Abstract
Background
Non-small cell lung cancer (NSCLC) is a common malignant tumor in humans. Long non-coding RNA (lncRNA) involved in cancer progression has been reported frequently. The objective of this study was to investigate the role of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and explore a novel mechanism in NSCLC development.
Materials and Methods
The expression of MALAT1, copper metabolism MURR1 domain-containing 8 (COMMD8) and microRNA-613 (miR-613) was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The protein levels of COMMD8, Cyclin D1, Ki67, B cell lymphoma/leukemia-2 (Bcl-2), Bcl-2 associated X protein (Bax), lactate dehydrogenase A (LDHA), CD63 and CD81 were determined by Western blot. Cell proliferation, the number of colonies and cell apoptosis were assessed by 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT), colony formation and flow cytometry assays, respectively. Glycolysis was distinguished based on glucose consumption, lactate production and LDHA activity. The role of MALAT1 in vivo was verified by animal experiments. The relationship between miR-613 and MALAT1 or COMMD8 was predicted by the bioinformatics tool starbase and verified by dual-luciferase reporter assay. The exosomes were isolated using the corresponding kit and identified by transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA).
Results
MALAT1 and COMMD8 were aberrantly upregulated in NSCLC tissues and cells. MALAT1 or COMMD8 knockdown blocked cell proliferation, colony formation and glycolysis but accelerated cell apoptosis in vitro. Besides, MALAT1 knockdown reduced tumor growth in vivo. We found that miR-613 was a target of MALAT1, and miR-613 could bind to the 3ʹ untranslated region (3ʹUTR) of COMMD8. MALAT1 regulated the expression of COMMD8 by absorbing miR-613. Moreover, the extracellular MALAT1 was transmitted by wrapping into exosomes.
Conclusion
MALAT1 promoted malignant activities of NSCLC cells through targeting miR-613/COMMD8 axis, and exosome-mediated transfer of NSCLC might be a novel approach for NSCLC treatment.
Keywords: MALAT1, COMMD8, miR-613, NSCLC, exosome
Introduction
Lung cancer is a global health burden and remains the major cause of cancer-related death.1 Lung cancer can be classified into two subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) according to its pathological histology.2 NSCLC accounts for 85% of lung cancer cases, containing adenocarcinoma, squamous cell carcinoma and large cell carcinoma.3 NSCLC is a molecularly heterogeneous disease, and patients are considered to be eligible for cure still maintaining a high recurrence rate.4 Progressive evidence indicates that chemotherapy is no longer the best treatment available for all patients, and targeted therapy has become an important means of disease management for patients with NSCLC.5 Currently, several oncogenes or tumor suppressor genes involved in the development of NSCLC have been extensively studied.2 The identification of novel biomarkers is of great significance for the cognition of NSCLC development and the targeted therapy of NSCLC.
Long non-coding RNA (lncRNA) is well known as non-coding RNA molecule with >200 nucleotides.6 LncRNA is an important regulator involved in a multitude of biological processes, and it has been gradually recognized that its dysfunction contributes to the initiation and progression of human cancers, including NSCLC.7,8 Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a widely studied lncRNA that participates in numerous cancers. It was initially characterized as a prognostic indicator of NSCLC.9 Subsequent reports revealed that MALAT1 regulated migration, colony formation, chemoresistance and other malignant behaviors in NSCLC cells, suggesting its carcinogenesis.10,11 However, its action mechanism is diverse and complex, which needs to be further elucidated in multiple approaches.
LncRNA can function in a variety of modes, including acting as sponges to inhibit the expression and function of microRNAs (miRNAs).12 MiRNAs are broadly known as non-coding RNAs with ~25 nucleotides, and miRNAs also play crucial roles in tumor initiation and development.13 Among these miRNAs, miR-613 was reported to be involved in numerous cancers, including NSCLC.14 However, the action mechanism of miR-613 in NSCLC was limitedly explored, and further regulatory networks of miR-613 need to be concluded.
Copper metabolism MURR1 domain-containing 8 (COMMD8) belongs to COMMD proteins that are pleiotropic factors existing in a wide range of eukaryotic organisms.15 COMMD proteins are widely conserved throughout evolution and play vital functions in intracellular membrane trafficking and transfection.16,17 Research on COMMD8 in cancers is rare. Interestingly, a previous study introduced the partial role and related action mechanism of COMMD8 in NSCLC.18 Overall, the evidence of COMMD8 function in cancers is still insufficient, and more research needs to be explored to reveal the role of COMMD8 in cancers.
The present study investigated the role of MALAT1 and COMMD8 on proliferation, colony formation, apoptosis and glycolysis in NSCLC cells and provided a novel action mechanism of MALAT1 associated with miR-613 and COMMD8. Besides, our study determined that MALAT1 was transmitted through NSCLC cell-derived exosomes. Our study enriched the role of MALAT1 and provided novel insights to understand the progression of NSCLC.
Materials and Methods
Tissue Collection
NSCLC patients (n=50) were recruited from Zaozhuang Tumor Hospital, and their tumor tissues and adjacent normal tissues were excised and collected for storage at −80°C. Informed consent was received from each patient, and all procedures got the approval of the Ethics Committee of Zaozhuang Tumor Hospital.
