenuated inversion recovery
- KBASE
Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer's Disease
- MCI
mild cognitive impairment
- MCL
minimum cost of living
- MR
magnetic resonance
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- WMH
white matter hyperintensities
Appendix 1. Authors
Appendix 2. Coinvestigators

Study funding
This study was supported by a grant from the Ministry of Science, ICT, and Future Planning, Republic of Korea (grant NRF-2014M3C7A1046042) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant HI18C0630 & HI19C0149).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Cummings JL. Alzheimer's disease. N Engl J Med 2004;351:56–67. [DOI] [PubMed] [Google Scholar]
- 2.Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med 1995;25:779–786. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter DC, Ho JX. Structure of serum albumin. Adv Protein Chem 1994;45:153–203. [DOI] [PubMed] [Google Scholar]
- 6.Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem 1996;271:32916–32922. [DOI] [PubMed] [Google Scholar]
- 7.Kuo YM, Kokjohn TA, Kalback W, et al. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun 2000;268:750–756. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem 2004;89:807–811. [DOI] [PubMed] [Google Scholar]
- 9.Stanyon HF, Viles JH. Human serum albumin can regulate amyloid-beta peptide fiber growth in the brain interstitium: implications for Alzheimer disease. J Biol Chem 2012;287:28163–28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milojevic J, Raditsis A, Melacini G. Human serum albumin inhibits Abeta fibrillization through a "monomer-competitor" mechanism. Biophys J 2009;97:2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuccala G, Marzetti E, Cesari M, et al. Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med 2005;118:496–502. [DOI] [PubMed] [Google Scholar]
- 12.Mizrahi EH, Blumstein T, Arad M, Adunsky A. Serum albumin levels predict cognitive impairment in elderly hip fracture patients. Am J Alzheimers Dis Other Demen 2008;23:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llewellyn DJ, Langa KM, Friedland RP, Lang IA. Serum albumin concentration and cognitive impairment. Curr Alzheimer Res 2010;7:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M, DeVos N, Wauters A, et al. Inflammatory markers in younger vs elderly normal volunteers and in patients with Alzheimer's disease. J Psychiatr Res 1999;33:397–405. [DOI] [PubMed] [Google Scholar]
- 15.Kim TS, Pae CU, Yoon SJ, et al. Decreased plasma antioxidants in patients with Alzheimer's disease. Int J Geriatr Psychiatry 2006;21:344–348. [DOI] [PubMed] [Google Scholar]
- 16.Xu WH, Dong C, Rundek T, Elkind MS, Sacco RL. Serum albumin levels are associated with cardioembolic and cryptogenic ischemic strokes: Northern Manhattan Study. Stroke 2014;45:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 1989;2:1434–1436. [DOI] [PubMed] [Google Scholar]
- 18.Byun MS, Yi D, Lee JH, et al. Korean brain aging study for the early diagnosis and prediction of Alzheimer's disease: methodology and baseline sample characteristics. Psychiatry Invest 2017;14:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 20.Lee DY, Lee KU, Lee JH, et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc 2004;10:72–81. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lee KU, Lee DY, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease assessment packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 2002;57:P47–P53. [DOI] [PubMed] [Google Scholar]
- 23.Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–122. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Eichner JE, Orchard TJ, Grandits GA, McCallum L, Tracy RP. The relation between serum albumin levels and risk of coronary heart disease in the Multiple Risk Factor Intervention Trial. Am J Epidemiol 1991;134:1266–1277. [DOI] [PubMed] [Google Scholar]
- 25.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology 2005;64:1371–1377. [DOI] [PubMed] [Google Scholar]
- 26.Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet 1991;337:1158–1159. [DOI] [PubMed] [Google Scholar]
- 27.Park JC, Han SH, Yi D, et al. Plasma tau/amyloid-beta1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer's disease. Brain 2019;142:771–786. [DOI] [PubMed] [Google Scholar]
- 28.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009;106:6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choe YM, Sohn BK, Choi HJ, et al. Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol Aging 2014;35:1519–1525. [DOI] [PubMed] [Google Scholar]
