ABSTRACT
Background
Information about the impact of HIV coinfection on clinical characteristics of COVID-19 patients remains limited.
Methods
Maximum body temperatures, fever duration, chest CT and viral shedding, lymphocyte counts, and titer of SARS-CoV-2 antibody were compared between COVID-19 patients with and without HIV infection in Zhongnan Hospital of Wuhan University from January 20th to February 14th, 2020.
Results
Compared with 53 COVID-19 patients without HIV infection, the patients with SARS-CoV-2 and HIV coinfection had higher maximum body temperatures (38.7°C vs 37.6°C, P = 0.044), longer duration of fever (8.7 ± 4.5 vs 4.2 ± 2.1 days, P = 0.038), longer time to have improvement of chest CT images (22 vs 15 days from the onset of illness, P = 0.011), lower level of SARS-CoV-2 IgG (5.11 ± 32.33 vs 37.45 ± 15.48 AU/ml, P = 0.042). However, no statistically significant difference of duration of SARS-CoV-2 shedding in the two groups was found (12.3 ± 2.6 vs 13.4 ± 2.4 days, , P = 0.813).
Conclusion
Lower level of CD4+ T lymphocyte counts caused by HIV infection itself might be one of reasons for relatively weak ability to produce SARS-CoV-2 specific antibodies. The effects of anti-HIV drugs in prevention and treatment of COVID-19 appears to be limited.
KEYWORDS: COVID-19, SARS-CoV-2, AIDS, viral shedding
1. Introduction
COVID-19 is a public health emergency that is spreading worldwide and seriously affecting global economy [1– , , ,5]. There are currently no drugs available to inhibit SARS-CoV-2 replication. Due to the limited experience of combination of lopinavir, ritonavir and interferon beta (LPV/RTV-IFNβ) in the treatment of MERS [6,7], the question of whether or not anti-HIV drugs can play a role in the the prevention and treatment of COVID-19 has been drawn attention by public [8].
In China, the permanent resident population was 1.4 billion. The number of people living with HIV/AIDS is approximate one million and the recently reported patients with COVID-19 is 85 thousand [9,10]. Wuhan was a high incidence of COVID-19 in the early stages of the epidemic, but the number of patients with SARS-CoV-2 and HIV infection is still limited. In this background, limited information on COVID-19/HIV coinfection patients were showed and analyzed in this study, in order to provide clinical clues for future prospective studies.
2. Methods
2.1. Study population
The patients confirmed with COVID-19 and HIV infection were hospitalized to Zhongnan Hospital of Wuhan University from January 20th to February 14th. Among COVID-19 patients who were hospitalized during the same period, those patients with same gender and age were used as control groups. This retrospective study was approved by the ethics committee of Zhongnan Hospital of Wuhan University (No. 2,020,011). Informed written consent was waived by the Ethics Commission of Zhongnan Hospital of Wuhan University in consideration of the retrospective nature of the study and the urgency of reporting on emerging infectious diseases.
2.2. Real-time reverse transcription polymerase chain reaction assay for SARS-CoV-2
COVID-19 was confirmed by detecting SARS-CoV-2 RNA in throat swab samples using a virus nucleic acid detection kit according to the manufacturer’s protocol (Shanghai BioGerm Medical Biotechnology Co.,Ltd). Briefly, the RT-PCR assay for SARS-CoV-2 amplifies simultaneously two target genes: open reading frame 1ab (ORF1ab) and the ORF for the nucleocapsid protein (N). Target 1 (ORF1ab): forward primer CCCTGTGGGTTTTACACTTAA; reverse primer ACGATTGTGCATCAGCTGA; probe 5ʹ-VICCCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3ʹ. Target 2 (N): forward primer GGGGAACTTCTCCTGCTAGAAT; reverse primer CAGACATTTTGCTCTCAAGCTG; probe 5ʹ-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3ʹ. Positive (pseudovirus with a fragment of ORF1ab and N) and negative (pseudovirus with a standard fragment) quality control samples were tested simultaneously. A cycle threshold (Ct) value of less than 37 was defined a positive test, while a Ct value of more than 40 was defined as a negative test. For the cases with an intermediate Ct value (37–40), a second sample was tested and weakly positive was defined as a recurrence of Ct value of 37–40. The diagnostic criteria were based on the recommendation from the National Institute for Viral Disease Control and Prevention (China) [11,12,13].