Cell Lines and Culture
NSCLC cell lines (A549 and H1299) and bronchial epithelial cell line (BEAS-2B) were purchased from BeNa Culture Collection (Suzhou, China) and maintained in 90% Roswell Park Memorial Institute 1640 (RPMI 1640; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen) and 90% Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen) containing 10% FBS (Invitrogen), respectively, in a humidified incubator containing 5% CO2 at 37°C.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA isolated from tissues and cells using the RNAiso Plus (Takara, Dalian, China) was examined using NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Then, Reverse Transcription Kit (Takara) and SYBR Premix Ex Taq (Takara) were used for the detection of MALAT1 and COMMD8. MiRNA First-Strand Synthesis Kit (Clontech, Mountain View, CA, USA) and miRNA qRT-PCR TB Green Kit (Clontech) were used for the detection of miR-613 under CFX96 System (Bio-Rad, Hercules, CA, USA). Internal reference genes were set using β-actin and U6. The normalized method was performed using 2−ΔΔct. The mentioned primers were list as follows:
MALAT1, sense: 5ʹ-GCTCTGTGGTGTGGGATTGA-3ʹ and anti-sense: 5ʹ-GTGGCAAAATGGCGGACTTT-3ʹ;
COMMD8, sense: 5ʹ-TGGCATTTGTGGTCGAGCTTA-3ʹ and anti-sense: 5ʹ-ACGTGCATCCATTCTTCTGATT-3ʹ;
β-actin, sense: 5ʹ-GCTTCTAGGCGGACTGTTAC-3ʹ and anti-sense: 5ʹ-CCATGCCAATGTTGTCTCTT-3ʹ;
miR-613, sense: 5ʹ-GTGAGTGCGTTTCCAAGTGT-3ʹ and anti-sense: 5ʹ-TGAGTGGCAAAGAAGGAACAT-3ʹ;
U6, sense: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and anti-sense: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
Western Blot
Total proteins were extracted using Radio Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China), and the proteins were loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). Then, the membranes were placed in skim milk for blockage and probed with the primary antibodies against COMMD8 (K12416; 1:500; bjbalb, Beijing, China), Cyclin D1 (ab16663; 1:200; Abcam, Cambridge, MA. USA), Ki67 (ab16667; 1:1000; Abcam), B cell lymphoma/lewkmia-2 (Bcl-2) (ab185002; 1:1000; Abcam), Bcl-2 associated X Protein (Bax) (ab32503; 1:1000; Abcam), lactate dehydrogenase A (LDHA) (ab125683; 1:1000; Abcam), CD63 (ab134045; 1:1000; Abcam), CD81 (ab109201; 1:1000; Abcam) and β-actin (ab8227; 1:2000; Abcam) at 4°C for overnight. After the probe of the goat-anti-rabbit secondary antibodies on the next day, the protein signals were emerged using the enhanced chemiluminescent reagent (Beyotime) under an imaging system (Bio-Rad).
Cell Transfection
For functional analysis, the expression of MALAT1 was depleted using the small interference RNA (siRNA) against MALAT1 (si-MALAT1) (Genechem; Shanghai, China), and its negative control (si-NC) acted as the comparison. Likewise, siRNA against COMMD8 (si-COMMD8) was used for COMMD8 knockdown (Genechem). COMMD8 overexpression plasmid (pcDNA-COMMD8) (Genechem) was also assembled, and pcDNA empty vector (pcDNA) acted as the comparison. For miR-613 reintroduction and inhibition, miR-613 mimics (miR-613), miRNA mimics negative control (miR-NC), miR-613 inhibitor and miRNA inhibitor negative control (inhibitor-NC) were purchased from Ribobio (Guangzhou, China). For animal experiment, stable MALAT1 knockdown was conducted using Lenti-short hairpin RNA against MALAT1 (sh-MALAT1) (Genechem) with sh-NC as the control. Subsequently, cell transfection was managed using Lipofectamine 3000 (Invitrogen) for 48 h.
Cell Proliferation Analysis
Transfected A549 and H1299 cells were loaded into 96-well plates (Corning Costar, Corning, NY, USA) at a density of 2×103 cells per well. Then a total of 10 µL 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) solution (Beyotime) was added into each well at the certain time points (24, 48 and 72 h), and cells were incubated for 4h at 37°C. Subsequently, 100 µL formazan solvent was added into each well to dissolve the formazan crystal. The optical density (OD) value at 490 nm was measured using Multiskan Ascent (Thermo Fisher Scientific).
Colony Formation Assay
Transfected A549 and H1299 cells were planted into 6-well plates at the density of 200 cells per well and maintained for 12 d. Afterwards, the cell medium was discarded, and the cells were immobilized with methanol and stained with crystal violet (Beyotime). The number of colonies was aggregated under a microscope (Olympus, Tokyo, Japan).
Cell Apoptosis Assay
The Annexin V-fluorescein isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis Detection Kit (KeyGen Biotech, Nanjing, China) was utilized to examine cell apoptosis. In brief, A549 and H1299 cells were reacted with 500 µL binding buffer, followed by infiltrating with 5 µL Annexin V-FITC and 5 µL PI for 15 min in the dark. Eventually, the apoptotic cells were monitored using Attune NxT Flow Cytometer (Invitrogen).
Glycolysis Process Assay
Glycolysis was investigated according to glucose consumption, lactate production and LDHA activity. Glucose consumption and lactate production was measured by Glucose Assay Kit (Biovision; Milpitas, CA, USA) and Lactate Assay Kit (Biovision) in line with the direction. LDH activity was examined using the LDH Kit (Beyotime) in line with the manufacturer’s protocol.
Animal Experiment
A total of 10 BALB/c nude mice (5-weeks-old, male) were purchased from Vitalriver Co., Ltd. (Beijing, China) and maintained in specific pathogen-free conditions. A549 cells transfected with sh-MALAT1 or sh-NC were subcutaneously inoculated into the right dorsal root of mice, dividing into the sh-MALAT1 group and the sh-NC group. After housing for 7 d, the tumor volume was recorded every 7 d using a vernier caliper according to the formula: length × width2 × 0.5. After 35 d, the mice were killed, and the tumor tissues were harvested for other experiments. Animal procedures were implemented with the authorization of the Animal Care and Use Committee of Zaozhuang Tumor Hospital, following the Guide for the Care and Use of Laboratory Animals (GB/T 35,892–2018).
Bioinformatics Analysis and Dual-Luciferase Reporter Assay
Online bioinformatics tool starbase (http://starbase.sysu.edu.cn/) was adopted to predict the target miRNAs of MALAT1 and analyze the interaction between miR-613 and COMMD8 3ʹ untranslated region (3ʹUTR).
The MALAT1 sequence fragments possessing the miR-613 binding sites were inserted into the pGL4 vector (Promega, Madison, WI, USA) to generate fusion plasmids, named as MALAT1-WT. Meanwhile, the same MALAT1 sequence fragments possessing the mutated miR-613 binding sites, named as MALAT1-MUT. The MALAT1-WT or MALAT1-MUT was introduced into A549 and H1299 cells transfected with miR-613 or miR-NC. At 48 h after transfection, the luciferase activity was investigated using Dual-Luciferase Reporter Assay System (Promega). For the verification of the relationship between COMMD8 and miR-613, wild-type COMMD8 3ʹUTR sequence fragment containing miR-613 binding site and mutant COMMD8 3ʹUTR sequence fragment containing mutated miR-613 binding site were amplified and cloned into pGL4 vector, and the recombinant plasmids were named as COMMD8 3ʹUTR-WT and COMMD8 3ʹUTR-MUT, respectively. The detection of luciferase activity were performed in line with the above details.