- 30.Westwood S, Baird AL, Hye A, et al. Plasma protein biomarkers for the prediction of CSF amyloid and tau and [(18)F]-Flutemetamol PET scan result. Front Aging Neurosci 2018;10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen L, Sobott F, De Deyn PP, Van Dam D. Signal loss due to oligomerization in ELISA analysis of amyloid-beta can be recovered by a novel sample pre-treatment method. MethodsX 2015;2:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol 2014;13:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai JZ, Peng SJ, Chen YW, et al. Automated segmentation and quantification of white matter hyperintensities in acute ischemic stroke patients with cerebral infarction. PLoS One 2014;9:e104011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng TP, Niti M, Feng L, Kua EH, Yap KB. Albumin, apolipoprotein E-epsilon4 and cognitive decline in community-dwelling Chinese older adults. J Am Geriatr Soc 2009;57:101–106. [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 2009;53:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota A, Kondo N, Murayama N, et al. Serum albumin levels and economic status in Japanese older adults. PLoS One 2016;11:e0155022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folsom AR, Ma J, Eckfeldt JH, Nieto FJ, Metcalf PA, Barnes RW. Low serum albumin: association with diabetes mellitus and other cardiovascular risk factors but not with prevalent cardiovascular disease or carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study Investigators. Ann Epidemiol 1995;5:186–191. [DOI] [PubMed] [Google Scholar]
- 38.Sui M, Jia X, Yu C, et al. Relationship between hypoalbuminemia, hyperlipidemia and renal severity in patients with lupus nephritis: a prospective study. Cent Eur J Immunol 2014;39:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattin L, Bordin P, Fonda M, et al. Factors associated with cognitive impairment among older Italian inpatients: Gruppo Italiano di Farmacovigilanza nell'Anziano (G.I.F.A.). J Am Geriatr Soc 1997;45:1324–1330. [DOI] [PubMed] [Google Scholar]
- 40.Hampel H, Kotter HU, Moller HJ. Blood-cerebrospinal fluid barrier dysfunction for high molecular weight proteins in Alzheimer disease and major depression: indication for disease subsets. Alzheimer Dis Assoc Disord 1997;11:78–87. [DOI] [PubMed] [Google Scholar]
- 41.Jeandel C, Nicolas MB, Dubois F, Nabet-Belleville F, Penin F, Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer's disease. Gerontology 1989;35:275–282. [DOI] [PubMed] [Google Scholar]
- 42.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science 2002;295:2264–2267. [DOI] [PubMed] [Google Scholar]
- 43.Boada M, Ortiz P, Anaya F, et al. Amyloid-targeted therapeutics in Alzheimer's disease: use of human albumin in plasma exchange as a novel approach for Abeta mobilization. Drug News Perspect 2009;22:325–339. [DOI] [PubMed] [Google Scholar]
- 44.Boada M, Lopez O, Nunez L, et al. Plasma exchange for Alzheimer's Disease Management by Albumin Replacement (AMBAR) trial: study design and progress. Alzheimers Dement 2019;5:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boada M, Ramos-Fernandez E, Guivernau B, et al. Treatment of Alzheimer disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: rationale and treatment approach of the AMBAR (Alzheimer Management by Albumin Replacement) study. Neurologia 2016;31:473–481. [DOI] [PubMed] [Google Scholar]
- 46.Campion EW, deLabry LO, Glynn RJ. The effect of age on serum albumin in healthy males: report from the Normative Aging Study. J Gerontol 1988;43:M18–M20. [DOI] [PubMed] [Google Scholar]
- 47.Leask RG, Andrews GR, Caird FI. Normal values for sixteen blood constituents in the elderly. Age Ageing 1973;2:14–23. [DOI] [PubMed] [Google Scholar]
- 48.Reed AH, Cannon DC, Winkelman JW, Bhasin YP, Henry RJ, Pileggi VJ. Estimation of normal ranges from a controlled sample survey, I: sex- and age-related influence on the SMA 12-60 screening group of tests. Clin Chem 1972;18:57–66. [PubMed] [Google Scholar]
- 49.Misra DP, Loudon JM, Staddon GE. Albumin metabolism in elderly patients. J Gerontol 1975;30:304–306. [DOI] [PubMed] [Google Scholar]
- 50.Andersen IB, Brasen CL, Christensen H, et al. Standardised resting time prior to blood sampling and diurnal variation associated with risk of patient misclassification: results from selected biochemical components. PLoS One 2015;10:e0140475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this analysis are owned by the KBASE research group. Requests for data access can be submitted to the administrative coordinator of the group by e-mail (kbasecohort@gmail.com).