2.3. Data collection
The following information, such as age, gender, COVID-19-related exposure history, symptoms, signs, severity assessment on admission, laboratory findings, and chest CT findings were collected. The data were reviewed by a trained team of physicians.
2.4. Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0.2 software. The comparison of highest body temperature, duration of fever and lung recovery, and levels of SARS-CoV-2 specific antibodies, changes of lymphocyte and CD4+ T lymphocyte counts were used independent group t tests. P < 0.05 was considered statistically significant.
3. Result
3.1. Baseline characteristics of COVID-19 patients with and without HIV infection
The selection of 56 COVID-19 patients in this study was shown in Figure 1. There were three patients with COVID-19 and HIV coinfection, and the other 53 COVID-19 patients without HIV infection were analyzed as control group. All the three patients with COVID-19 and HIV coinfection were male and receiving ART therapy, and the patients in control group were comparable in gender and age. Their baseline characteristics, such as fever, lymphocyte count, CD4+ T lymphocyte counts and chest CT manifestation, were compared in Table 1.
Figure 1.
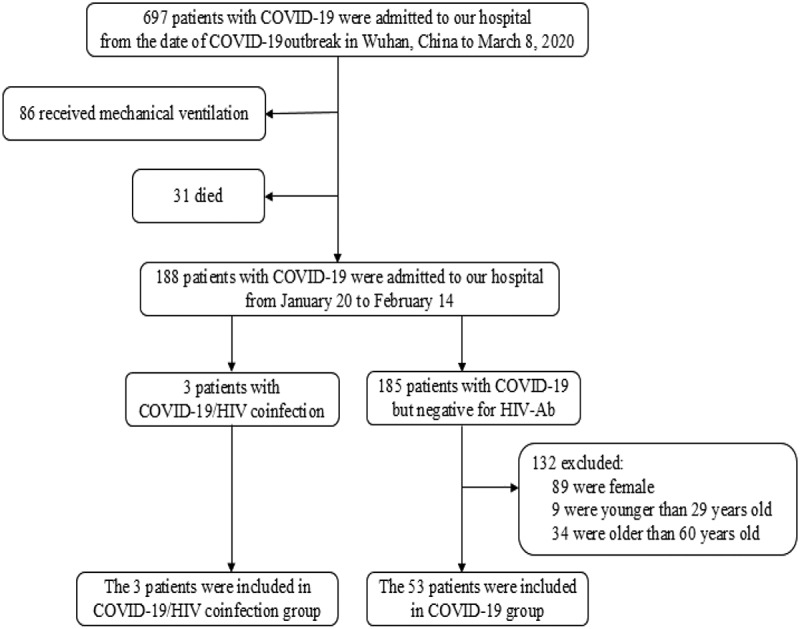
Flow chart of the study subjects
Table 1.
Baseline characteristics of 56 COVID-19 patients with or without HIV infection
| With HIV infection | Without HIV infection |
Test | P | |||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | 53 Patients | - | - | |
| Age/Mean age(years) | 31 | 60 | 29 | 49 | 0.584 | 0.630 |
| Gender | Male | Male | Male | Male | - | - |
| Lymphocyte count, ×109/L | 0.95 | 0.9 | 0.43 | 0.89 ± 0.21 | 0.631 | 0.531 |
| CD4 + T lymphocyte counts(/ul) | 420 | 550 | 21 | 638 | 2.126 | 0.038 |
| Fever | Yes | Yes | No | 49 Yes and 4 No | - | - |
| Chest CT | Radiographic findings of viral pneumonia |
Radiographic findings of viral pneumonia |
Normal | Radiographic findings of viral pneumonia |
- | - |
| ART regimens | AZT/3TC/NVP | TDF/3TC/EFV | STRIBILD | - | - | - |
| ART duration(months) | 96 | 75 | 1.7 | - | - | - |
STRIBILD, TRUVADA (emtricitabine and tenofovir disoproxil fumarate) + TYBOST (cobicistat) + VITEKTA (elvitegravir).