RNase A and Triton X 100 Treatment
A549 and H1299 cells were treated with 1 g/mL RNase A alone or the combination of 1 g/mL RNase A and 0.1% Triton X 100 at 37°C for 30 min, and blank treatment acted as the control. Then, qRT-PCR was conducted to ascertain the expression of MALAT1.
Exosome Isolation
Exosomes were isolated from A549 and H1299 cell culture supernatant using the MagCapture™ Exosome Isolation Kit PS (Wako, Tokyo, Japan) in accordance with the instructions. In brief, the culture medium was thawed on ice and centrifuged at 300×g for 5 min at 4°C to desert cells and cell debris. Then, the supernatant was collected and centrifuged at 1200×g for 20 min at 4°C to desert cell debris. Next, the supernatant was collected and centrifuged at 10,000×g for 30 min at 4°C. The supernatant obtained from 10,000×g centrifugation was exposed to exosomes binding enhancer and interacted with streptomycin affinity magnetic beads. Subsequently, the reactant was eluted with exosomes elution buffer. The final products containing exosomes were collected for identification.
Transmission Electron Microscopy (TEM)
The exosome pellets were placed on a carbon-coated 200-mesh copper electron microscopy grid extra-film for 5 min at room temperature. Then, the grid was removed and the excess liquid was expelled by touching the grid edge against a clean piece of filter paper. The grid was then placed in a 2% phosphotungstic acid with pH 7.0 for about 5 seconds, and the excess liquid was expelled. The grid was allowed to semi-dry for several minutes and then observed using a transmission electron microscope (Hitachi, Tokyo, Japan).
Nanoparticle Tracking Analysis (NTA)
NTA was performed to analyze the absolute size distribution and concentration of exosomes. In brief, exosomes were exposed to 1 mL PBS and then loaded into the sample chamber of the Nanosight NS300 (Nanosight, Malvern, UK). Data analysis was performed using NTA 2.1 software (Nanosight). In NTA, the particles were automatically tracked and underwent Brownian motion. Each sample was measured in triplicate at the camera, which recorded and tracked each visible particle. The number and size distribution of exosomes were subsequently determined using the Stokes-Einstein equation, and results are displayed as the exosomes concentration (particles/mL).
Statistical Analysis
All statistical analyses were proceeded using SPSS 20.0 software (SPSS Incorporation, Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD). The Student’s t-test or one-way ANOVA was used to evaluate the difference between 2 or over 3 variables. The Spearman correlation analysis was utilized to assess correlations between MALAT1, COMMD8 and miR-613. All experiments were repeated at least 3 times. The differences were considered statistically significant at P < 0.05.
Result
The Expression of MALAT1 and COMMD8 Was Elevated in NSCLC Tissues
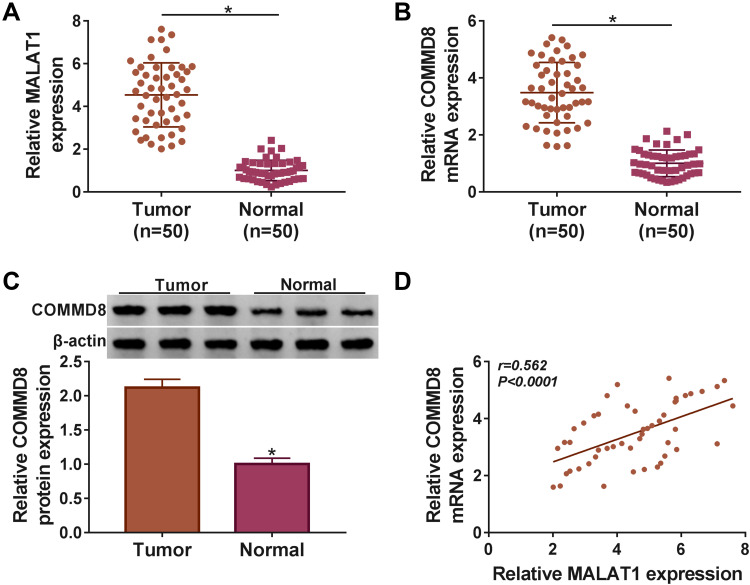
The expression of MALAT1 and COMMD8 was detected in NSCLC tissues to determine whether they functioned in NSCLC. Data from qRT-PCR presented that MALAT1 was significantly upregulated in NSCLC tissues (n=50) relative to adjacent normal tissues (n=50) (Figure 1A). The expression of COMMD8 was also enhanced in NSCLC tissues (n=50) compared with adjacent normal tissues (n=50) at both mRNA and protein levels (Figure 1B and C). Besides, we noticed that the expression of COMMD8 at the mRNA level was positively associated with the MALAT1 expression in NSCLC tissues (Figure 1D). The data showed that dysregulated MALAT1 and COMMD8 might play important parts in NSCLC.
Figure 1.
MALAT1 and COMMD8 were aberrantly upregulated in NSCLC tissues. (A) The expression of MALAT1 in tumor tissues (n=50) and normal tissues (n=50) was detected by qRT-PCR. (B and C) The expression of COMMD8 in tumor tissues (n=50) and normal tissues (n=50) was detected by qRT-PCR and Western blot at both mRNA and protein levels. (D) The correlation between the expression of COMMD8 and MALAT1 was depicted according to the Spearmen’s correlation coefficient. *P < 0.05.
