Abstract
There is a scarcity of data on lung ultrasound (LUS) in SARS-CoV-2 pneumonia. As with many other pulmonary conditions, ultrasound may be a better diagnostic tool than routine chest radiography. In an era where computed tomography scanning is deferred because of the potential for cross-contamination, we evaluated the ability of LUS to detect a pattern of lung injury in SARS-CoV-2 pneumonia. A limited anterolateral LUS was performed to limit time spent in isolation rooms by ultrasound operators. We chose to use a hand-held ultrasound device due to portability and superior confidence in infection control. Both linear and phased array probes were used to obtain images of the pleura and lung. Of 69 patients who had lung ultrasound images saved and were included in the analysis, 36 were positive for SARS-CoV-2. Multifocal confluent B-lines, pleural irregularities, and the absence of moderate or large pleural effusions were the predominant pattern observed in most (86%) of SARS-CoV-2–positive patients. We evaluated the accuracy of the above criteria (LUS-CoV) and report a high sensitivity (91%) and specificity (86%) for SARS-CoV-2 pneumonia. In conclusion, a characteristic sonographic pattern of multifocal confluent B-lines with irregular pleural markings was seen on LUS in patients with SARS-CoV-2 pneumonia.
Keywords: COVID-19, lung ultrasound, SARS-CoV-2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has created a need for a rapid and sensitive testing modality to identify suspected cases and triage and manage them efficiently. The characteristic chest computed tomography (CT) findings in SARS-CoV-2 have a high sensitivity for SARS-CoV-2 pneumonia; however, the logistical obstacles with transporting critically ill patients, the risks of infection transmission during transport, and the postimaging decontamination that may take up to 60 minutes render this modality available only for a select few.1,2 Lung ultrasound (LUS) has gained popularity for the evaluation of various acute lung pathologies with higher sensitivity and specificity than portable chest radiographs (CXR).3 While viral pneumonia and acute respiratory distress syndrome findings on LUS are documented, there is a paucity of data on LUS in SARS-CoV-2 pneumonia.4–6 Our study aimed to determine a constellation of findings on lung ultrasound that are suggestive of SARS-CoV-2 infection.
METHODS
A retrospective analysis of patients admitted to our institution between March 29, 2020, and June 26, 2020, was performed after obtaining institutional review board approval. LUS was performed prospectively on the first encounter (within the first 24 h of hospitalization) in patients with suspected SARS-CoV-2 infection by one of the three critical care physicians (BJ, MI, IK) proficient in performing and interpreting LUS. We used a hand-held ultrasound device (GE Vscan Extend Dual Probe) and followed a modified scanning protocol with four scanning areas—three anterior (upper, mid, and lower lung zones) and the posterolateral costophrenic recess on each hemithorax—using both phased and linear array probes. Patients were only scanned in the supine position, and the posterior lung fields were skipped to reduce time spent in isolation rooms and conserve the personal protective equipment required for additional staff to assist with repositioning patients. Images were deidentified and cross-reviewed by all three operators independently. Disagreements in LUS findings were reviewed as a group, and if disagreements persisted, each operator’s findings were aggregated and LUS findings were only deemed to be present or absent if two of the three operators agreed.