3.2. Comparative of clinical and laboratory findings between COVID-19 patients with and without HIV infection
In COVID-19 patients with and without HIV infection, the highest body temperatures were 38.7°C and 37.6°C, and the difference was statistically significant (t = 2.059, P = 0.044). The duration of fever in COVID-19 patients with HIV infection was 8.7 ± 4.5 days, which was longer than that in COVID-19 patients without HIV infection (8.7 ± 4.5 vs 4.2 ± 2.1 days, t = 2.129, P = 0.038). Moreover, the levels of SARS-CoV-2 IgG in COVID-19 patients with and without HIV infection were 5.11 ± 32.33 and 37.45 ± 15.48 AU/ml, and the difference was statistically significant(t = 2.089, P = 0.042). However, no statistically significant difference of duration of SARS-CoV-2 shedding in the two groups was found (12.3 ± 2.6 vs 13.4 ± 2.4 days, t = 0.238, P = 0.813). These data were shown in Figure 2.
Figure 2.
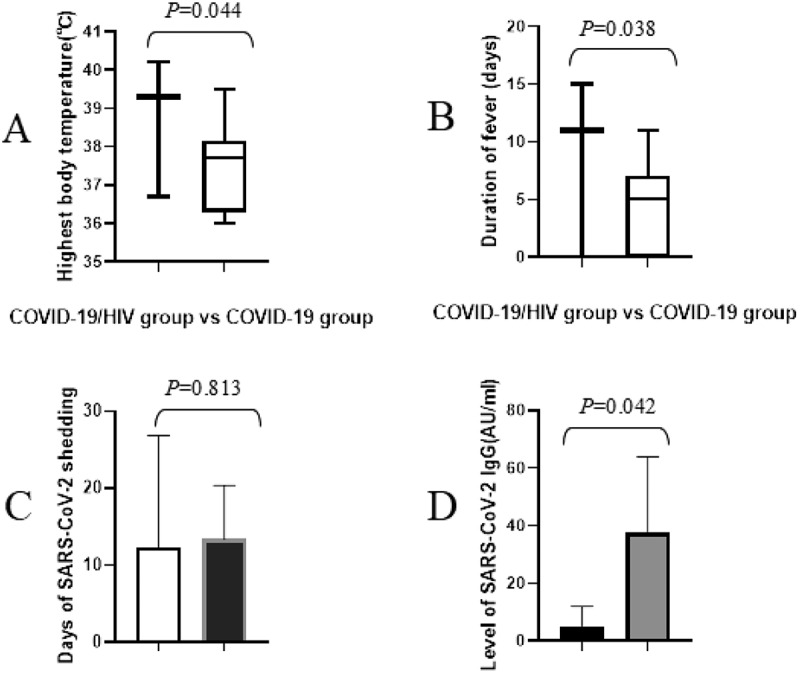
The comparison of highest body temperatures, duration of fever and viral shedding, and levels of SARS-CoV-2 IgG between COVID-19 patients with and without HIV infection
3.3. Comparative of radiological findings between COVID-19 patients with and without HIV infection
Chest CT images of the three patients with SARS-CoV-2 and HIV coinfection were shown in Figure 3. Two of them showed typical clinical and radiological manifestations on admission while chest CT manifestation of the third patient showed no abnormalities. After 10 and 14 days, respectively, imaging findings of the two patients with SARS-CoV-2 and HIV coinfection were deteriorated, while chest CT manifestation of the third patient didn’t show any signs of viral pneumonia during the course of the disease.
Figure 3.