MALAT1 Knockdown Inhibited Proliferation and Glycolysis and Induced Apoptosis of NSCLC Cells
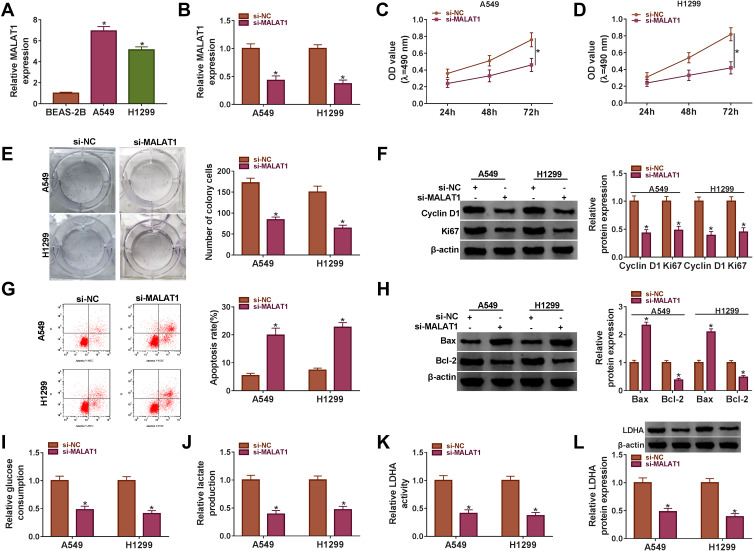
The endogenous level of MALAT1 was knocked down to investigate the role of MALAT1 in NSCLC. At first, we examined the expression of MALAT1 in NSCLC cells (A549 and H1299) and normal lung epithelial cells (BEAS-2B), and the result exhibited that the expression of MALAT1 in A549 and H1299 cell was notably higher than that in BEAS-2B cells (Figure 2A). Then, the interference efficiency of MALAT1 was checked, and we found that the expression of MALAT1 was obviously reduced in A549 and H1299 cells transfected with si-MALAT1 but not si-NC (Figure 2B). The proliferation of A549 and H1299 cells with si-MALAT1 transfection was inhibited compared with that with si-NC transfection (Figure 2C and D). The number of colonies was also decreased in A549 and H1299 cells transfected with si-MALAT1 relative to si-NC (Figure 2E). Next, the proliferation-associated protein markers (Cyclin D1 and Ki67) were quantified, and the data suggested that the protein levels of Cyclin D1 and Ki67 were both declined in A549 and H1299 cells with MALAT1 knockdown (Figure 2F). Oppositely, MALAT1 knockdown induced cell apoptosis (Figure 2G). The apoptosis-related proteins (Bax and Bcl-2) were also quantified, and the data suggested that MALAT1 knockdown promoted the level of Bax but dwindled the level of Bcl-2 in A549 and H1299 cells (Figure 2H). Moreover, MALAT1 knockdown suppressed glucose consumption and lactate production in A549 and H1299 cells (Figure 2I and J). The LDHA activity was inhibited in A549 and H1299 cells transfected with si-MALAT1 relative to si-NC, and the protein level of LDHA presented the same idea (Figure 2K and L). These data indicated that MALAT1 knockdown blocked the malignant processes in NSCLC cells.
Figure 2.
MALAT1 knockdown inhibited cell proliferation, colony formation and glycolysis but induced cell apoptosis. (A) The expression of MALAT1 in NSCLC cell lines (A549 and H1299) and normal lung cells (BEAS-2B) was measured using qRT-PCR. A549 and H1299 cells were transfected with si-MALAT1 or si-NC. (B) The interference efficiency was examined using qRT-PCR. (C and D) Cell proliferation was assessed by MTT assay. (E) The number of colonies was monitored according to the colony formation assay. (F) The protein levels of Cyclin D1 and Ki67 were determined by Western blot. (G) Cell apoptosis was observed using flow cytometry assay. (H) The protein levels of Bax and Bcl-2 were ascertained by Western blot. (I–K) The glycolysis processes were assessed according to glucose consumption, lactate production and LDHA activity. (L) The protein level of LDHA was determined by Western blot. *P < 0.05.
MALAT1 Knockdown Inhibited Tumor Growth in vivo
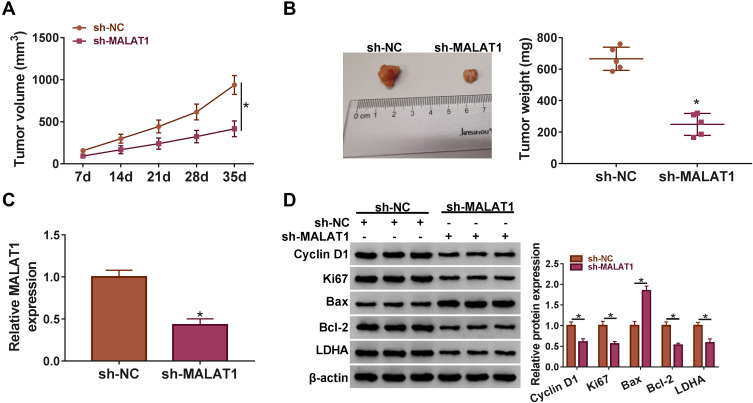
A549 cells transfected with sh-MALAT1 or sh-NC were subcutaneously injected into the nude mice to monitor the role of MALAT1 in vivo. The tumor volume was recorded once a week, and we found that the tumor volume was significantly reduced in the sh-MALAT1 group relative to sh-NC (Figure 3A). After 35 d, the excised tumor tissues were weighed, and we found that tumor size and tumor weight were notably reduced in the sh-MALAT1 group compared with that in the sh-NC group (Figure 3B). Besides, the expression of MALAT1 in the excised tumor tissues from the sh-MALAT1 group was declined compared with that from the sh-NC group (Figure 3C). Additionally, we examined the protein level of proliferation-, apoptosis- and glycolysis-related markers in these tissues. The data showed that Cyclin D1, Ki67, Bcl-2 and LDHA were all downregulated, while Bax was upregulated in tumor tissues from the sh-MALAT1 group compared to sh-NC (Figure 3D). The above data suggested that MALAT1 knockdown blocked tumor growth in vivo.
Figure 3.
MALAT1 knockdown inhibited tumor growth in vivo. (A) The tumor volume was recorded once a week. (B) The tumor weight was measured in excised tumor tissues. (C) The expression of MALAT1 was detected in excised tumor tissues by qRT-PCR. (D) The protein levels of Cyclin D1, Ki67, Bax, Bcl-2 and LDHA were determined by Western blot in removed tumor tissues. *P < 0.05.
