Abstract
Objective
To summarize the published evidence regarding the association between maternal infection during pregnancy and childhood leukemia.
Study design
In this systematic review and meta-analysis (PROSPERO number, CRD42018087289), we searched PubMed and Embase to identify relevant studies. We included human studies that reported associations of at least one measure of maternal infection during pregnancy with acute lymphoblastic leukemia (ALL) or all childhood leukemias in the offspring. One reviewer extracted the data first using a standardized form, and the second reviewer independently checked the data for accuracy. Two reviewers used the Newcastle-Ottawa Scale to assess the quality of included studies. We conducted random effects meta-analyses to pool the ORs of specific type of infection on ALL and childhood leukemia.
Results
This review included 20 studies (ALL, n = 15; childhood leukemia, n = 14) reported in 32 articles. Most (>65%) included studies reported a positive association between infection variables and ALL or childhood leukemia. Among specific types of infection, we found that influenza during pregnancy was associated with higher risk of ALL (pooled OR, 3.64; 95% CI, 1.34–9.90) and childhood leukemia (pooled OR, 1.77; 95% CI, 1.01–3.11). Varicella (pooled OR, 10.19; 95% CI, 1.98–52.39) and rubella (pooled OR, 2.79; 95% CI, 1.16–6.71) infections were also associated with higher childhood leukemia risk.
Conclusions
Our findings suggest that maternal infection during pregnancy may be associated with a higher risk of childhood leukemia.
Leukemia, the most common cancer in children, accounts for about one-third of all childhood cancers worldwide.1 Evidence has shown that acquired genetic mutations that initiate childhood leukemia occur in utero.2 Factors affecting genetic stability and cell growth pathways in the fetus may be responsible for a significant proportion of childhood leukemia.
Maternal infection during pregnancy has long been a suspected risk factor for childhood leukemia.3,4 Infectious agents with oncogenic potential may transfer from mother to fetus, leading to genomic instability.5 Alternately, fetal infection may lead to immune tolerance because the adaptive immune response in the fetus is immature. This tolerance would allow the long-term persistence of the virus and proliferation of infected cells, resulting in a high viral load.6,7 Maternal infection may also affect the development of the immune system in the fetus without transplacental transmission.8 Levels of several cytokines at birth are different between children who develop acute lymphoblastic leukemia (ALL) and matched controls,9,10 suggesting a role for dysregulated immune function at birth in the development of leukemia. There are, therefore, several plausible mechanisms that might explain the contribution of a specific type of maternal infection or infection in general to childhood leukemia.
The epidemiologic evidence for the association between maternal infection during pregnancy and childhood leukemia has accumulated steadily over the past 6 decades, but with inconsistent findings. Investigated types of infections range from specific pathogens (eg, cytomegalovirus [CMV]) to more general systemic infection (eg, urinary tract infection), based on data collected using laboratory tests, self-report, or medical records. A narrative review published in 2013 found that 11 of 16 articles reported that maternal infection was associated with an increased risk of childhood leukemia.11 Other reviews of the association with infection at any point before childhood leukemia have only highlighted a small number of studies on the contribution of maternal infection.12–14 However, there has been no systematic review or meta-analysis of this association. Furthermore, evidence from studies using laboratory techniques (eg, examining viral DNA15–17) has not been summarized and reviewed. We, therefore, conducted a systematic review and meta-analysis to determine whether any maternal infection or specific types of infection during pregnancy was associated with childhood leukemia risk in the offspring.
Methods
The protocol of this systematic review and meta-analysis was registered in PROSPERO (CRD42018087289). We followed the PRISMA guidelines. Because ALL is the largest subgroup of childhood leukemia and relatively homogenous with regards to cell lineage, we used ALL as the primary outcome and all childhood leukemias (not categorized) as the secondary outcome.
We searched PubMed and Embase from inception through January 16, 2018, without language restriction. The search terms and strategy are shown in Table I (available at www.jpeds.com). We also screened the reference lists of included studies. All identified items were imported into Covidence (Veritas Health Innovation, Melbourne, Victoria, Australia), a systematic review software. An updated search was performed through July 17, 2018, with no additional eligible studies identified.
Table I.
Search term in PubMed and EMBASE
| Nos. | Search terms |
|---|---|
| PubMed (searched on 16 January 2018) | |
| #1 | “Bacterial Infections and Mycoses”[Mesh] OR “Virus Diseases”[Mesh] OR “Parasitic Diseases”[Mesh] OR Infect*[TIAB] OR bacteri*[TIAB] OR virus[TIAB] OR viral[TIAB] OR fungus[TIAB] OR fungi[TIAB] OR fungal[TIAB] OR parasite*[TIAB] |
| #2 | “Maternal Exposure”[Mesh] OR maternal[TIAB] |
| #3 | intrauterine[TIAB] OR “in utero”[TIAB] OR prenatal[TIAB] OR antenatal[TIAB] |
| #4 | “Pregnancy”[Mesh] OR “Pregnant Women”[Mesh] OR “Pregnancy Trimesters”[Mesh] OR pregnan* [TIAB] OR gestation*[TIAB] OR conception*[TIAB] OR trimester*[TIAB] |
| #5 | “Fetus”[Mesh] OR fetus*[TIAB] OR foetus*[TIAB] OR foetal*[TIAB] OR fetal*[TIAB] |
| #6 | “Fetal Blood”[Mesh] OR “Dried Blood Spot Testing”[Mesh] OR cord blood*[TIAB] OR Guthrie card*[TIAB] OR blood spot*[TIAB] |
| #7 | #2 OR #3 OR #4 OR #5 OR #6 |
| #8 | #1 AND #7 |
| #9 | “Leukemia”[Mesh] OR leukemia[TIAB] OR leukemia[TIAB] |
| #10 | “Infant”[Mesh] OR “Child”[Mesh] OR “Adolescent”[Mesh] OR newborn*[TIAB] OR neonat*[TIAB] OR infan*[TIAB] OR toddler*[TIAB] OR child*[TIAB] OR adolescen*[TIAB] OR juvenile[TIAB] OR teen*[TIAB] OR girl*[TIAB] OR boy*[TIAB] OR youth*[TIAB] OR paediatric*[TIAB] OR pediatric*[TIAB] |
| #11 | #9 AND #10 |
| #12 | #8 AND #11 |
| EMBASE via Ovid (1974 to 15 January 2018) | |
| #1 | exp infection/ or (infect* or bacteri* or virus or viral or fungus or fungi or fungal or parasite*).tw. |
| #2 | maternal exposure/ or maternal.tw. |
| #3 | prenatal exposure/ or prenatal period/ or (intrauterine or in utero or prenatal or antenatal).tw. |
| #4 | exp pregnancy/ or exp conception/ or pregnant woman/ or (pregnan* or gestation* or conception* or trimester*).tw. |
| #5 | fetus/ or (fetus* or foetus* or foetal* or fetal*).tw. |
| #6 | fetus blood/ or umbilical cord blood/ or dried blood spot testing/ or (cord blood* or Guthrie card* or blood spot*).tw. |
| #7 | 2 or 3 or 4 or 5 or 6 |
| #8 | 1 and 7 |
| #9 | exp leukemia/ or (leukemia or leukemia).tw. |
| #10 | exp juvenile/ or (newborn* or neonat* or infan* or toddler* or child* or adolescen* or juvenile or teen* or girl* OR boy* or youth* or paediatric* or pediatric*).tw. |
| #11 | 9 and 10 |
| #12 | 8 and 11 |
We included studies that were conducted in humans, reported associations between ≥1 measure of maternal infection during pregnancy, and used ALL or childhood leukemia as the outcome in the offspring up to the age of 19 years. Maternal infection during pregnancy can be measured using self-reported questionnaires, medical records, or biospecimens (eg, maternal blood during pregnancy, cord blood, or neonatal dried blood spot). It is possible that any infectious agent detected by dried blood spot was acquired after birth. However, because most causes of early onset neonatal sepsis (ie, clinical infection in the first week of life) are acquired from the mother either in utero or during the birth process, it was felt that infections detected on dried blood spot were most likely to be from maternal infection rather than postnatal exposures. We excluded studies that (1) were reviews, comments, case reports, or conference abstracts, (2) did not measure maternal infection at the individual level (eg, ecological study), (3) only reported results for maternal infection at times other than during pregnancy, (4) used indirect markers for maternal infections (eg, antimicrobial use, vaccination), (5) were restricted to children of specific subgroups (eg, children with trisomy 21), or (6) did not have a full-text article in English. Conference abstracts18 were excluded from the final review and analysis, but were used to trace relevant full reports via additional searches or by contacting the authors. We also tracked full reports for research letters.19
The authors first screened titles and abstracts independently to identify potentially relevant papers, and then assessed the full text for eligibility. Any disagreement was resolved by discussion between the 2 reviewers and/or a third reviewer.