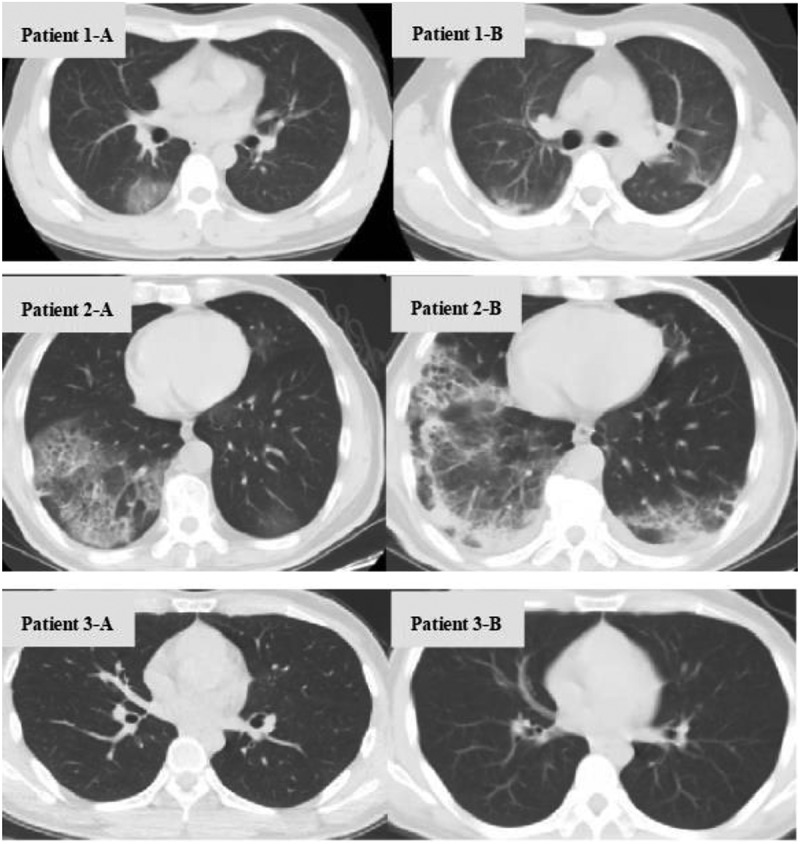
Chest computed tomography findings of three patients with COVID-19/HIV coinfection patient 1-A, patient 2-A, and patient 3-A were chest CT fingdings examined on admission, and patient 1-B, patient 2-B, and patient 3-B were reexamined 10, 14, and 12 days later, respectively
Moreover, compared with COVID-19 patients without HIV infection, the patients with SARS-CoV-2 and HIV coinfection took longer to have improvement of chest CT images (22 vs 15 days from the onset of illness, t = 2.655, P = 0.011). The data was shown in Figure 4.
Figure 4.
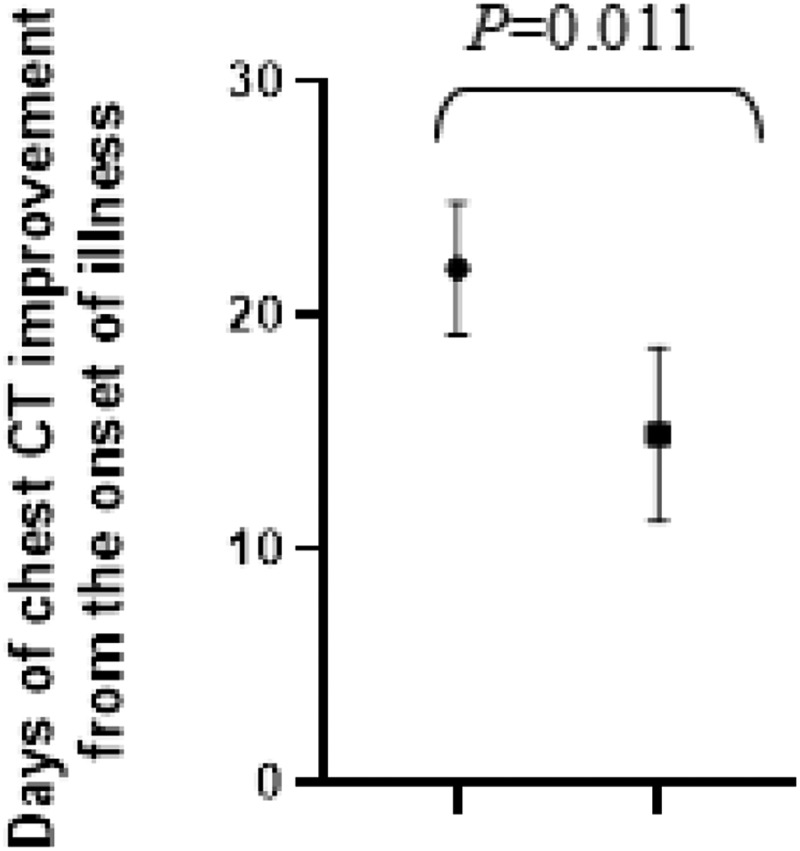
The comparison of days of chest CT improvement from the onset of illness between COVID-19 patients with and without HIV infection
3.4. Changes of total lymphocyte and CD4+ T lymphocyte counts in COVID-19 patients with or without HIV infection
Within 2 weeks of illness, the difference of levels of lymphocyte counts in COVID-19 patients with and without HIV infection were not statistical significance. However, in the following stages of the disease, the level of lymphocyte counts in COVID-19 patients with HIV infection was lower than that in COVID-19 patients without HIV infection[2–4 weeks of illness (0.91 vs 1.57 × 109/L, t = 2.110, P = 0.040); 4–6 weeks of illness (0.94 vs 1.81 × 109/L, t = 2.805, P = 0.007)].