COMMD8 Knockdown Also Inhibited Proliferation and Colony Formation, Induced Apoptosis and Blocked Glycolysis in NSCLC Cells
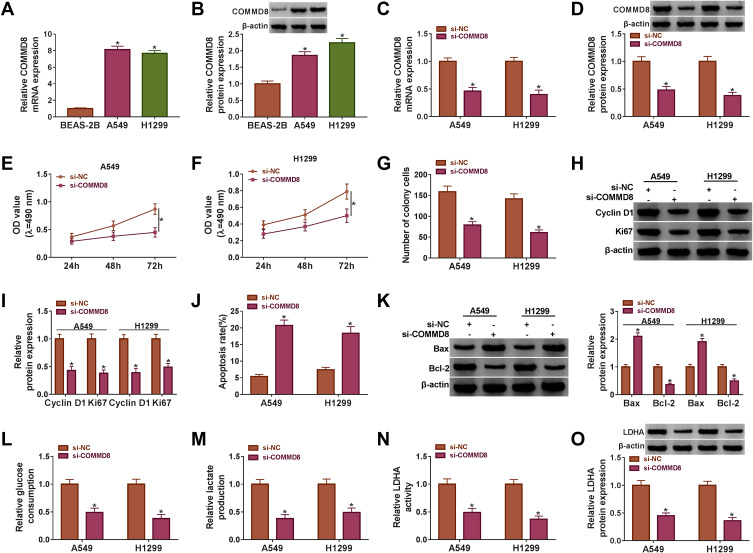
We also explored the role of COMMD8 in NSCLC cells. The expression of COMMD8 in A549 and H1299 cells was prominently increased compared with that in BEAS-2B cells at both mRNA and protein levels (Figure 4A and B). Then, the interference efficiency of COMMD8 was detected, and we discovered that the expression of COMMD8 was pronouncedly declined in A549 and H1299 cells transfected with si-COMMD8 but not si-NC at both mRNA and protein levels (Figure 4C and D). Noticeably, COMMD8 knockdown repressed the proliferation and the number of colonies of A549 and H1299 cells (Figure 4E-G). The protein levels of Cyclin D1 and Ki67 verified the above results and presented a markedly declined tendency in A549 and H1299 cells with COMMD8 knockdown (Figure 4H and I). Inversely, the apoptosis rate was elevated in A549 and H1299 cells transfected with si-COMMD8 relative to si-NC (Figure 4J). The protein level of Bax was strikingly strengthened, while the level of Bcl-2 was depleted with the knockdown of COMMD8 (Figure 4K). Moreover, the glucose consumption and lactate production were restrained in A549 and H1299 cells transfected with si-COMMD8 relative to si-NC (Figure 4L and M). The activity of LDHA and the protein level of LDHA were also inhibited with the COMMD8 knockdown (Figure 4N and O). The above data manifested that COMMD8 knockdown also suppressed the progression of NSCLC cells.
Figure 4.
COMMD8 knockdown blocked cell proliferation, colony formation and glycolysis but induced cell apoptosis. (A and B) The expression of COMMD8 in NSCLC cell lines (A549 and H1299) and normal lung cells (BEAS-2B) was measured using qRT-PCR and Western blot. A549 and H1299 cells were transfected with si-COMMD8 or si-NC. (C and D) The interference efficiency of COMMD8 was checked using qRT-PCR and Western blot. (E and F) Cell proliferation was evaluated by MTT assay. (G) The number of colonies was observed according to the colony formation assay. (H and I) The protein levels of Cyclin D1 and Ki67 were determined by Western blot. (J) Cell apoptosis was investigated using flow cytometry assay. (K) The protein levels of Bax and Bcl-2 were quantified by Western blot. (L–N) The glycolysis processes were assessed according to glucose consumption, lactate production and LDHA activity. (O) The protein level of LDHA was detected by Western blot. *P < 0.05.
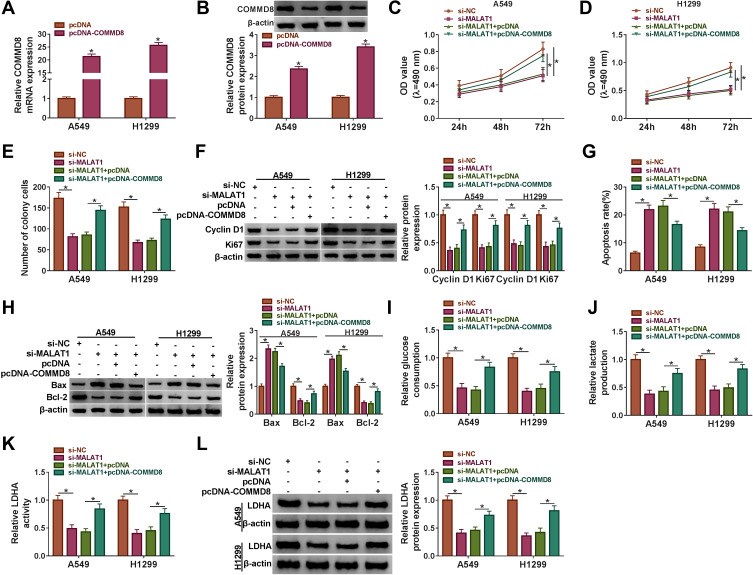
COMMD8 Overexpression Reversed the Role of MALAT1 Knockdown
To ascertain the relationship between COMMD8 and MALAT1, the rescue experiment was performed. The overexpression efficiency of COMMD8 was examined, and we found that the expression of COMMD8 was elevated in A549 and H1299 cells with pcDNA-COMMD8 transfection but not pcDNA transfection (Figure 5A and B). MALAT1 knockdown-inhibited cell proliferation and colony formation were recovered in A549 and H1299 cells transfected with si-MALAT1+pcDNA-COMMD8 (Figure 5C-E). The protein levels of Cyclin D1 and Ki67 were reduced by si-MALAT1 but restored by si-MALAT1+pcDNA-COMMD8 (Figure 5F). MALAT1 knockdown-induced cell apoptosis was repressed by the combination of MALAT1 knockdown and COMMD8 overexpression (Figure 5G). The protein level of Bax promoted by MALAT1 knockdown was regained by COMMD8 overexpression, while the level of Bcl-2 was opposite to the Bax level (Figure 5H). Furthermore, the glucose consumption and lactate production were blocked in A549 and H1299 cells transfected with si-MALAT1 but elevated in cells transfected with si-MALAT1+pcDNA-COMMD8 (Figure 5I and J). Moreover, the LDHA activity and LDHA level restrained by si-MALAT1 transfection were promoted by si-MALAT1+pcDNA-COMMD8 transfection in A549 and H1299 cells (Figure 5K and L). These data suggested that COMMD8 overexpression could rescue the role of MALAT1 knockdown.