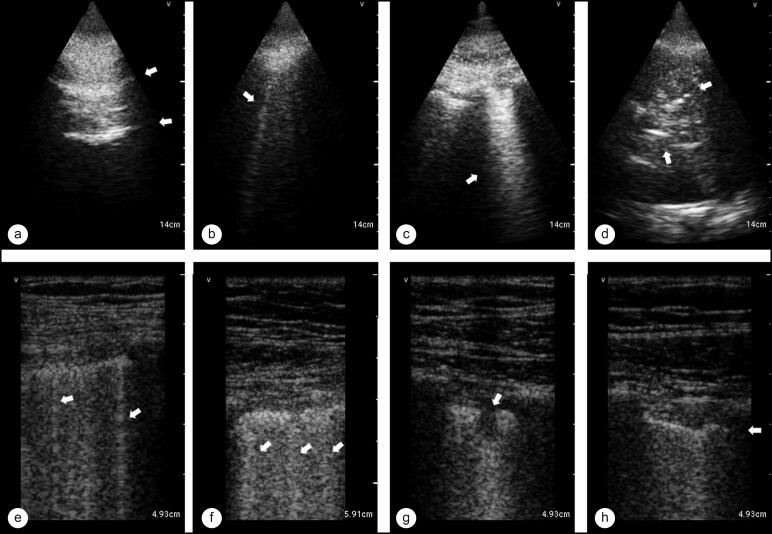
The presence of specific findings was recorded: A-lines (Figure 1a, repetitive vertical lines equidistantly placed), no confluent B-lines (Figure 1b and 1e, discrete vertical comet tail artifacts), confluent B-lines (Figure 1c and 1f, dense vertical artifact >5 mm in width at the pleural line by visual estimate), pleural effusion (a hypoechoic area between diaphragm and lung), costophrenic consolidation (Figure 1g, hepatization of lung with punctate hyperechoic artifacts at the costophrenic recess), subpleural consolidation (Figure 1g, small, well-defined hypoechoic areas immediately beneath the pleural line), and pleural irregularities (Figure 1h, loss of smooth contour of pleural line or discontinuities in the pleural line).
Figure 1.
A summary of LUS findings represented by arrows, divided into phased array probe images in the top row and linear array probe images in the bottom row. (a) A-lines, repetitive equidistant horizontal artifacts. (b, e) Nonconfluent B-lines, discrete vertical comet-tail artifacts. (c, f) Confluent B-lines, dense vertical artifacts >5 m width at the pleural line by visual estimate. (d) Pleural effusion and costophrenic consolidation, with the hypoechoic area representing pleural fluid and punctate hyperechoic artifacts within consolidated lung representing airbronchograms. (g) Subpleural consolidation, small, well-defined hypoechoic areas immediately beneath the pleural line. (h) Pleural irregularities, loss of smooth contour of pleura or discontinuities of the pleural line.
For analyses, the sample population was divided into two groups based on results of SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) testing. Given the poor sensitivity of RT-PCR testing,7 all patients who tested negative on their first test were retested in 48 hours. Patients included in this study were deemed to be SARS-CoV-2 negative only if two consecutive RT-PCR tests were negative and an alternate diagnosis explained the clinical picture.
Continuous variables are presented as medians and interquartile ranges, and categorical variables are reported as percentages. Data were analyzed using R Studio Version 1.1.383. Accuracy measurements were calculated using standard formulae. Differences in ultrasound patterns and features between the two groups were tested with Fisher’s exact test, and a two-sided P value < 0.05 was considered statistically significant.
RESULTS
The demographic and clinical characteristics of the 69 study patients are summarized in Table 1. Most (92%) patients in this study did not have chest CT scans during their stay; however, all patients had a portable anteroposterior CXR within 24 hours of performing LUS, the findings of which are summarized in Table 2. In all study patients, LUS was performed within 5 days of symptom onset. In patients who were RT-PCR positive for SARS-CoV-2, multifocal confluent B-lines (92% vs. 15%, P < 0.001) and pleural irregularities (92% vs. 42%, P < 0.001) were observed more frequently than in those who tested negative. All pleural effusions were in the SARS-CoV-2–negative group (0% vs. 24%, P = 0.002) and were moderate or large effusions.
Table 1.