During the course of disease, the levels of CD4 + T lymphocyte counts in COVID-19 patients with HIV infection were persistently lower than that in COVID-19 patients without HIV infection[within 2 weeks of illness (330 vs 638/ul, t = 2.126, P = 0.038); 2–4 weeks of illness (337 vs 705/ul, t = 2.366, P = 0.012); 4–6 weeks of illness (370 vs 768/ul, t = 2.726, P = 0.006)]. These data were shown in Table 2.
Table 2.
Comparison on changes of lymphocyte counts and CD4+ T lymphocyte counts in COVID-19 patients with or without HIV infection
| With HIV infection |
Without HIV infection |
Test |
P |
|||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | 53 Patients | - | - | |
| Lymphocyte counts, ×109/L | ||||||
| Within 2 weeks of illness | 0.65 | 0.76 | 0.40 | 0.86 ± 0.24 | 0.436 | 0.650 |
| 2–4 weeks of illness | 0.98 | 1.12 | 0.64 | 1.57 ± 0.62 | 2.110 | 0.040 |
| 4–6 weeks of illness | 0.89 | 1.24 | 0.70 | 1.81 ± 0.53 | 2.805 | 0.007 |
| CD4 + T lymphocyte counts(/ul) | ||||||
| Within 2 weeks of illness | 420 | 550 | 21 | 638 ± 102 | 2.126 | 0.038 |
| 2–4 weeks of illness 4–6 weeks of illness |
453 480 |
523 584 |
35 47 |
705 ± 105 768 ± 116 |
2.366 2.726 |
0.012 0.006 |
4. Discussion
Cases of COVID-19/HIV coinfection were not common until now. Although a few of studies involving patients with SARS‐CoV‐2 and HIV coinfection were reported [null–null ,15], information about the impact of HIV coinfection and anti-HIV drugs on the clinical characteristics and prognosis of COVID-19 patients remains limited.
In this study, even if COVID-19 patients coinfected with HIV, they have symptoms and imaging manifestations of typical viral pneumonia [1], or they are asymptomatic SARS‐CoV‐2 carrier, which suggesting that HIV coinfection does not affect the diagnosis and clinical typing of COVID-19.
Fever and cough are the initial symptoms and primary cause of hospitalization for most COVID-19 patients [16]. Compared with COVID-19 patients without HIV infection, except for the highest body temperature, those patients with COVID-19 and HIV coinfection had longer duration of fever and lung imaging recovery. We hypothesized that the delayed SARS-CoV-2 specific antibody response caused by HIV infection may affect the recovery of lung lesions, but this hypothesis needs to be confirmed by in-depth randomized controlled observation.
In terms of blood test indicators, lymphocytopenia is one of the characteristics of advanced stage of AIDS after HIV infection. Meanwhile lymphocytopenia is common among COVID-19 patients [17]. In this study, the levels of lymphocyte counts in COVID-19 patients without HIV infection returned to normal in later stage of the disease, but persistently lymphocytopenia was common in patients with COVID-19 and HIV infection throughout the observation period. The target cells for HIV infection are CD4+ T lymphocyte cells. As a component of lymphocyte subsets, the changes of CD4+ T lymphocyte counts can affects the level of total lymphocyte counts. For patients with COVID-19 and HIV infection, we hypothesized that, both the extremely low CD4+ T lymphocyte cells levels and the persistent lymphocytopenia after recovery from COVID-19 were associated with immunodeficiency by HIV infection itself. Moreover, we can preliminarily speculate that the lymphocytopenia associated with SARS-CoV-2 infection can return to normal along with the improvement of the disease. This is different from the lymphocytopenia caused by chronic HIV infection which requires ART for a long time to recovery.