Figure 5.
COMMD8 overexpression reversed the role of MALAT1 knockdown. A549 and H1299 cells were introduced with si-MALAT1 or si-MALAT1+pcDNA-COMMD8, si-NC or si-MALAT1+pcDNA serving as the control, respectively. (A and B) The overexpression efficiency of COMMD8 was ascertained by qRT-PCR and Western blot. (C and D) Cell proliferation was assessed by MTT assay. (E) The number of colonies was monitored according to the colony formation assay. (F) The protein levels of Cyclin D1 and Ki67 were determined by Western blot. (G) Cell apoptosis was observed using flow cytometry assay. (H) The protein levels of Bax and Bcl-2 were ascertained by Western blot. (I–K) The glycolysis processes were assessed according to glucose consumption, lactate production and LDHA activity. (L) The protein level of LDHA was determined by Western blot. *P < 0.05.
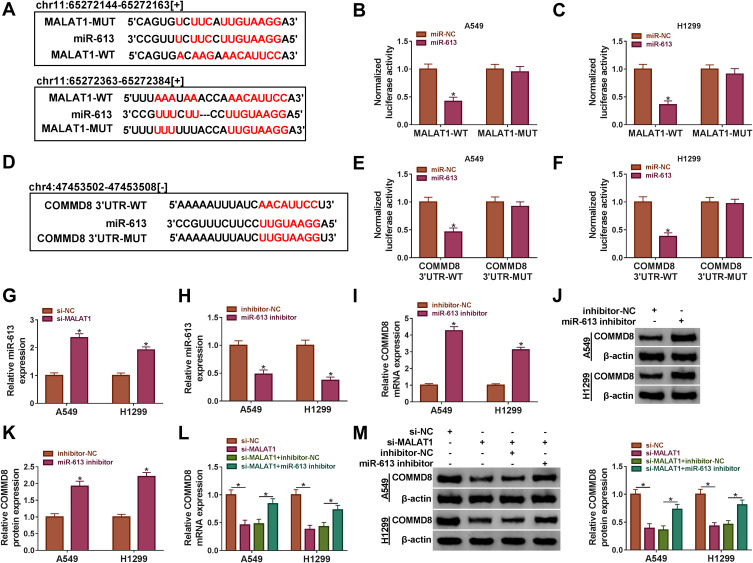
COMMD8 Was Modulated by MALAT1 Through miR-613
MALAT1 was forecasted to harbor the several binding sites with miR-613 by the online bioinformatics software starbase (http://starbase.sysu.edu.cn/) (Figure 6A). Besides, the dual-luciferase reporter assay confirmed this forecast and showed that the miR-613 enrichment notably diminished the luciferase activity in A549 and H1299 cells transfected with MALAT1-WT but not MALAT1-MUT compared with miR-NC (Figure 6B and C). Interestingly, COMMD8 was predicted as a target of miR-613 with a specific binding site at the 3ʹUTR of COMMD8 by starbase (Figure 6D). Likewise, this prediction was verified by the dual-luciferase reporter assay, and the result presented that miR-613 enrichment substantially dwindled the luciferase activity in A549 and H1299 cells transfected with COMMD8 3ʹUTR-WT but not COMMD8 3ʹUTR-MUT relative to miR-NC (Figure 6E and F). Moreover, the expression of miR-613 was rapidly elevated with the downregulation of MALAT1 in A549 and H1299 cells (Figure 6G). The examination of miR-613 inhibition efficiency showed that the expression of miR-613 was conspicuously decreased in A549 and H1299 cells transfected with miR-613 inhibitor relative to inhibitor-NC (Figure 6H). The inhibition of miR-613 remarkably strengthened the expression of COMMD8 at both mRNA and protein levels (Figure 6I-K). Next, A549 and H1299 cells were introduced with si-MALAT1 or si-MALAT1+miR-613 inhibitor, si-NC or si-MALAT1+inhibitor-NC acting as the control, respectively. The expression of COMMD8 was suppressed in cells transfected with si-MALAT1 but promoted in cells transfected with si-MALAT1+miR-613 inhibitor at both mRNA and protein levels (Figure 6L-M). These analyses demonstrated that MALAT1 sponged miR-613 to modulate the expression of COMMD8.
Figure 6.
MALAT1 targeted miR-613 to regulate the expression of COMMD8. (A) The binding sites between MALAT1 and miR-613 were forecasted by the online tool starbase. (B and C) The relationship between MALAT1 and COMMD8 was verified by dual-luciferase reporter assay. (D) COMMD8 was predicted to be a target of miR-613 with a specific binding site at its 3ʹUTR. (E and F) The relationship between miR-613 and COMMD8 was confirmed by dual-luciferase reporter assay. (G) The expression of miR-613 in A549 and H1299 cells transfected with si-MALAT1 or si-NC was detected by qRT-PCR. (H) The expression of miR-613 in A549 and H1299 cells transfected with miR-613 inhibitor or inhibitor-NC was detected by qRT-PCR. (I–K) The expression of COMMD8 in A549 and H1299 cells transfected with miR-613 inhibitor or inhibitor-NC was examined by qRT-PCR and Western blot at both mRNA and protein levels. (L and M) The expression of COMMD8 in A549 and H1299 cells transfected with si-MALAT1, si-NC, si-MALAT1+miR-613 inhibitor or si-MALAT1+inhibitor-NC was examined by qRT-PCR and Western blot at both mRNA and protein levels. *P < 0.05.