Data Extraction and Quality Assessment
One reviewer extracted the data first using a standardized form, and the second reviewer independently checked the data for accuracy. Disagreements were resolved by discussion between the 2 reviewers and/or a third reviewer. Extracted data items included authors, publication year; study design, study period, sources of participants, matching variables; sample size, country of origin, age at diagnosis of childhood leukemia; type, timing, and measurement method of maternal infection; type (ALL and/or childhood leukemia) and ascertainment method of leukemia; effect size (point estimate and 95% CI) and adjustment variables. When the effect estimate was not reported, we collected raw data of cell counts (details in the Appendix; available at www.jpeds.com). Duplicated publications were identified based on the description of study population, data source, study period, and authors. When multiple articles from a study were identified on the same exposure and outcome variables, only the report with the largest sample size was included; however, if the study samples were independent, they were treated as separate studies.20,21 When multiple reports from the same study reported different exposures (infections), data were first extracted from each report separately and then linked across multiple reports. These multiple reports of a study were treated as a single study. Authors of 7 studies reported in 14 articles were contacted for additional information or clarification,17,20,22–33 and 5 studies17,22,25–33 responded, among which 2 provided clarification.17,25–31
Two reviewers used the Newcastle-Ottawa Scale (NOS) to assess the quality of included studies. Consisting of 9 items, the NOS assesses the following domains34: selection (4 items), comparability (2 items), and ascertainment of exposure/outcome (3 items). One star is awarded if the study meets 1 assessment item. We used birth date and maternal socioeconomic status for comparability assessment. High-quality studies were defined as those with a NOS score of ≥7, medium-quality as a score of 4–6, and low-quality as a score of ≤3.
Statistical Analyses
Based on the potential mechanisms we mentioned elsewhere in this article, it is possible that both infections in general and specific types of infection can contribute to childhood leukemia. We performed qualitative and quantitative syntheses depending on the infection variables examined to examine the possible broad effects (including immunologic response) of maternal infection; we used an aggregate variable referred to as a “proxy for any infection”. For those studies that reported effect estimates for more than one infection, we included only the infection variable with the highest prevalence in that study as a proxy for any infection. In studies where the association was reported only for a specific infection, we included this infection variable as proxy for any infection in the meta-analysis. Owing to substantial differences in the definition of proxy for any infection and high heterogeneity in effect estimates across studies, a formal meta-analysis was not appropriate; hence, we only qualitatively summarized results for this exposure variable. For each specific type of infection, we conducted quantitative synthesis (meta-analyses) if ≥2 studies presented effect estimates. When studies reported multiple types of immunoglobulins, we selected IgM as the measure of the specific infection given high level of IgM represents recent occurrence of infection (details in the Appendix). We used random effect models (DerSimonian-Laird method35) to pool study-level effect measure of association between specific infections and childhood leukemia (ALL and childhood leukemia separately). We used the Cochran Q test and the I2 statistic to assess the heterogeneity across studies. A P value of <.10 for the Cochran Q test was used to indicate heterogeneity. An I2 value of ≥50% suggests substantial heterogeneity.36
We used the ORs to perform data syntheses (qualitative and quantitative syntheses) as it was the only effect measure reported in included studies. If the OR was not reported, we used data of cell counts, constructed 2 × 2 tables and calculated the crude OR. We added 0.5 to all cells before OR calculation when there was a null value in 1 of the 4 cells. Two studies, 1 for ALL24 and 1 for childhood leukemia,23 were excluded from the meta-analysis because the effect estimates and/or 95% CIs was not reported and could not be computed. An additional study was excluded from the data synthesis for childhood leukemia because of its low quality.37 When ≥2 types of controls were reported, we only used results for the one that was closest to the concept of population-based controls (community controls rather than cancer controls24) or, alternatively, with the results with the best matching criteria.
Prespecified subgroup analyses were performed based on exposure measurement method (self-report, medical record, or laboratory test), study design (case-control, nested case-control, or cohort study), study region (Europe, North America, or others), and study quality (high or medium quality). Differences in effect estimates across subgroups were explored by random effects meta-regression. Prespecified sensitivity analysis was performed by excluding studies with less than ten subjects with exposure/outcome. We also repeated the meta-analysis by excluding studies that included cases >19 years of age or that combined the exposure periods 3 months before pregnancy and pregnancy. We used the Egger bias test38 and a funnel plot to assess the publication bias. Subgroup analysis, sensitivity analysis, and publication bias analysis were performed for the most frequently reported type of infection (ie, influenza). We did not conduct subgroup, sensitivity or publication bias analyses for the other specific types of infection due to the small number of studies. All analyses were performed using STATA software (version 14.0, Stata Corp LP, College Station, Texas).
Results
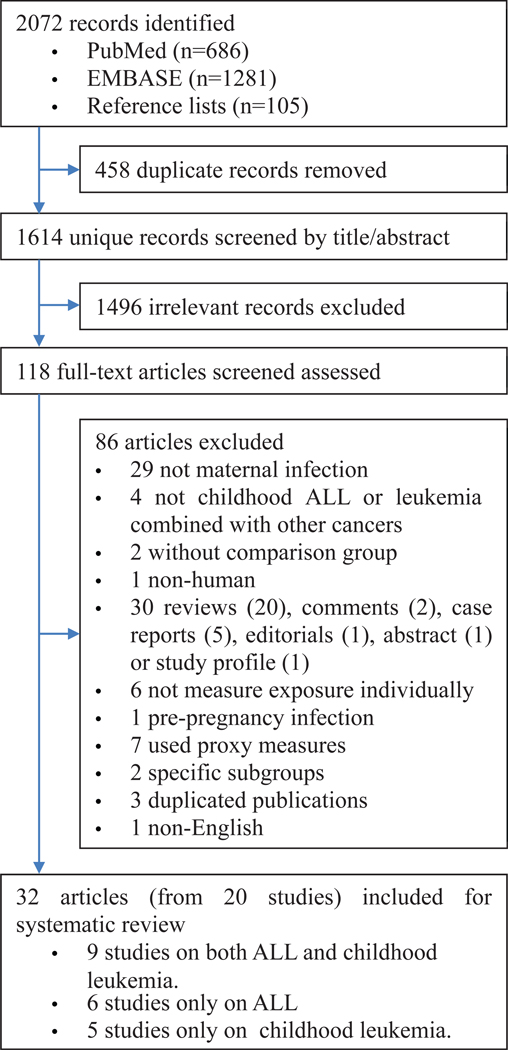
Of 2072 records identified, 118 were eligible for full-text assessment. After further excluding 86 articles based on our exclusion criteria, the remaining 32 articles3,4,15–17,20–33,37,39–50 consisted of 20 independent studies included in this review (Figure 1; available at www.jpeds.com). A selection of excluded studies and the reasons for exclusion are listed in Table II (available at www.jpeds.com).
Figure 1.
Study selection flow chart.
Table II.
A selection of excluded studies and the corresponding exclusion reasons
| Nos. | Citation | Exclusion reasons |
|---|---|---|
| 1 | Influenza during pregnancy in relation to subsequent childhood leukemia and lymphoma. Am J Epidemiol 1974;100:399–409. | Not measure at the individual level |
| 2 | Maternal health conditions during pregnancy and acute leukemia in children with Down syndrome: A Children’s Oncology Group study. Pediatr Blood Cancer 2009;52:602–8. | Restricted to children of specific subgroups |
| 3 | Childhood cancer in relation to infections in the community during pregnancy and around the time of birth. Int J Cancer 2003;104:772–7. | Not measure at the individual level |
| 4 | Markers of infection, breast-feeding and childhood acute lymphoblastic leukemia. Br J Cancer 2000;83:1559–64. | Used indirect markers for maternal infections |
| 5 | Association between influenza during pregnancy and childhood leukemia. Br Med J 1973;4:265–7. | Not measure at the individual level |
| 6 | Antibiotic use from conception to diagnosis of child leukemia as compared to the background population: a nested case-control study. Pediatr Blood Cancer 2015;62:1155–61. | Used indirect markers for maternal infections |
| 7 | Letter: sequelae of virus infection in pregnancy. Br Med J 1974;2:502. | Duplicated publication |
| 8 | Childhood leukemia and maternal infectious diseases during pregnancy. J Natl Cancer Inst 1974;53:943–7. | Not measure at the individual level |
| 9 | Childhood and maternal infections and risk of acute leukemia in children with Down syndrome: a report from the Children’s Oncology Group. Br J Cancer 2004;91:1866-72. | Restricted to children of specific subgroups |
| 10 | Prescription drug use during pregnancy and risk of childhood cancer - is there an association?. Cancer Epidemiol 2015;39:8–73. | Used indirect markers for maternal infections |
| 11 | Foetal infection, childhood leukemia and cancer. Br J Cancer 1983;48:849–52. | Used indirect markers for maternal infections |
| 12 | Childhood leukemia and mother-foetus infection. Br J Cancer 1980;42:158–61. | Maternal infection that did not occur during pregnancy |
| 13 | Maternal use of antibiotics and cancer in the offspring: results of a case-control study in Germany. Cancer Causes Control 2010;21:1335–45. | Used indirect markers for maternal infections |
| 14 | Excess leukemia in cohorts of children born following influenza epidemics. Am J Epidemiol 1975;101:77–83. | Not measure at the individual level |
| 15 | Incidence of neoplasms in children born after influenza epidemics. Br Med J 1972;4:631–4. | Not measure at the individual level |
| 16 | Malignant disease in children whose mothers had chickenpox, mumps, or rubella in pregnancy. Br Med J 1972;4:629–31. | Duplicated publication |
| 17 | Sequelae of virus infection in pregnancy. Child Health 1976;31:72–83. | Duplicated publication |
| 18 | Childhood cancers and their association with pregnancy drugs and illnesses. Paediatr Perinat Epidemiol 1989;3:66–94. | Combined with other childhood cancers |
Characteristics of Included Studies
The characteristics of 20 studies (32 individual publications) are shown in Table III. Of these, 13 were case-control, 4 nested case-control, and 3 cohort studies. Twelve studies used nonlaboratory data (self-report or medical records) for exposure assessment, 6 used laboratory data (DNA or immunoglobulin presence in maternal serum during pregnancy or neonatal blood spot at birth), and 2 used both nonlaboratory and laboratory data depending on the type of infection investigated. A total of 3559 ALL cases and 79 619 non-ALL controls (from 15 studies), 7115 childhood leukemia cases and 88 611 non-childhood leukemia controls (from 14 studies) were included. Out of a maximum of 9, the NOS scores of the 32 articles published from the 20 studies, ranged from 3 to 8 (median, 7): 17 articles were high quality (score of ≥7), 14 medium quality (score of 4–6), and 1 low quality (score of ≤3) (Table IV; available at www.jpeds.com). For ALL, 15 studies investigated 37 different infection variables: 26 (70%) were viral or virus-related infection, 7 (19%) systemic symptoms (eg, urinary tract infection), 3 bacterial (including 1 chlamydia and 1 mycoplasma), and 1 fungal infection (Table V; available at www.jpeds.com). For childhood leukemia, 14 studies investigated 29 infections: 17 (59%) viral or virus-related infection, 8 (28%) systemic symptoms, 3 bacterial (including 1 chlamydia and 1 mycoplasma), and 1 fungal infection (Table VI; available at www.jpeds.com).