In generally, it is difficult to obtain an effective treatment options during a short time of the outbreak of a new emerging viral diseases. As such, treatments designed and approved for other diseases are administered to patients with emerging viral syndromes empirically based on limited clinical or laboratory data. By referring the limited experience on Middle East respiratory syndrome coronavirus (MERS-CoV) treatment [6], a combination of lopinavir, ritonavir and interferon beta (LPV/RTV-IFNβ) is speculated to be valid for severe COVID-19 patients. This has led to speculation about the use of anti-HIV drugs to treat COVID-19. However, no statistically significant difference in their duration of SARS-CoV-2 shedding from the onset of symptoms was found between COVID-19 patients with and without HIV coinfection in this study. Although we cannot confirm or exclude the same incubation period between the two groups, we speculated that the anti-HIV drugs taken by patients with COVID-19 and HIV coinfection in this cluster failed to shorten the duration of SARS-CoV-2 shedding. In addition, all COVID-19/HIV coinfection patients in this study acquired SARS-CoV-2 infection during ART process, suggesting that anti-HIV drugs have limited effect on the prevention of SARS-CoV-2 infection. Of course, to draw the final conclusion, systematical observations are needed by comparing the SARS-CoV-2 infection rate in patients with ART and without ART under the same exposure environment.
Lymphocytes can play an important role in the maintenance of immune system function [18]. In this study, both on admission and after pulmonary symptoms recovery, the level of CD4+ T lymphocyte counts of patients with COVID-19 and HIV coinfection was lower than that of the COVID-19 patients without HIV infection. Moreover, the level of SARS-CoV-2 IgG was found to be lower in patients with COVID-19 and HIV coinfection. We speculated that the low level of CD4+ T lymphocyte counts in HIV coinfection group might be one of the reasons for the relatively weak ability to produce antibodies. In view of the previous reports about hepatitis B vaccination in HIV-infected patients, it was found that the lower the CD4+ T lymphocyte count, the lower proportion of patients to acquire protective HBsAb [19–23]. Therefore, as for those HIV-infected patients with severe immune deficiency, we speculated that their ability to produce SARS-CoV-2 antibodies could also be weakened. As for this particular population of HIV infection, the impact of CD4+ T lymphocyte counts on SARS-CoV-2 antibodies screening results should be taken into account, when conducting an epidemiological investigation of COVID-19 herd immunity.
There are some limitations in this study. First, the number of patients with COVID-19/HIV coinfections is limit. The situation of COVID-19/HIV coinfection did not receive enough attention in the early stage of COVID-19 outbreak. As far as we know, the total number of reported patients with SARS-CoV-2 and HIV coinfection is 142 cases all over the world [24]. Second, the three patients with COVID-19/HIV coinfection and the control group reported in this study were all male, which could not fully reflect the clinical characteristics of the overall population. A recent review reported that, of those patients with COVID-19 and HIV coinfection, 84.5% were male [24], which was consistent with the situation that all the three cases in this study were male. Future research on the effects of immunodeficiency on COVID-19 can be expanded to follow the clues provided in this study.
5. Conclusion
For COVID-19 patients coinfected with HIV coinfection, their highest body temperature were higher, and they had longer duration of fever and lung imaging recovery. Their lower level of CD4+ T lymphocyte counts caused by HIV infection itself might be one of the reasons for the relatively weak ability to produce SARS-CoV-2 specific antibodies. The effects of anti-HIV drugs in prevention and treatment of COVID-19 appears to be limited, but larger sample size of systematic observations are needed to draw conclusions.
6. Expert opinion
The authors present an interesting report on COVID-19 in HIV positive patients. Compared to 50 COVID-19 cases, the authors report that the co-infections were characterized by higher and longer duration fever. They also took longer to recover from their lung disease. Their lung lesions took additional days to resolve. This report, although limited by the low number of subjects, will add clinical insights to the literature, which will be needed for future studies.
Acknowledgments
We thank the patients, the nurses and physicians who provided care for the patients, and the investigators at Zhongnan Hospital of Wuhan University.
Funding Statement
This project was supported by National Natural Science Foundation of China (Grant No. 81572902).
Author contributions
All authors collected the clinical data. Rongrong Yang, Yongxi Zhang, Yong Xiong and Shicheng Gao drafted the manuscript. Xien Gui and Hengning Ke supervised the final manuscript. All authors were responsible for summarising all data related to this study.