Demographic and clinical characteristics of study patients based on positivity for SARS-CoV-2
| Variable | All (n = 69) | SARS-CoV-2 RT-PCR |
P value | |
|---|---|---|---|---|
| Positive (n = 36) | Negative (n = 33) | |||
| Age, median (IQR) (years) | 64 (51–76) | 62.5 (51–75.25) | 65 (51–80) | – |
| Male | 34 (49%) | 19 (52%) | 15 (45%) | – |
| Female | 35 (51%) | 17 (47%) | 18 (55%) | – |
| BMI, median (IQR) (kg/m2) | 30 (26–35) | 30.5 (27–37) | 29 (24–33) | – |
| Black | 19 (28%) | 13 (36%) | 6 (18%) | – |
| White | 40 (58%) | 17 (47%) | 23 (70%) | – |
| Hispanic | 10 (14%) | 6 (17%) | 4 (12%) | – |
| Diabetes mellitus | 32 (46%) | 20 (56%) | 12 (36%) | – |
| Hypertension | 46 (67%) | 25 (69%) | 21 (64%) | – |
| COPD | 16 (23%) | 6 (17%) | 10 (30%) | – |
| Interstitial lung disease | 2 (3%) | 0 (0%) | 2 (6%) | – |
| Chronic kidney disease | 20 (29%) | 7 (19%) | 13 (39%) | – |
| Coronary artery disease | 15 (22%) | 7 (19%) | 8 (24%) | – |
| Symptom onset (IQR) | 3 (2–4.75) | 3 (2–5) | 4 (2–4.25) | – |
| Fever | 38 (55%) | 27 (75%) | 11 (33%) | – |
| Cough | 35 (51%) | 20 (56%) | 15 (45%) | – |
| Dyspnea | 60 (87%) | 32 (89%) | 28 (85% | – |
| Fatigue | 38 (55%) | 20 (56%) | 18 (55%) | – |
| Admitted to ICU | 37 (54%) | 24 (67%) | 13 (39%) | – |
| Mechanical ventilation | 18 (26%) | 11 (31%) | 7 (21%) | – |
| High-flow nasal cannula | 19 (28%) | 11 (31%) | 8 (24%) | – |
| Highest SOFA score, median (IQR) | 4 (2–6) | 5 (2–10) | 3 (2–4) | – |
BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; RT-PCR, reverse-transcription polymerase chain reaction; SOFA, sequential organ failure assessment.
Table 2.
Chest radiograph and lung ultrasound findings of study patients based on positivity for SARS-CoV-2
| Imaging findings | All (n = 69) | SARS-CoV-2 RT-PCR |
P value | |
|---|---|---|---|---|
| Positive (n = 36) | Negative (n = 33) | |||
| Chest radiograph | ||||
| Normal | 11 (16%) | 4 (11%) | 7 (21%) | 0.330 |
| Bilateral infiltrates | 37 (54%) | 27 (75%) | 10 (30%) | <0.001 |
| Vascular congestion | 7 (10%) | 1 (3%) | 6 (18%) | 0.049 |
| Right lung infiltrates | 9 (13%) | 3 (8%) | 6 (18%) | 0.294 |
| Left lung infiltrates | 5 (7%) | 1 (3%) | 4 (12%) | 0.186 |
| Lung ultrasound | ||||
| A-lines | 50 (72%) | 20 (56%) | 30 (91%) | 0.001 |
| Nonconfluent B-lines | 45 (65%) | 24 (67%) | 21 (64%) | 0.806 |
| Confluent B-lines | 38 (55%) | 33 (92%) | 5 (15%) | <0.001 |
| Pleural effusion | 8 (12%) | 0 (0%) | 8 (24%) | 0.002 |
| Pleural irregularities | 47 (68%) | 33 (92%) | 14 (42%) | <0.001 |
| Subpleural consolidation | 29 (42%) | 21 (58%) | 8 (24%) | 0.007 |
| Costophrenic consolidation | 7 (10%) | 2 (6%) | 7 (15%) | 0.247 |
| LUS-CoV | 34 (49%) | 31 (86%) | 3 (9%) | <0.001 |
LUS indicates lung ultrasound; RT-PCR, reverse-transcription polymerase chain reaction.
We devised the LUS-CoV (Lung UltraSound in CoV) criteria, which include the presence of multifocal confluent B-lines, irregular pleura, and the absence of a moderate or large pleural effusion. Most patients with SARS-CoV pneumonia met the LUS-CoV criteria (86% vs. 9%, P < 0.001). Five patients in the SARS-CoV-2–positive group did not meet LUS-CoV criteria; two of these patients had pleural irregularities without confluent B-lines, and three had confluent B-lines without pleural irregularities. Comparing LUS-CoV criteria with SARS-CoV-2 RT-PCR, the sensitivity and specificity of the LUS-CoV criteria for SARS-CoV-2 pneumonia were 91% and 86%, respectively, with a positive predictive value of 86% and negative predictive value of 91%. The positive likelihood ratio was 6.38, and the negative likelihood ratio was 0.10.