Table III.
Characteristics of included studies
| Study nos. | Publication | Year | Country | Study design | Study period* | Subject source | Matching/adjusting variables | Sample size | Investigated exposure (agents) | Measurement method and exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Francis et al15 | 2015 | USA | Nested case- control† | 1982–2006 (birth year) | Population based | Birth date, race and sex/race, age, and sex | 268 ALL and 270 controls | CMV,‡ EBV (DNA) | Digital droplet PCR in neonatal blood spot |
| #2 | Bogdanovic et al17 | 2016 | Sweden | Case-control | 1992–2006 | Population based | Age or birthdate and birthplace | 95 ALL and 95 controls | HHV-6,‡ parvovirus B19, HERV (DNA) | Next-generation sequencing in pooled samples and real-time PCR in individual samples of neonatal blood spots |
| Gustafsson et al31 | 2012 | 50 ALL and 100 controls | KIPyV, WUPyV, MCPyV (DNA) | Nested PCR in neonatal blood spots | ||||||
| Honkaniemi et al30 | 2010 | 243 ALL and 484 controls | Species C adenovirus (DNA) | |||||||
| #3 | Bzhalava et al16 | 2016 | Sweden | Nested case-control | 1977–2005 | Population based | None | 26 ALL cases and 47 controls | Anelloviridae,‡ viruses from environmental samples, papillomaviridae, “unclassified” viruses (DNA) | Next generation sequencing in first trimester maternal sera |
| #4 | Kumar et al37 | 2014 | India | Case-control | 2008–2012 | Hospital based | Age, sex and residency | 132 leukemia and 132 controls | Infection‡ | Self-report interview on exposure during pregnancy |
| #5 | Tedeschi et al21 | 2009 | Finland and Iceland | Nested case-control | 1983–2006 | Population based | Mother’s country, age at serum sampling, date of specimen collection and children’s birth date and sex/birth order and sibship size | 705 leukemia (561 ALL) and 2105 controls | EBV (VCA IgM,‡ EA and ZEBRA IgG) | ELISA in first trimester maternal sera |
| #6 | Vasconcelos et al39 | 2008 | USA | Case-control | 1995–2002 | Population based | Birth date, sex, ethnicity, maternal | 89 ALL and 100 controls | Species C adenoviruses (DNA) | Seminested PCR in neonatal blood spots |
| Kwan et al32 | 2007 | county of residence at birth, or plus maternal race/household income, maternal education, and maternal age at the birth of the child | 365 leukemia (311 ALL) and 460 controls | Influenza/pneumonia,‡ urinary tract infection, sexually transmitted diseases | Self-report interview on exposure from 3 months before pregnancy through the end of pregnancy | |||||
| #7 | Gustafsson et al29 | 2007 | Sweden | Case-control | 1980–2001 | Hospital based | Birthdate or age and birthplace | 49 ALL and 47 controls | Species C adenoviruses DNA‡ | Nested PCR in neonatal blood spots |
| Gustafsson et al28 | 2006 | 48 ALL and 46 controls | CMV (DNA) | |||||||
| Bogdanovic et al27 | 2004 | 54 ALL and 47 controls | HHV-6, EBV (DNA) | |||||||
| Isa et al26 | 2004 | 54 ALL and 50 controls | Parvovirus B19 (DNA) | |||||||
| Priftakis et al25 | 2003 | 54 ALL and 37 controls | BKV, JCV (DNA) | |||||||
| #8 | Leppik et al33 | 2007 | Finland and Iceland | Nested case-control | 1975–1997 | Population based | Mothers’ country, age at serum sampling, date of specimen collection and children’s birth date and sex | 30 leukemia and 30 controls | TT virus (DNA) | PCR in first trimester maternal sera |
| Tedeschi et al20 | 2007 | 343 leukemia (304 ALL) and 973 controls | EBV (VCA IgM, EA IgG and IgM, ZEBRA IgG and IgM) | ELISA in first trimester maternal sera | ||||||
| Lehtinen et al40 | 2005 | 402 leukemia (341 ALL) and 1212 controls | Mycoplasma pneumoniae, chlamydia trachomatis, helicobacter pylori (IgM and IgG) | |||||||
| Lehtinen et al41 | 2003 | 403 leukemia (342 ALL) and 1216 controls | HHV-6 (IgM,‡ IgG); CMV, EBV VCA (IgM, IgG) | |||||||
| #9 | Naumburg et al42 | 2002 | Sweden | Case-control | 1973–1989 | Population based | Sex and birth in the same year and month/adjustments for potential confounders only marginally influenced the risk estimates, such as mothers age, parity, mode of delivery (vaginal or caesarian), maternal smoking, elapsed time from rupture of the membranes to delivery, gestational age at birth, birth weight, birth weight for gestational age, and type of birth (single or multiple). | 652 leukemia (578 ALL) and 652 controls | Infection,‡ lower genital tract infection, urinary tract infection, other infection | Data extracted from medical record on exposure during pregnancy |
| #10 | Dockerty et al44 | 1999 | New Zealand | Case-control | 1990–1993 | Population based | None | 121 leukemia and 303 controls | Influenza,‡ cystitis or kidney infection, cold sores/oral herpes, any other infection | Self-reported questionnaire on exposure during pregnancy or in the 3 months before the pregnancy |
| #11 | McKinney et al43 | 1999 | UK | Case-control | 1991–1994 | Population based | Age, sex and health board area of residence | 144 leukemia (124 ALL) and 271 controls | Any infection,‡ respiratory tract infection, viral infection, genitourinary infection, fungal infection | Data abstracted from medical record on exposure during pregnancy |
| #12 | Roman et al45 | 1997 | UK | Case-control | 1962–1992 | Hospital based | Hospital catchment area of birth, sex and year and month of birth | 143 leukemia (113 ALL) and 286 controls | Viral infection,‡ influenza, vulvar warts, herpes simplex, rubella | Data abstracted from medical record on exposure during pregnancy |
| #13 | Buckley et al24,§ | 1994 | USA and Canada | Case-control | 1982–1991 | Hospital based | Birth year, race, income, geographical region and family size | 404 ALL and 440 community controls | Infection | Self-administered questionnaire on exposure during pregnancy |
| #14 | Gardner et al46 | 1990 | UK | Case-control | 1950–1985 | Population based | Sex, birth date and residence | 35 leukemia and 103 local controls | Viral infection‡ | Self-reported questionnaire on exposure during pregnancy |
| #15 | McKinney et al23,§ | 1987 | UK | Case-control | 1980–1983 | Population based | Sex and age | 171 leukemia and 342 controls | Influenza, urinary tract infection | Self-reported interview on exposure during pregnancy |
| #16 | Fine et al48 | 1985 | UK | Prospective and retrospective cohorts | 1946–1972 | Population based | Sex and date and area of birth | 6 leukemia (4 ALL) and 5039 nonleukemia | Viral infection,‡ influenza, varicella, herpes zoster, mumps, rubella, measles, infectious hepatitis, CMV, miscellaneous viruses | Self-reported interview or medical records from general practice, local authority, hospital and virus laboratory sources |
| #17 | Van Steensel-Moll et al47 | 1985 | Netherlands | Case-control | 1973–1980 | Population based | Birth date, sex and municipality/age and sex | 519 ALL and 507 controls | Viral infections‡ | Self-administered questionnaires on exposure during pregnancy |
| #18 | Madden et al49 | 1983 | USA | Nested case-control | 1959–1966 | Hospital based | Maternal age, race, date of delivery, hospital, socioeconomic relationships | 7 leukemia deaths and 7 controls | BKV IgM | ELISA in maternal sera series |
| Heinonen et al50 | 1973 | Prospective cohort | None | 8 leukemia (including 2 ALL¶) deaths and 50 889 controls | Spontaneous viral infections‡ | Self-reported interview and review of hospital and other records on exposure during pregnancy | ||||
| #19 | Bithell et al22 | 1973 | UK | Case-control | 1953–1967 | Population based | None | 4219 leukemia deaths and 9074 controls | Influenza,‡ rubella, varicella, other virus infections | Self-reported interviews and review of antenatal records (whenever possible) on exposure during pregnancy |
| Stewart et al3 | 1958 | Age (birth in the same month or half year), sex, locality | 619 leukemia deaths and 619 controls | Genitourinary tract, virus infection, rubella, mumps, herpes zoster, infective hepatitis | ||||||
| #20 | Fedrick and Alberman4 | 1972 | UK | Prospective cohort | 1958 | Population based | Birth date | 11 leukemia (10 ALL**) and 17 739 nonleukemia | Influenza‡ | Self-reported questionnaires on exposure during pregnancy |
BKV, BK virus; EBV, Epstein-Barr virus; EA, early antigen; ELISA, enzyme-linked immunosorbent assay; HHV-6, human herpesvirus type 6; HERV, human endogenous retroviruses; JCV, JC virus; KIPyV, Karolinska Institutet polyomavirus; MCPyV, Merkel cell polyomavirus; PCR, polymerase chain reaction; TT virus, Torque teno viruses; VCA, viral capsid antigen; WUPyV, Washington University polyomavirus.