Article highlights
HIV coinfection does not affect the diagnosis and clinical typing of COVID-19.
Patients coinfection with SARS-CoV-2 and HIV had longer duration of fever and lung imaging recovery.
Lymphocytopenia associated with SARS-CoV-2 infection can return to normal along with the improvement of the disease, which is different from lymphocytopenia caused by HIV infection which requires ART for a long time to recovery.
No statistically significant difference in the duration of SARS-CoV-2 shedding was found between COVID-19 patients with and without HIV coinfection in this study.
For HIV-infected patients with severe immune deficiency, the ability to produce SARS-CoV-2 antibodies could also be weakened.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Consent for publication
Written informed consent for publication can be obtained from all participants.
Availability of data and materials
All data analyzed during this study are included in this article.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. LANCET. 2020. January 24;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of the important studies that presenting the clinical features of COVID-19 patients in Wuhan.
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important background paper on the distribution of COVID-19 patients in designed hospital.
- 3.Lu H, Stratton CW, Tang YW.. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020. January 16;92(4):401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillie PJ, Samson A, Li A, et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J Infect. 2020. February 28;80:578–606. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spina S, Marrazzo F, Migliari M, et al. The response of Milan’s emergency medical system to the COVID-19 outbreak in Italy. LANCET. 2020. February 28;395:e49-e50. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim UJ, Won EJ, Kee SJ, et al. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for middle east respiratory syndrome. Antivir Ther. 2016;21(5):455–459. [DOI] [PubMed] [Google Scholar]
- 8.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020. May 7;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyu P, Chen FF. National HIV/AIDS epidemic estimation and interpretation in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(10):1191–1196. [DOI] [PubMed] [Google Scholar]
- 10.Update on COVID-19 as of 1 September. National Health Commission of the People’s Republic of China; 2020. cited 2020 September02. Available from: http://www.nhc.gov.cn/xcs/yqfkdt/202009/c9e6ec635c2447a7a467934b1b98aa7a.shtml [DOI] [PMC free article] [PubMed]
- 11.National Institute for Viral Disease Control and Prevention (China) . Specific primers and probes for detection 2019 novel coronavirus. Beijing: National Institute for Viral Disease Control and Prevention (China); [Chinese]. 2020. January 21. Available from http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html [Google Scholar]
- 12.Zhu F, Cao Y, Xu S, et al. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92(6):529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the important studies that presented the clinical of patients with SARS-CoV-2 and HIV co-infection.
- 13.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the important studies that presented the clinical characteristics of SARS-CoV-2 and HIV co-infection.
- 14.Guo W, Ming FZ, Feng Y, et al. Patterns of HIV and SARS-CoV-2 co-infection in Wuhan, China. J Int AIDS Soc. 2020;23:e25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao JJ, Liao XJ, Wang HY, et al. Early virus clearance and delayed antibody response in a case of COVID-19 with a history of co-infection with HIV-1 and HCV. Clin Infect Dis. 2020. April 9:ciaa408. Online ahead of print. DOI: 10.1093/cid/ciaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. published online Feb 7. DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: A randomized controlled trial. JAMA. 2011;305:1432–1440. [DOI] [PubMed] [Google Scholar]
- 20.De Vries-Sluijs TE, Hansen BE, van Doornum GJ, et al. A randomized controlled study of accelerated versus standard hepatitis B vaccination in HIV-positive patients. J Infect Dis. 2011;203:984–991. [DOI] [PubMed] [Google Scholar]
- 21.Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. Standard doses of hepatitis B vaccination in HIV-infected adults: A randomized, controlled trial. PLoS One. 2013;8:e80409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psevdos G, Kim JH, Groce V, et al. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS. 2010;24(7):403–407. . [DOI] [PubMed] [Google Scholar]
- 23.Sayad B, Alavian SM, Najafi F, et al. Effects of oral levamisole as an adjuvant to hepatitis B vaccine in HIV/AIDS patients: a randomized controlled trial. Hepat Mon. 2012;12(9):e6234. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costenaro P, Minotti C, Barbieri E, et al. SARS-CoV-2 infection in people living with HIV: a systematic review. Rev Med Virol. 2020. September 1:e2155. Online ahead of print. DOI: 10.1002/rmv.2155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this article.