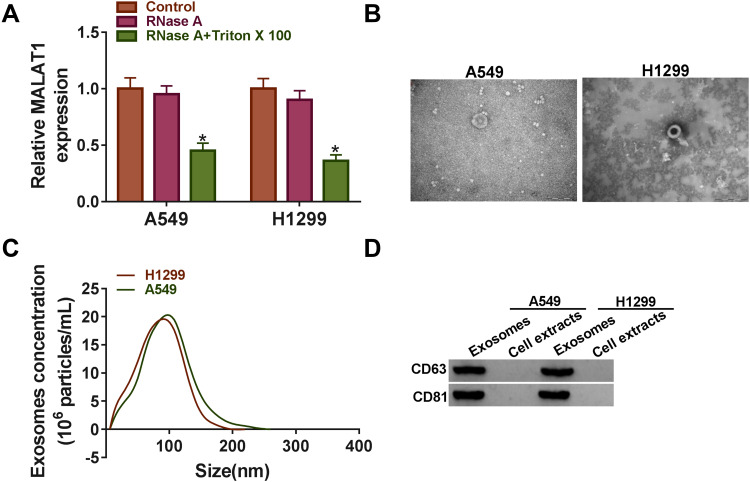
Extracellular MALAT1 Was Transferred Through Incorporation into Exosomes
Exosomes can be actively secreted from a variety of cell types.19 To determine whether MALAT1 was secreted through packaging into exosomes from A549 and H1299 cells, the expression of MALAT1 was detected after the treatment with RNase A in medium, and the result showed that the expression of MALAT1 in the culture medium had no noticeable change with the treatment of RNase A alone compared with Control, while the expression of MALAT1 was significantly reduced with the treatment of RNase A and Triton X 100, indicating that extracellular MALAT1 was mainly packed in membranes rather than direct secretion (Figure 7A). Then, the exosomes derived from A549 and H1299 cells were isolated and identified using transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) and Western blot analysis. The representative phenotype was observed with a typical lipid bilayer membrane (Figure 7B), and the size of exosomes isolated from A549 and H1299 cells was confirmed by TEM and NTA (Figure 7C). Moreover, the identity of exosomes was further verified by Western blot analysis, and the data showed that the exosome protein (CD63 and CD81) were abundantly expressed in exosomes but not cell extracts (Figure 7D). These data indicated that the extracellular MALAT1 could be released by packaging into exosomes.
Figure 7.
MALAT1 was secreted by packaging into exosomes. (A) The expression of MALAT1 in A549 and H1299 cell medium treated with RNase A or RNase A+Triton X 100 was measured using qRT-PCR. (B) The representative characteristic of exosomes was observed using TEM. (C) The size distribution of exosomes was analyzed by NTA using Zetasizer. (D) Exosome marker proteins (CD63 and CD81) were quantified by Western blot. *P < 0.05.
Discussion
Accumulating efforts in the past few years are conducive to understanding the progression and molecular mechanism of NSCLC.20 Currently, targeted therapy has aroused much attention for NSCLC treatment with less adverse reactions for patients.5 For example, epidermal growth factor receptor (EGFR) is a vital molecular target and serves as the first-line treatment opinion for NSCLC.5,21 However, the pathological features of NSCLC are incredibly complex, and there are a multitude of carcinogenic factors. Therefore, more biomarkers or predictive factors responded to targeted therapy should be identified to provide additional therapeutic opinions for NSCLC.
In our study, we noticed that MALAT1 was aberrantly overexpressed in NSCLC tissues and cell lines. MALAT1 was a common oncogene in different cancers, including ovarian cancer, breast cancer and colon cancer.22–24 The comprehensive role of MALAT1 and relevant action modes in NSCLC have been documented in numerous papers. For example, Schmidt et al held the view that MALAT1 was richly expressed in several NSCLC cell lines, and functional analysis revealed that MALAT1 contributed to cell migration and invasion in vitro and promoted tumor formation and growth in vivo.10,25 Consistent with the previous studies, we found that MALAT1 knockdown suppressed proliferation but induced apoptosis in vitro and impeded tumor growth in vivo. Besides, we also innovatively discovered that MALAT1 knockdown inhibited glycolysis metabolism, and this character of MALAT1 was mentioned in hepatocellular carcinoma in a former study,26 suggesting that glycolysis was an important pathway by which MALAT1 participated in cancer progression. Recently, the use of exosomes as biomarkers or therapeutic targets has attracted increasing interest, especially in cancer. Exosomes can protect lncRNAs or miRNAs from degradation in circulation and may be used for cancer surveillance at the early stage.27 Oncogenes (such as MALAT1) or tumor suppressor genes (such as miR-34c-3p) packaged into exosomes from NSCLC tumor cells directly mediate the effect of NSCLC cell on migration and invasion.28,29 Consistently, our data suggested that extracellular MALAT1 could be released by packing into exosomes, and exosomes containing MALAT1 might act as a valuable therapeutic tool for the diagnosis and treatment of NSCLC. In short, our findings greatly enriched the function of MALAT1 in NSCLC.
LncRNAs function at the post-transcriptional level through a variety of mechanisms, including as a precursor of miRNAs and other mediators, and jointly regulate mRNA expression with miRNAs.30 In our findings, miR-613 was identified as a linkup between MALAT1 and COMMD8. The underlying participation of COMMD8 in human cancers was rarely mentioned throughout the previous research. Ji et al highlighted the view that COMMD8 was aberrantly upregulated in hepatocellular carcinoma, and its restoration rescued the role of lncRNA MNX1-AS1 knockdown, leading to the aggressive properties of cancer cells.31 Crucially, Zhang et al mentioned the similar regulatory manner that COMMD8 was modulated by lncRNA LINC00657 through miR-26b-5p, and its overexpression stimulated proliferation and migration of NSCLC cells.18 In accordance with the existing research, we also observed a high abundance of COMMD8 in NSCLC tissues and cells, and its depletion suppressed malignant behaviors, leading to the inhibition of cell proliferation, colony formation and glycolysis in NSCLC cells. Besides, COMMD8 overexpression reversed the effects of MALAT1 knockdown, suggesting that COMMD8 played a carcinogenic role in NSCLC.
Conclusion
Collectively, the expression of MALAT1 and COMMD8 was abnormally elevated in NSCLC tissues and cells. MALAT1 exerted carcinogenic effects in NSCLC by upregulating the level of COMMD8 through competitively targeting miR-613. Besides, the tumorigenicity of MALAT1 was verified in vivo. Moreover, we found that the extracellular MALAT1 was released by packaging into exosomes. The evidence provides new insights into the treatment of NSCLC, and the MALAT1/miR-613/COMMD8 axis will be a promising pathway for future therapeutic opinion.