Diagnosis year of cases for case-control study or recruitment year of mothers or babies for cohort study.
This study was categorized as nested case-control study because the cases and controls were drawn from the same source population at birth (Childhood Cancer Record Linkage Program).
Variables that were selected as the “proxy of any infection” variable in qualitative summary in Figure 2.
The studies of Buckley et al24 in 199424 and McKinney et al23 in 198723 were not included in any meta-analysis because the effect estimate or 95% CI was not reported and could not be computed.
Two cases of “leukemia, lymphoblastic” or “leukemia, lymphocytic” in the nonexposed group were treated as ALL.
One case of lymphoblastic leukemia in the non-exposed group was treated as ALL.
Table IV.
Quality assessment of included studies using the NOS*
| Study nos. | Publication | Year | Selection (4 items) | Comparability† (2 items) | Exposure/outcome assessment (3 items) | Total score |
|---|---|---|---|---|---|---|
| #1 | Francis et al15 | 2017 | ★★★★ | ★ | ★★★ | 8 |
| #2 | Bogdanovic et al17 | 2016 | ★★★★ | ★★★ | 7 | |
| Gustafsson et al31 | 2012 | ★★★ | ★ | ★★★ | 7 | |
| Honkaniemi et al30 | 2010 | ★★★★ | ★ | ★★★ | 8 | |
| #3 | Bzhalava et al16 | 2016 | ★★★★ | ★★★ | 7 | |
| #4 | Kumar et al37 | 2014 | ★ | ★★ | 3 | |
| #5 | Tedeschi et al21 | 2009 | ★★★★ | ★ | ★★★ | 8 |
| #6 | Vasconcelos et al39 | 2008 | ★★★ | ★ | ★★★ | 7 |
| Kwan et al32 | 2007 | ★★★★ | ★★ | ★★ | 8 | |
| #7 | Gustafsson et al29 | 2007 | ★★★ | ★ | ★★★ | 7 |
| Gustafsson et al28 | 2006 | ★★★ | ★★★ | 6 | ||
| Bogdanovic et al27 | 2004 | ★★★ | ★★★ | 6 | ||
| Isa et al26 | 2004 | ★★★ | ★★★ | 6 | ||
| Priftakis et al25 | 2003 | ★★★ | ★ | ★★★ | 7 | |
| #8 | Leppik et al33 | 2007 | ★★★ | ★ | ★★★ | 7 |
| Tedeschi et al20 | 2007 | ★★★★ | ★ | ★★★ | 8 | |
| Lehtinen et al40 | 2005 | ★★★★ | ★ | ★★★ | 8 | |
| Lehtinen et al41 | 2003 | ★★★★ | ★ | ★★★ | 8 | |
| #9 | Naumburg et al42 | 2002 | ★★★★ | ★ | ★★ | 7 |
| #10 | Dockerty et al44 | 1999 | ★★★ | ★ | ★★ | 6 |
| #11 | McKinney et al43 | 1999 | ★★★ | ★★ | 5 | |
| #12 | Roman et al45 | 1997 | ★★ | ★ | ★★ | 5 |
| #13 | Buckley et al24 | 1994 | ★★★ | ★ | ★ | 5 |
| #14 | Gardner et al46 | 1990 | ★★★★ | ★ | ★ | 6 |
| #15 | McKinney et al23 | 1987 | ★★★ | ★★ | 5 | |
| #16 | Fine et al48 | 1985 | ★★★ | ★ | ★★ | 6 |
| #17 | Van Steensel-Moll et al47 | 1985 | ★★★ | ★ | ★★ | 6 |
| #18 | Madden et al49 | 1983 | ★★★ | ★★ | ★★★ | 8 |
| Heinonen et al50 | 1973 | ★★★★ | ★★ | 6 | ||
| #19 | Bithell et al22 | 1973 | ★★★★ | ★★ | 6 | |
| Stewart et al3 | 1958 | ★★★★ | ★ | 5 | ||
| #20 | Fedrick and Alberman4 | 1972 | ★★★★ | ★ | ★★★ | 8 |
One star is awarded if the study meets 1 assessment item.
The 2 items used for comparability assessment were birth date and maternal socioeconomic status (eg, educational level, income).
Table V.
Results of studies on ALL that were not included into any data synthesis (qualitative or quantitative) and reasons for exclusion
| Infection variables | No. of study Investigating | No. of study included in data synthesis | Studies not included in any data synthesis | |||
|---|---|---|---|---|---|---|
| Study ID | Year | Results | Reason for exclusion | |||
| Proxy of any infection | 15* | 14* | Buckley et al24 | 1994 | OR, 1.0, P ≥.1 | Did not provide exact CI for OR |
| Viral infection | 5† | 5† | None | – | ||
| Influenza | 4‡ | 4‡ | None | – | ||
| CMV | 4 | 3 | Gustafsson et al28 | 2006 | Not found in both case and control group | OR cannot be calculated |
| EBV | 4 | 3 | Bogdanovic et al27 | 2004 | Not found in both case and control group | OR cannot be calculated |
| HHV-6 | 3 | 2 | Bogdanovic et al27 | 2004 | Not found in both case and control group | OR cannot be calculated |
| Species C adenovirus | 3 | 3 | None | – | ||
| Rubella | 2 | 0 | Roman et al45 | 1997 | None of case or control had exposure | Only 1 study had a valid OR and 95% CI for rubella |
| Fine et al48 | 1985 | OR, 1.25; 95% CI, 0.05–30.79 | ||||
| Urinary tract infection | 2 | 2 | None | – | ||
| Parvovirus B19 | 2 | 0 | Isa et al26 | 2004 | Not found in both case and control group | Only 1 study had valid OR and 95% CI for Parvovirus B19 |
| Bogdanovic et al17 | 2016 | OR, 3.06; 95% CI, 0.12–76.20 | ||||
| Varicella | 1 | Fine et al48 | 1985 | OR, 17.2; 95% CI, 1.55–190.07 | Only 1 study reporting | |
| Genitourinary infection | 1 | McKinney et al43 | 1999 | OR, 1.18; 95% CI, 0.5–2.79 | Only 1 study reporting | |
| Herpes zoster | 1 | Fine et al48 | 1985 | OR, 12.4; 95% CI, 0.50–307.32 | Only 1 study reporting | |
| Infectious hepatitis | 1 | Fine et al48 | 1985 | OR, 4.03; 95% CI, 0.16–99.33 | Only 1 study reporting | |
| Mumps | 1 | Fine et al48 | 1985 | OR, 1.87; 95% CI, 0.08–45.94 | Only 1 study reporting | |
| BKV | 1 | Priftakis et al25 | 2003 | Not found in both case and control group | OR cannot be calculated | |
| Chlamydia trachomatis | 1 | Lehtinen et al40 | 2005 | OR, 0.7; 95% CI, 0.2–2.3 | Only 1 study reporting | |
| Fungal infection | 1 | McKinney et al43 | 1999 | OR, 0.34; 95% CI, 0.07–1.54 | Only 1 study reporting | |
| Helicobacter pylori | 1 | Lehtinen et al40 | 2005 | OR, 0.8; 95% CI, 0.3–1.9 | Only 1 study reporting | |
| Herpes simplex | 1 | Roman et al45 | 1997 | None of cases or controls had exposure | OR cannot be calculated | |
| Lower genital tract infection | 1 | Naumburg et al42 | 2002 | OR, 1.63; 95% CI, 1.04–2.53 | Only 1 study reporting | |
| Sexually transmitted diseases | 1 | Kwan et al32 | 2007 | OR, 6.65; 95% CI, 1.37–32.38 | Only 1 study reporting | |
| Vulvar warts | 1 | Roman et al45 | 1997 | OR, 7.64; 95% CI, 0.31–188.95 | Only 1 study reporting | |
| Measles | 1 | Fine et al48 | 1985 | OR, 20.88; 95% CI, 0.84–520.58 | Only 1 study reporting | |
| Miscellaneous viruses | 1 | Fine et al48 | 1985 | OR, 3.85; 95% CI, 0.16–94.69 | Only 1 study reporting | |
| Mycoplasma pneumoniae | 1 | Lehtinen et al40 | 2005 | OR, 1.6; 95% CI, 1.0–2.6 | Only 1 study reporting | |
| Respiratory tract infection | 1 | McKinney et al43 | 1999 | OR, 1.64; 95% CI, 0.60–4.46 | Only 1 study reporting | |
| Anelloviridae | 1 | Bzhalava et al16 | 2016 | OR, 0.58; 95% CI, 0.17–1.97 | Only 1 study reporting | |
| HERV | 1 | Bogdanovic et al17 | 2016 | The same HERV composition was found in both case and control groups | OR cannot be calculated | |
| JCV | 1 | Priftakis et al25 | 2003 | Not found in both case and control group | OR cannot be calculated | |
| KIPyV | 1 | Gustafsson et al31 | 2012 | Not found in both case and control group | OR cannot be calculated | |
| MCPyV | 1 | Gustafsson et al31 | 2012 | Not found in both case and control group | OR cannot be calculated | |
| Papillomaviridae | 1 | Bzhalava et al16 | 2016 | OR, 0.58; 95% CI, 0.02–14.88 | Only 1 study reporting | |
| Viruses from environmental samples | 1 | Bzhalava et al16 | 2016 | OR, 0.51; 95% CI, 0.16–1.61 | Only 1 study reporting | |
| WUPyV | 1 | Gustafsson et al31 | 2012 | Not found in both case and control group | ||
| “Unclassified” viruses | 1 | Bzhalava et al16 | 2016 | OR, 1.56; 95% CI, 0.50–4.82 | Only 1 study reporting | |
| Other infection | 1 | Naumburg et al42 | 2002 | OR, 1.13; 95% CI, 0.81–1.60 | Only 1 study reporting | |
BKV, BK virus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus type 6; HERV, human endogenous retroviruses; JCV, JC virus; KIPyV, Karolinska Institutet polyomavirus; MCPyV, Merkel cell polyomavirus; OR, odds ratio; WUPyV, Washington University polyomavirus.