DISCUSSION
Imaging is crucial in helping clinicians make confident decisions while awaiting testing results in patients suspected of having SARS-CoV-2 pneumonia. It can help monitor the progression of lung injury and response to treatments. We simplified the approach to performing and interpreting LUS to be able to generalize the use of this method of detection of lung injury patterns and successfully showed the value of using hand-held ultrasound in evaluating injury patterns on LUS in SARS-CoV-2 patients. Early in disease, we identified a consistent pattern of lung injury on LUS denoted by multifocal confluent B-lines and pleural irregularities, similar to previous reports of sonographic findings in SARS-CoV-2.4,5 While small pleural effusions are seen in some SARS-CoV-2 patients, moderate or large effusions are considered an unlikely consequence of a viral illness.8 Based on these findings, we devised the LUS-CoV criteria: multifocal confluent B-lines, pleural irregularities, and the absence of a moderate to large pleural effusion. These findings likely represent pleural and interstitial inflammation in SARS-CoV-2, similar to prior viral infections such as H1N1.9,10 The LUS-CoV criteria showed high sensitivity and specificity for SARS-CoV-2 pneumonia.
Four patients with SARS-CoV-2 had normal CXRs but met the LUS-CoV criteria. This demonstrates the potential ability of LUS to detect lung injury from SARS-CoV-2 early, even before radiographic abnormalities appear on CXR. Two patients in the SARS-CoV-2–negative group met the LUS-CoV criteria and also carried a diagnosis of advanced pulmonary fibrosis. Patients with chronic interstitial lung disease have pleural and subpleural disease that may be difficult to distinguish from acute changes of SARS-CoV-2 pneumonia on LUS.
In addition to the retrospective study design and a low number of patients with existing lung disease, we acknowledge three significant limitations of this study. First, we report on a small sample size. Second, RT-PCR testing is flawed with low sensitivity, and despite repeat testing some patients could have had false-negative results. Finally, while scanning was performed within 24 hours of presentation, patients were likely at different stages of lung injury. Even with these limitations, the LUS-CoV criteria in the context of acute infectious symptoms during this COVID-19 pandemic could aid in the diagnosis of SARS-CoV-2 pneumonia. We stress that the findings seen on LUS are a consequence of lung injury and inflammation caused by the SARS-CoV-2 pathogen and could be seen in other viral infections and disease processes that cause a similar lung injury pattern.
In conclusion, although larger studies are needed to validate our findings, we encourage the use of LUS in patients suspected of having SARS-CoV-2 pneumonia to strengthen clinical suspicions and increase the level of confidence in decision making.
References
- 1.Li X, Zeng W, Li X, et al. CT imaging changes of corona virus disease 2019 (COVID-19): a multi-center study in Southwest China. J Transl Med. 2020;18(1):154. doi: 10.1186/s12967-020-02324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Radiology . ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. Updated March 22, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection.
- 3.Winkler MH, Touw HR, van de Ven PM, Twisk J, Tuinman PR.. Diagnostic accuracy of chest radiograph, and when concomitantly studied lung ultrasound, in critically ill patients with respiratory symptoms: a systematic review and meta-analysis. Crit Care Med. 2018;46(7):e707–e714. doi: 10.1097/CCM.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 4.Yasukawa K, Minami T.. Point-of-care lung ultrasound findings in patients with COVID-19 pneumonia. Am J Trop Med Hyg. 2020;102(6):1198–1202. doi: 10.4269/ajtmh.20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group . Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F.. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 7.Tahamtan A, Ardebili A.. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou S, Wang Y, Zhu T, Xia L.. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 9.Testa A, Soldati G, Copetti R, Giannuzzi R, Portale G, Gentiloni-Silveri N.. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16(1):R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargani L, Forfori F, Giunta F, Picano E.. [Lung ultrasound imaging of H1N1 influenza]. Recenti Prog Med. 2012;103(1):23–25. doi: 10.1701/1022.11154. [DOI] [PubMed] [Google Scholar]