Funding Statement
No funding was received.
Highlights
MALAT1 and COMMD8 are aberrantly upregulated in NSCLC tissues and cells.
MALAT1 knockdown or COMMD8 knockdown inhibits proliferation, colony formation, glycolysis but promotes apoptosis of NSCLC cells.
The interaction between MALAT1 and COMMD8 is linked by miR-613.
MALAT1 knockdown represses the tumor growth in vivo.
Extracellular MALAT1 is released by packaging into exosomes.
Data Sharing Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the ethical review committee of Zaozhuang Tumor Hospital. Written informed consent was obtained from all enrolled patients.
Patient Consent for Publication
All patients agree to participate in this work.
Author Contributions
Conceptualization and Methodology: Tao Wang and Darui Liu; Formal analysis and Data curation: Darui Liu and Haiyang Kong; Validation and Investigation: Shouzhong Wang and Darui Liu; Writing - original draft preparation and Writing - review and editing: Shouzhong Wang, Tao Wang and Haiyang Kong; Approval of final manuscript: all authors. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Dorantes-Heredia R, Ruiz-Morales JM, Cano-Garcia F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5(4):401–412. doi: 10.21037/tlcr.2016.07.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165172. doi: 10.1016/S1470-2045(14)71180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farhat FS, Houhou W. Targeted therapies in non-small cell lung carcinoma: what have we achieved so far? Ther Adv Med Oncol. 2013;5(4):249–270. doi: 10.1177/1758834013492001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan Y, Zang H, Feng J, Lu J, Chen L, Fan S. Long non-coding RNAs associated with non-small cell lung cancer. Oncotarget. 2017;8(40):69174–69184. doi: 10.18632/oncotarget.20088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricciuti B, Mencaroni C, Paglialunga L, et al. Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33(2):18. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Zhang J, Wen L, Lin A. Membrane-lipid associated lncRNA: A new regulator in cancer signaling. Cancer Lett. 2018;419:(27–29. doi: 10.1016/j.canlet.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 9.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt LH, Spieker T, Koschmieder S, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6(12):1984–1992. doi: 10.1097/JTO.0b013e3182307eac [DOI] [PubMed] [Google Scholar]
- 11.Guo F, Guo L, Li Y, Zhou Q, Li Z. MALAT1 is an oncogenic long non-coding RNA associated with tumor invasion in non-small cell lung cancer regulated by DNA methylation. Int J Clin Exp Pathol. 2015;8(12):15903–15910. [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18(1):206. doi: 10.1186/s13059-017-1348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi: 10.1172/JCI84424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Li DQ, Liu D, Tang XJ. MiR-613 induces cell cycle arrest by targeting CDK4 in non-small cell lung cancer. Cell Oncol. 2016;39(2):139–147. doi: 10.1007/s13402-015-0262-4 [DOI] [PubMed] [Google Scholar]
- 15.Mao X, Gluck N, Chen B, et al. COMMD1 (copper metabolism MURR1 domain-containing protein 1) regulates Cullin RING ligases by preventing CAND1 (Cullin-associated Nedd8-dissociated protein 1) binding. J Biol Chem. 2011;286(37):32355–32365. doi: 10.1074/jbc.M111.278408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maine GN, Burstein E. COMMD proteins: cOMMing to the scene. Cell Mol Life Sci. 2007;64(15):1997–2005. doi: 10.1007/s00018-007-7078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy MD, Hospenthal MK, Hall RJ, et al. Structural insights into the architecture and membrane interactions of the conserved COMMD proteins. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Niu Z, Pei H, Peng Z. Long noncoding RNA LINC00657 induced by SP1 contributes to the non-small cell lung cancer progression through targeting miR-26b-5p/COMMD8 axis. J Cell Physiol. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Pefanis E, Wang J, Rothschild G, et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161(4):774–789. doi: 10.1016/j.cell.2015.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Ying S, Hu S, et al. EGFR TKIs impair lysosome-dependent degradation of SQSTM1 to compromise the effectiveness in lung cancer. Signal Transduct Target Ther. 2019;4:25. doi: 10.1038/s41392-019-0059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L, Wang A, Zhang Y, Xu X, Zhang X. Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer cells to cisplatin through inhibiting the Notch1 signaling pathway. Exp Cell Res. 2018;366(2):161–171. doi: 10.1016/j.yexcr.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Katsaros D, Biglia N, et al. High expression of long non-coding RNA MALAT1 in breast cancer is associated with poor relapse-free survival. Breast Cancer Res Treat. 2018;171(2):261–271. doi: 10.1007/s10549-018-4839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Meng WY, Jie Y, Zhao H. LncRNA MALAT1 induces colon cancer development by regulating miR-129-5p/HMGB1 axis. J Cell Physiol. 2018;233(9):6750–6757. doi: 10.1002/jcp.26383 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt LH, Gorlich D, Spieker T, et al. Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 lncRNA in non-small-cell lung cancer. J Thorac Oncol. 2014;9(9):1294–1304. doi: 10.1097/JTO.0000000000000243 [DOI] [PubMed] [Google Scholar]
- 26.Malakar P, Stein I, Saragovi A, et al. Long Noncoding RNA MALAT1 Regulates Cancer Glucose Metabolism by Enhancing mTOR-Mediated Translation of TCF7L2. Cancer Res. 2019;79(10):2480–2493. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Shao Y, Zhang X, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36(3):2007–2012. doi: 10.1007/s13277-014-2807-y [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Xia Y, Wang Z, et al. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490(2):406–414. doi: 10.1016/j.bbrc.2017.06.055 [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Yan Y, Liu Y, et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin alpha2beta1. Signal Transduct Target Ther. 2020;5(1):39. doi: 10.1038/s41392-020-0133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan M, Chen J, Zhang D. Exploring the secrets of long noncoding RNAs. Int J Mol Sci. 2015;16(3):5467–5496. doi: 10.3390/ijms16035467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D, Wang Y, Sun B, Yang J, Luo X. Long non-coding RNA MNX1-AS1 promotes hepatocellular carcinoma proliferation and invasion through targeting miR-218-5p/COMMD8 axis. Biochem Biophys Res Commun. 2019;513(3):669–674. doi: 10.1016/j.bbrc.2019.04.012 [DOI] [PubMed] [Google Scholar]