Three studies (Naumburg et al42 in 2002, McKinney et al43 in 1999, Buckley et al24 in 1994) used a variable of total infection; for other studies, we selected specific type of infection that had the highest prevalence as any infection.
One study used a variable of spontaneous viral infection.
One study used a variable of influenza/pneumonia.
Table VI.
Results of studies on all childhood leukemias that were not included into any data synthesis (qualitative or quantitative) and reasons for exclusion
| Infection variables | No. of study Investigating | No. of study included in data synthesis | Studies not included in any data synthesis | |||
|---|---|---|---|---|---|---|
| Study ID | Year | Results | Reason for exclusion | |||
| Proxy of any infection | 14* | 12* | McKinney et al23 | 1987 | None of relative risks for influenza and urinary tract infection was >2 or reached significance at the 5% level | Did not provide exact OR and CI |
| Kumar et al37 | 2014 | OR, 0.86; 95% CI, 0.40–1.84 | Low quality assessed by the NOS | |||
| Influenza | 7† | 6† | McKinney et al23 | 1987 | None of the relative risks was greater than 2 or reached significance at the 5% level | Did not provide exact OR and CI |
| Viral infection | 6‡ | 6‡ | None | – | ||
| Urinary tract infection | 4§ | 3§ | McKinney et al23 | 1987 | None of the relative risks was greater than 2 or reached significance at the 5% level | Did not provide exact OR and CI |
| Rubella | 3 | 3 | None | – | ||
| CMV | 2 | 2 | None | – | ||
| Varicella | 2 | 2 | None | – | ||
| EBV | 2 | 2 | None | – | ||
| Genitourinary infection | 2 | 2 | None | – | ||
| Herpes simplex | 2¶ | 2¶ | None | – | ||
| Herpes zoster | 2 | 2 | None | – | ||
| Infectious hepatitis | 2 | 2 | None | – | ||
| Mumps | 2 | 2 | None | – | ||
| BKV | 1 | Madden et al49 | 1983 | OR, 0.29; 95% CI, 0.01–8.39 | Only 1 study reporting | |
| Chlamydia trachomatis | 1 | Lehtinen et al40 | 2005 | OR, 0.7; 95% CI, 0.2–2 | Only 1 study reporting | |
| Fungal infection | 1 | McKinney et al43 | 1999 | OR, 0.28; 95% CI, 0.06–1.26 | Only 1 study reporting | |
| Helicobacter pylori | 1 | Lehtinen et al40 | 2005 | OR, 1.1; 95% CI, 0.5–2.4 | Only 1 study reporting | |
| HHV-6 | 1 | Lehtinen et al41 | 2003 | OR, 0.8; 95% CI, 0.6–1.2 | Only 1 study reporting | |
| Lower genital tract infection | 1 | Naumburg et al42 | 2002 | OR, 1.78; 95% CI, 1.17–2.72 | Only 1 study reporting | |
| Sexually transmitted diseases | 1 | Kwan et al32 | 2007 | OR, 7.59; 95% CI, 1.58–36.56 | Only 1 study reporting | |
| vulvar warts | 1 | Roman et al45 | 1997 | OR, 6.03; 95% CI, 0.24–149.00 | Only 1 study reporting | |
| Measles | 1 | Fine et al48 | 1985 | OR, 12.5; 95% CI, 0.59–265.15 | Only 1 study reporting | |
| Miscellaneous viruses | 1 | Fine et al48 | 1985 | OR, 2.31; 95% CI, 0.11–48.20 | Only 1 study reporting | |
| Mycoplasma pneumoniae | 1 | Lehtinen et al40 | 2005 | OR, 1.6; 95% CI, 1.0–2.5 | Only 1 study reporting | |
| Respiratory tract infection | 1 | McKinney et al43 | 1999 | OR, 1.46; 95% CI, 0.58–3.67 | Only 1 study reporting | |
| TT virus | 1 | Leppik et al33 | 2007 | No obvious differences between cases and controls | OR cannot be calculated | |
| Any other infection | 1 | Dockerty et al44 | 1999 | OR, 1.45; 95% CI, 0.55–3.82 | Only 1 study reporting | |
| Other infection | 1 | Naumburg et al42 | 2002 | OR, 1.13; 95% CI, 0.83–1.55 | Only 1 study reporting | |
| Other virus infection | 1 | Bithell et al22 | 1973 | OR, 0.69; 95% CI, 0.34–1.41 | Only 1 study reporting | |
TT virus, Torque teno viruses.
Three studies (Kumar et al37 in 2014, Naumburg et al42 in 2002, McKinney et al43 in 1999) used a variable of total infection; for other studies, we selected a specific type of infection that had the highest prevalence as any infection.
One study used a variable of influenza/pneumonia.
One study used a variable of spontaneous viral infection.
One study used a variable of cystitis or kidney infection.
One study used a variable of cold sores/oral herpes.
Qualitative Evaluation for a “Proxy for Any Infection”
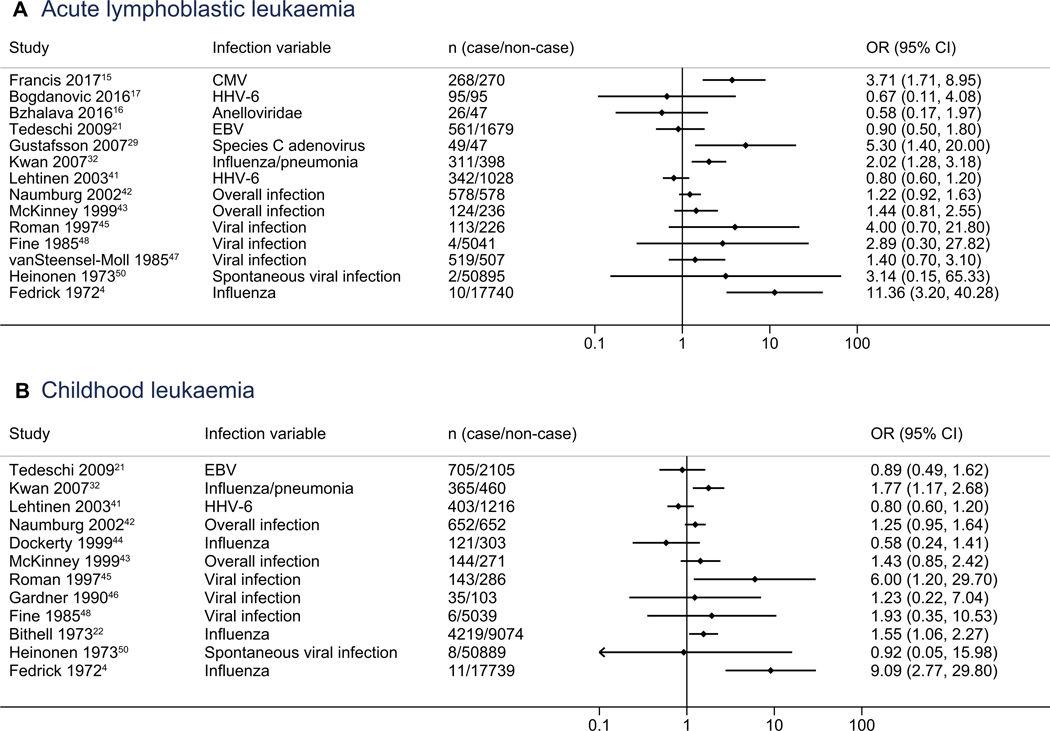
Fourteen studies provided data on effect estimates for ALL and proxy for any infection, with study-level ORs ranging from 0.58 to 11.36 (Figure 2). Of these studies, 10 (71%) reported point estimate ORs of >1, of which 4 were statistically significant (ORs ranging from 2.02 to 11.36). For the 12 studies that had data on ORs for childhood leukemia, 8 (67%) showed proxy for any infection as a risk factor (OR > 1), of which 4 were statistically significant (ORs ranging from 1.55 to 9.09).
Figure 2.
Qualitative summary of a proxy of any infection for A, ALL and B, all childhood leukemias. EBV, Epstein-Barr virus; HHV-6, human herpesvirus type 6.
Meta-analysis of Specific Types of Infection
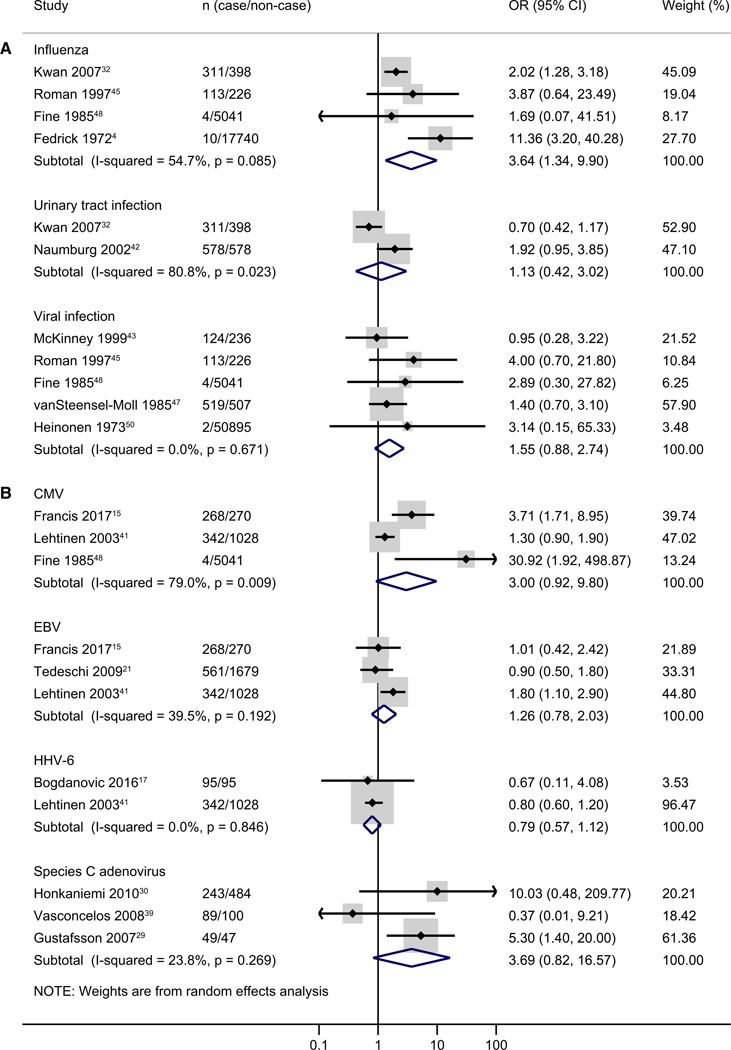
Seven types of infections were included in the meta-analyses for ALL. These included influenza (n = 4), urinary tract infection (n = 2), viral infection (n = 5), CMV (n = 3), Epstein-Barr virus (n = 3), human herpesvirus type 6 (n = 2), and species C adenovirus (n = 3). Among them, influenza during pregnancy was associated with a higher risk of ALL (pooled OR, 3.64; 95% CI, 1.34–9.90; I2 = 54.7%). Both CMV (pooled OR, 3.00; 95% CI, 0.92–9.80; I2 = 79.0%) and species C adenovirus (pooled OR, 3.69; 95% CI, 0.82–16.57; I2 = 23.8%) showed an increased risk, but associations were not statistically significant and numbers of studies were low (Figure 3).
Figure 3.
Meta-analysis of specific type of infection for ALL (A, nonlaboratory data; B, laboratory data). The study of Fine et al 1985 used both self-report or medical record data for CMV.
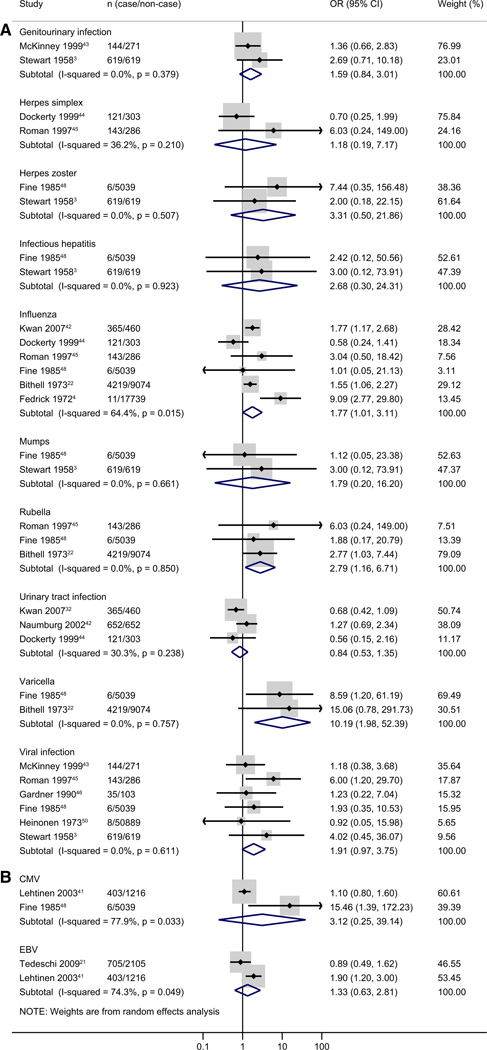
For childhood leukemia, 12 infections were included in meta-analyses, including genitourinary infection (n = 2), herpes simplex (n = 2), herpes zoster (n = 2), infectious hepatitis (n = 2), influenza (n = 6), mumps (n = 2), rubella (n = 3), urinary tract infection (n = 3), varicella (n = 2), viral infection (n = 6), CMV (n = 2), and Epstein-Barr virus (n = 2). Influenza (pooled OR, 1.77; 95% CI, 1.01–3.11; I2 = 64.4%), rubella (pooled OR, 2.79; 95% CI, 1.16–6.71; I2 = 0%), and varicella (pooled OR, 10.19; 95% CI, 1.98–52.39; I2 = 0%) infections were significantly associated with a higher risk of childhood leukemia (Figure 4). There were no significant associations observed for other types of infections.
Figure 4.
Meta-analysis of specific type of infection for all childhood leukemias (A, nonlaboratory data; B, laboratory data). The study of Fine et al 1985 used both self-report or medical record data for CMV.
Subgroup analyses for influenza showed that pooled ORs among cohort studies seemed to be higher than those in case-control studies (eg, pooled OR, 7.91 [95% CI, 1.83–34.24]4,48 vs pooled OR, 2.10 [95% CI, 1.35–3.27]32,45 for ALL), but the P value for the interaction was >.1; both groups had lower heterogeneity compared with the overall analysis (Table VII; available at www.jpeds.com). The pooled ORs across different stratum of measurement method, study region, or study quality were similar. Sensitivity analyses excluding studies with <10 subjects with exposure/outcome or excluding studies with cases >19 years of age had similar results to the overall analysis, although the CIs were wider (Table VIII; available at www.jpeds.com). When excluding studies that combined the exposure periods 3 months before pregnancy and pregnancy, the pooled ORs (eg, pooled OR, 6.87 [95% CI, 2.57–18.42] for ALL) seemed to be higher than those in overall analysis (pooled OR, 3.64 [95% CI, 1.34–9.90] for ALL).
Table VII.
Meta-analysis of influenza by subgroups
| ALL | All childhood leukemias | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | No. | I2 | OR (95% CI) | Pi* | No. | I2 | OR (95% CI) | Pi* |
| Measurement method | ||||||||
| Self-report | 2 | 84.2% | 4.31 (0.80–23.12) | Ref | 3 | 85.0% | 1.96 (0.59–6.48) | Ref |
| Medical record† | 2 | 0% | 3.17 (0.66–15.26) | .81 | 3 | 0% | 1.59 (1.10–2.30) | .92 |
| Study design | ||||||||
| Case-control study | 2 | 0% | 2.10 (1.35–3.27) | Ref | 4 | 46.9% | 1.43 (0.93–2.20) | Ref |
| Cohort study | 2 | 15% | 7.91 (1.83–34.24) | .16 | 2 | 42.5% | 4.82 (0.69–33.85) | .12 |
| Study region | ||||||||
| Europe | 3 | 0% | 6.87 (2.57–18.42) | Ref | 4 | 63.2% | 2.82 (0.97–8.19) | Ref |
| North America | 1 | – | 2.02 (1.28–3.18) | .17 | 1 | – | 1.77 (1.17–2.68) | .66 |
| Others | – | – | – | – | 1 | – | 0.58 (0.24–1.41) | .22 |
| Study quality | ||||||||
| High quality | 2 | 84.2% | 4.31 (0.80–23.12) | Ref | 2 | 84.6% | 3.63 (0.74–17.84) | Ref |
| Medium quality | 2 | 0% | 3.17 (0.66–15.26) | .82 | 4 | 37.8% | 1.22 (0.63–2.35) | .26 |
Table VIII.
Sensitivity analysis for influenza
| ALL | All childhood leukemias | |||||
|---|---|---|---|---|---|---|
| Sensitivity analysis | No. | I2 | OR (95% CI) | No. | I2 | OR (95% CI) |
| After excluding studies with <10 subjects with exposure/outcome | 2 | 84.2% | 4.31 (0.80–23.12) | 4 | 77.8% | 1.74 (0.92–3.32) |
| After excluding studies that included cases >19 years of age | 3 | 68.7% | 3.69 (0.96–14.28) | 5 | 70.5% | 1.70 (0.93–3.12) |
| After excluding studies that combined the exposure periods 3 months before pregnancy and pregnancy | 3 | 0% | 6.87 (2.57–18.42) | 4 | 63.2% | 2.82 (0.97–8.19) |
Assessment of Publication Bias
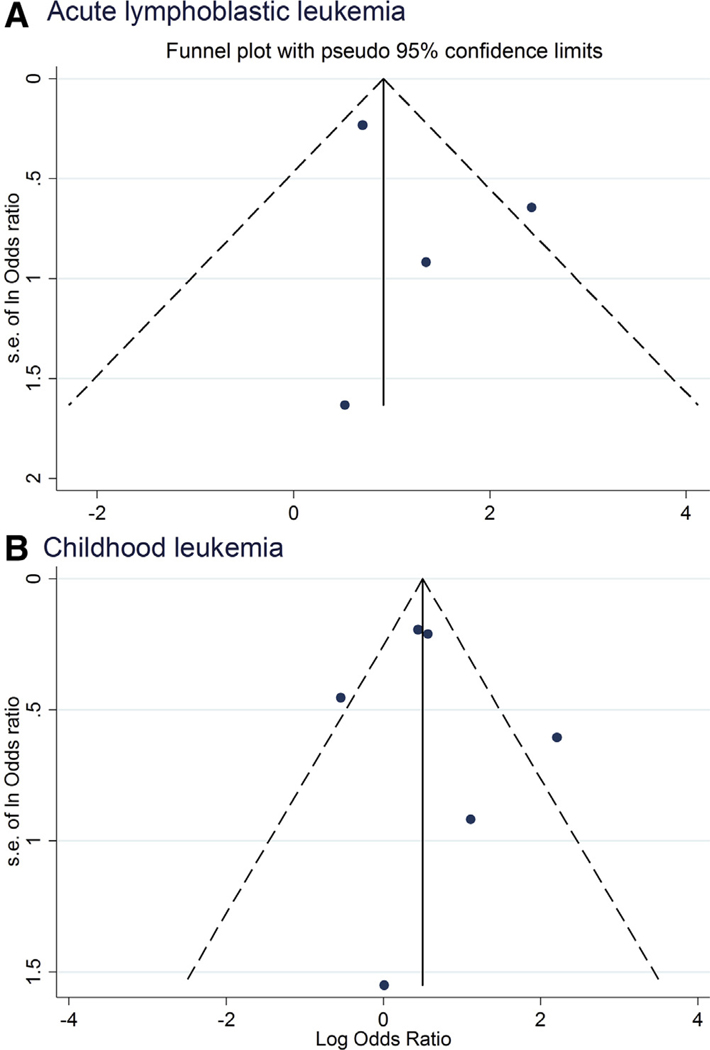
Funnel plots appear symmetrical (Figure 5; available at www.jpeds.com), and the Egger test revealed that there was no evident publication bias or small-study effects (P values of >.4). The potential for publication bias for other infections could not be assessed owing to the small number of studies.
Figure 5.
Funnel plots of influenza for A, ALL and B, all childhood leukemias.
Results for Studies Not Included in Data Synthesis (Qualitative or Quantitative)
Among the infections not included in the meta-analysis because only 1 study reported the effect estimate, an increased risk of ALL and childhood leukemia was observed for lower genital tract infection (pooled OR, 1.63 [95% CI, 1.04–2.53] for ALL, 1.78 (pooled OR, 1.17–2.72) for childhood leukemia), sexually transmitted diseases (pooled OR, 6.65 [95% CI, 1.37–32.38] for ALL, 7.59 [95% CI, 1.58–36.56] for childhood leukemia), and mycoplasma pneumoniae (pooled OR, 1.6 [95% CI, 1.0–2.6] for ALL, 1.6 [95% CI, 1.0–2.5] for childhood leukemia) (Tables V and VI). Varicella infection was associated with higher risk of ALL (pooled OR, 17.2; 95% CI, 1.55–190.07) (Table V).
Discussion
In this systematic review and meta-analysis, we found that 3 types of infection (influenza, varicella, and rubella) were significantly associated with a higher risk of ALL and/or childhood leukemia, although numbers of studies were small. For the hypothesis of any infection, we were unable to perform a quantitative synthesis because of the high heterogeneity across studies; however, most of included studies reported a positive association of childhood leukemia when we used a variable of proxy for any infection.
Among the specific infections, the meta-analysis results for influenza seemed to be more robust, based on the number and quality of studies and sample size. However, the results should be interpreted with some degree of caution given there was substantial heterogeneity across studies reporting on influenza. This finding may be explained by study design (cohort vs case-control study) and different methods (self-reports, medical records, or 2 combined) used to collect influenza data across studies. We also found rubella and varicella infections during pregnancy were associated with increased risk of childhood leukemia, despite the small number of studies. It has been shown that these 2 infections may be transmitted from the mother to the fetus and cause congenital or neonatal infection51,52; thus, they are more likely to directly affect the fetus/child. However, further studies are needed to confirm the findings on these 2 infections.
The strengths of our review include its adherence to a registered protocol, methodologic advantages, no obvious evidence publication bias, and consistent results in sensitivity analyses, suggesting the robustness and validity of our findings. Several limitations, however, should be noted. First, few studies reported results for a variable of any infection; thus, it was difficult to test our hypothesis that any infection might be linked with childhood leukemia via general immunologic response. To address this issue, we used a specific type of infection as a proxy to represent any infection for studies that did not report a variable of any infection. However, the high heterogeneity across studies precluded a meta-analysis for this proxy variable. Second, for the meta-analysis of specific infections, and relatively strong associations were observed for varicella and rubella and childhood leukemia, the number of studies were too small to draw confident conclusions. Third, we were unable to perform subgroup analyses by exposure timing, age at diagnosis, and race/ethnicity because very few studies reported results stratified on these variables. Fourth, most of the included studies failed to adjust for socioeconomic status or other maternal characteristics such as age and obesity, which are potential confounders.53–55 Therefore, the associations observed in the original individual studies as well as in our meta-analyses might be confounded by ≥1 factors.
The findings from this systematic review highlight the lack of consistent epidemiologic evidence to support an association for specific infections and childhood leukemias. However, plausible biological mechanisms have been reported in studies examining in utero exposures to maternal infections and childhood cancer. Besides the genetic and immunologic mechanisms that we outlined elsewhere in this article, fetal infection may initiate immune dysregulation, through initial B-cell sensitization during programming of central tolerance, thereby altering adaptive immune response independent of viral load and cryptically contributing to leukemia. Another possibility is that maternal infections (especially those leading to severe illnesses) might result in adverse and other pregnancy outcomes, for example, cesarean delivery56,57 and shorter gestational length58 that, in turn, increase the risk of childhood leukemia.54,59 In addition, it is possible that the use of antibiotics or other medications to treat infection and related symptoms, rather than the infection itself, could be related to the risk of leukemia. Whether the medication use acts as a mediator or directly on the causal pathway requires further investigation.
Although this review does not include postnatal infections, it is important to distinguish timing of infection and leukemia risk. A popular theory known as the delayed infection or hygiene hypothesis posits that a paucity of infections early in life may increase the risk of childhood leukemia.2,60 Our analysis focused on the pregnancy window and suggests that maternal infections during pregnancy are associated with childhood leukemia. As mentioned, maternal infection might alter the fetal immune system development, and one might hypothesize that this alteration may become overt in childhood if there is no correction by the early (postnatal) exposure to infection. However, we were not able to investigate this possibility with the evidence currently available. Further research is needed to understand critical windows of infection, and interaction by timing of infections to further elucidate temporally varying risk and the underlying mechanism of leukemogenesis.
Our findings suggest directions for future epidemiologic research on this topic. The observation that the association of influenza was stronger in cohort studies than case-control studies suggests that there should be more emphasis on this study design in the future. Not only would this approach reduce the possibility of recall bias, it would provide a greater opportunity for obtaining predisease biospecimens. However, adequately powered prospective studies in this field are scarce owing to the rarity of childhood leukemia. In addition, given that limited information was available on exposure timing, age at diagnosis and race/ethnicity among the studies included in our review, future studies should also explore whether the relationship between maternal infection and childhood leukemia varies across different strata of these factors.
Our findings suggest that, although based on a small number of studies, specific infections during pregnancy are associated with childhood leukemia. These findings justify the need for further research; however, they should not be used as a basis for supporting specific preventive measures. Further studies are needed to confirm our findings, ideally with larger sample sizes, including a greater collection of prospective evidence, and more accurate methods for detecting and measuring infection. ■
Supplementary Material
Acknowledgments
We thank Nia Wyn Roberts (librarian at the University of Oxford) and Helen Elwell (librarian at the British Medical Association library in London) for help with the search terms.
J-R.H. is supported by the Chinese Scholarship Council. P.M. and S.H. are supported by the Research Council of Norway through its Centers of Excellence funding scheme (262700) and by the Norwegian Institute of Public Health.
Glossary
- ALL
Acute lymphoblastic leukemia
- CMV
Cytomegalovirus
- NOS
Newcastle-Ottawa Scale
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 2017;18:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer 2018;18:471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart A, Webb J, Hewitt D. A survey of childhood malignancies. Br Med J 1958;1:1495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedrick J, Alberman ED. Reported influenza in pregnancy and subsequent cancer in the child. Br Med J 1972;2:485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M. Considerations on a possible viral etiology for B-precursor acute lymphoblastic leukemia of childhood. J Immunother 1997;20: 89–100. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H, de Villiers EM. Prenatal infections with subsequent immune tolerance could explain the epidemiology of common childhood cancers. Lyon: IARC; 2014. [Google Scholar]
- 7.zur Hausen H, de Villiers EM. Virus target cell conditioning model to explain some epidemiologic characteristics of childhood leukemias and lymphomas. Int J Cancer 2005;115:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis 2012;12:330–40. [DOI] [PubMed] [Google Scholar]
- 9.Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev 2011;20:1736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soegaard SH, Rostgaard K, Skogstrand K, Wiemels JL, Schmiegelow K, Hjalgrim H. Neonatal inflammatory markers are associated with childhood B-cell precursor acute lymphoblastic leukemia. Cancer Res 2018;78:5458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maia Rda R, Wunsch Filho V. Infection and childhood leukemia: review of evidence. Rev Saude Publica 2013;47:1172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor SM, Boneva RS. Infectious etiologies of childhood leukemia: plausibility and challenges to proof. Environ Health Perspect 2007;115: 146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eden T. Aetiology of childhood leukaemia. Cancer Treat Rev 2010;36: 286–97. [DOI] [PubMed] [Google Scholar]
- 14.McNally RJ, Eden TO. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br J Haematol 2004;127:243–63. [DOI] [PubMed] [Google Scholar]
- 15.Francis SS, Wallace AD, Wendt GA, Li L, Liu F, Riley LW, et al. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood 2017;129:1680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bzhalava D, Hultin E, Arroyo Muhr LS, Ekstrom J, Lehtinen M, de Villiers EM, et al. Viremia during pregnancy and risk of childhood leukemia and lymphomas in the offspring: nested case-control study. Int J Cancer 2016;138:2212–20. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanovic G, Pou C, Barrientos-Somarribas M, Bjerkner A, Honkaniemi E, Allander T, et al. Virome characterisation from Guthrie cards in children who later developed acute lymphoblastic leukaemia. Br J Cancer 2016;115:1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nency Y, Fardhani R, Wibawa S, Farida H, Cayami F. The correlation between history of breastfeeding and the incidence of childhood acute leukemia in Semarang, Indonesia. Asia Pac J Clin Oncol 2014;10:144. [Google Scholar]
- 19.Donovan J, Adelstein AM, Leighton P. Letter: sequelae of virus infection in pregnancy. Br Med J 1974;2:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedeschi R, Bloigu A, Ogmundsdottir HM, Marus A, Dillner J, dePaoli P, et al. Activation of maternal Epstein-Barr virus infection and risk of acute leukemia in the offspring. Am J Epidemiol 2007;165:134–7. [DOI] [PubMed] [Google Scholar]
- 21.Tedeschi R, Luostarinen T, Marus A, Bzhalava D, Ogmundsdottir HM, Dillner J, et al. No risk of maternal EBV infection for childhood leukemia. Cancer Epidemiol Biomarkers Prev 2009;18:2790–2. [DOI] [PubMed] [Google Scholar]
- 22.Bithell JF, Draper GJ, Gorbach PD. Association between malignant disease in children and maternal virus infections. Br Med J 1973;1:706–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney PA, Cartwright RA, Saiu JM, Mann JR, Stiller CA, Draper GJ, et al. The inter-regional epidemiological study of childhood cancer (IRESCC): a case control study of aetiological factors in leukaemia and lymphoma. Arch Dis Child 1987;62:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley JD, Buckley CM, Ruccione K, Sather HN, Waskerwitz MJ, Woods WG, et al. Epidemiological characteristics of childhood acute lymphocytic leukemia. Analysis by immunophenotype. The Childrens Cancer Group. Leukemia 1994;8:856–64. [PubMed] [Google Scholar]
- 25.Priftakis P, Dalianis T, Carstensen J, Samuelsson U, Lewensohn-Fuchs I, Bogdanovic G, et al. Human polyomavirus DNA is not detected in Guthrie cards (dried blood spots) from children who developed acute lymphoblastic leukemia. Med Pediatr Oncol 2003;40:219–23. [DOI] [PubMed] [Google Scholar]
- 26.Isa A, Priftakis P, Broliden K, Gustafsson B. Human parvovirus B19 DNA is not detected in Guthrie cards from children who have developed acute lymphoblastic leukemia. Pediatr Blood Cancer 2004;42:357–60. [DOI] [PubMed] [Google Scholar]
- 27.Bogdanovic G, Jernberg AG, Priftakis P, Grillner L, Gustafsson B. Human herpes virus 6 or Epstein-Barr virus were not detected in Guthrie cards from children who later developed leukaemia. Br J Cancer 2004;91:913–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafsson B, Jernberg AG, Priftakis P, Bogdanovic G. No CMV DNA in Guthrie cards from children who later developed ALL. Pediatr Hematol Oncol 2006;23:199–205. [DOI] [PubMed] [Google Scholar]
- 29.Gustafsson B, Huang W, Bogdanovic G, Gauffin F, Nordgren A, Talekar G, et al. Adenovirus DNA is detected at increased frequency in Guthrie cards from children who develop acute lymphoblastic leukaemia. Br J Cancer 2007;97:992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honkaniemi E, Talekar G, Huang W, Bogdanovic G, Forestier E, von Doblen U, et al. Adenovirus DNA in Guthrie cards from children who develop acute lymphoblastic leukaemia (ALL). Br J Cancer 2010;102:796–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsson B, Honkaniemi E, Goh S, Giraud G, Forestier E, von Dobeln U, et al. KI, WU, and Merkel cell polyomavirus DNA was not detected in Guthrie cards of children who later developed acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2012;34:364–7. [DOI] [PubMed] [Google Scholar]
- 32.Kwan ML, Metayer C, Crouse V, Buffler PA. Maternal illness and drug/medication use during the period surrounding pregnancy and risk of childhood leukemia among offspring. Am J Epidemiol 2007;165:27–35. [DOI] [PubMed] [Google Scholar]
- 33.Leppik L, Gunst K, Lehtinen M, Dillner J, Streker K, de Villiers EM. In vivo and in vitro intragenomic rearrangement of TT viruses. J Virol 2007;81:9346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009. [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 36.Core principles and methods for conducting a systematic review of health interventions, Systematic reviews: CRD’s guidance for undertaking reviews in health care. University of York: Centre for Reviews and Dissemination; 2009. p. 67.
- 37.Kumar A, Vashist M, Rathee R. Maternal factors and risk of childhood leukemia. Asian Pac J Cancer Prev 2014;15:781–4. [DOI] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasconcelos GM, Kang M, Pombo-de-Oliveira MS, Schiffman JD, Lorey F, Buffler P, et al. Adenovirus detection in Guthrie cards from paediatric leukaemia cases and controls. Br J Cancer 2008;99: 1668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehtinen M, Ogmundsdottir HM, Bloigu A, Hakulinen T, Hemminki E, Gudnadottir M, et al. Associations between three types of maternal bacterial infection and risk of leukemia in the offspring. Am J Epidemiol 2005;162:662–7. [DOI] [PubMed] [Google Scholar]
- 41.Lehtinen M, Koskela P, Ogmundsdottir HM, Bloigu A, Dillner J, Gudnadottir M, et al. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. Am J Epidemiol 2003;158: 207–13. [DOI] [PubMed] [Google Scholar]
- 42.Naumburg E, Bellocco R, Cnattingius S, Jonzon A, Ekbom A. Perinatal exposure to infection and risk of childhood leukemia. Med Pediatr Oncol 2002;38:391–7. [DOI] [PubMed] [Google Scholar]
- 43.McKinney PA, Juszczak E, Findlay E, Smith K, Thomson CS. Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study. Br J Cancer 1999;80:1844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dockerty JD, Skegg DC, Elwood JM, Herbison GP, Becroft DM, Lewis ME. Infections, vaccinations, and the risk of childhood leukaemia. Br J Cancer 1999;80:1483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman E, Ansell P, Bull D. Leukaemia and non-Hodgkin’s lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer 1997;76:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardner MJ, Snee MP, Hall AJ, Powell CA, Downes S, Terrell JD. Results of case-control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ 1990;300:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Steensel-Moll HA, Valkenburg HA, Vandenbroucke JP, van Zanen GE. Are maternal fertility problems related to childhood leukaemia? Int J Epidemiol 1985;14:555–9. [DOI] [PubMed] [Google Scholar]
- 48.Fine PE, Adelstein AM, Snowman J, Clarkson JA, Evans SM. Long term effects of exposure to viral infections in utero. Br Med J (Clin Res Ed) 1985;290:509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madden DL, Iltis J, Tzan N, Sever JL. Frequency of antibody to BK antigen in women whose children developed malignancies and women who developed detectable carcinoma in situ of the cervix during this pregnancy. Prog Clin Biol Res 1983;105:149–56. [PubMed] [Google Scholar]
- 50.Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenberg L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol 1973;2:229–35. [DOI] [PubMed] [Google Scholar]
- 51.Leeper C, Lutzkanin A. Infections During Pregnancy. Prim Care 2018;45: 567–86. [DOI] [PubMed] [Google Scholar]
- 52.Eppes C. Management of infection for the obstetrician/gynecologist. Obstet Gynecol Clin North Am 2016;43:639–57. [DOI] [PubMed] [Google Scholar]
- 53.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006;6: 438–46. [DOI] [PubMed] [Google Scholar]
- 54.Stacy SL, Buchanich JM, Ma ZQ, Mair C, Robertson L, Sharma RK, et al. Maternal obesity, birth size, and risk of childhood cancer development. Am J Epidemiol 2019;188:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sergentanis TN, Thomopoulos TP, Gialamas SP, Karalexi MA, Biniaris-Georgallis SI, Kontogeorgi E, et al. Risk for childhood leukemia associated with maternal and paternal age. Eur J Epidemiol 2015;30:1229–61. [DOI] [PubMed] [Google Scholar]
- 56.Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 2011;342:d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brito V, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med 2011;32:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis 2006;25:562–9. [DOI] [PubMed] [Google Scholar]
- 59.Marcotte EL, Thomopoulos TP, Infante-Rivard C, Clavel J, Petridou ET, Schuz J, et al. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol 2016;3:e176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinlen L. Childhood leukaemia, nuclear sites, and population mixing. Br J Cancer 2011;104:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.