ABSTRACT
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has quickly spread all over the globe from China. Pleural involvement is not common; around 5–10% of patients can develop pleural effusion and little is known about the involvement of pleural structures in this new infection.
A 61-year-old male kidney transplant patient with a history of multiple biopsy-confirmed acute rejections and chronic allograft rejection was admitted to our COVID-19 Unit with dry cough, exertional dyspnea, oliguria, and abdominal distension. Lung ultrasound imaging, chest X-ray, and CT scan showed left pleural effusion and atelectasis of the neighboring lung parenchyma. RT-PCR was positive for SARS-CoV-2 in the pleural fluid and cytology showed mesothelial cells with large and multiple nuclei, consistent with a cytopathic effect of the virus.
This is one of few reports describing detection of SARS-CoV-2 in the pleural fluid and to the best of our knowledge, is the first to document the simultaneous presence of a direct cytopathic effect of the virus on mesothelial cells in a kidney transplant patient with COVID-19 pneumonia. The pleura proved to be a site of viral replication where signs of a direct pathological effect of the virus on cells can be observed, as we report here. RT-PCR for SARS-CoV-2 should be part of routine examination of pleural effusion even in patients with mild respiratory symptoms or with comorbidities that seem to explain the cause of effusion.
KEYWORDS: COVID-19, SARS-CoV-2, pleural fluid, transplant
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection spread rapidly around the world from China. Common symptoms of Coronavirus disease 2019 (COVID-19) at presentation include fever, dyspnea, dry cough, fatigue, and diarrhea [1]. Nasal congestion and anosmia have also been reported [1,2]. Interstitial pneumonia is the major clinical manifestation and around 10% of patients develop severe acute respiratory distress syndrome (ARDS) [3,4]. Pleural involvement is not common; around 5–10% of patients can develop pleural effusion but little is known about involvement of pleural structures in this new infection [5].
This is one of few reports describing detection of SARS-CoV-2 in pleural fluid, and to the best of our knowledge, it is the first to document the simultaneous presence of a direct cytopathic effect of the virus on mesothelial cells in a kidney transplant patient with COVID-19 pneumonia.
Case report
A 61-year-old male kidney transplant patient with a history of multiple biopsy-confirmed acute rejections and chronic allograft rejection was admitted to our COVID-19 Unit on 9 April 2020. Renal function was severely reduced and serum creatinine measured 3 weeks before admission was 7.59 mg/dl (glomerular filtration rate 16 ml/min), although he was not yet on dialysis. His maintenance immunosuppressant therapy consisted of prednisone, tacrolimus, and mycophenolate mofetil.
On admission, the patient presented with mild respiratory symptoms, oliguria, and abdominal distension. He reported a dry cough and exertional dyspnea in the previous week and a nasopharyngeal swab was positive for SARS-CoV-2. Since serum creatinine was 11.1 mg/dl, hemodialysis was immediately begun. He also showed severe anemia and leukopenia while C-reactive protein was 4.00 mg/dl (see Table 1 for complete lab data). Chest auscultation revealed wheezing, basal right crackles, basal left reduction of physiological vesicular murmur, and dullness to percussion. Lung ultrasound imaging indicated an interstitial syndrome with bilateral diffuse multiple B lines and left basal pleural effusion. Chest X-ray showed left pleural effusion and a CT scan confirmed the left pleural effusion and atelectasis of the neighboring lung parenchyma with no signs of viral parenchymal involvement (Figure 1(a)).
Table 1.
Lab findings on admission to hospital
| C-reactive protein | 4.00 mg/dL |
|---|---|
| Lactate dehydrogenase | 262 IU/L |
| D-dimer | 478 ug/L |
| Ferritin | 925 ng/mL |
| White blood cells | 2.63 * 10^3/mmc |
|
67.8% |
|
20.5% |
|
9.1% |
|
1.5% |
|
1.15 |
| Red blood cells | 2.19 * 10^6/mmc |
| Hemoglobin | 6.2 g/dL |
| Hematocrit | 19.4% |
| Mean Corpuscular Volume | 88.6 fL |
| Mean Corpuscular Hemoglobin | 28.3 pg |
| Mean Corpuscular Hemoglobin Concentration | 32.0 g/dL |
| Red blood cell Distribution Width | 16.8% |
| Platelets | 148 10^3/mmc |
| Glucose | 71 mg/dL |
| Creatinine | 11.1 mg/dL |
| Blood Urea Nitrogen | 212 mg/dL |
| Cholesterol | 161 mg/dL |
| Total proteins | 4.2 g/dL |
| Albumin | 2.4 g/dL |
| Bilirubin | 0.3 mg/dL |
| Glutamic oxaloacetic transaminase | 10 IU/L |
| Glutamate-pyruvate transaminase | 8 IU/L |
Figure 1.
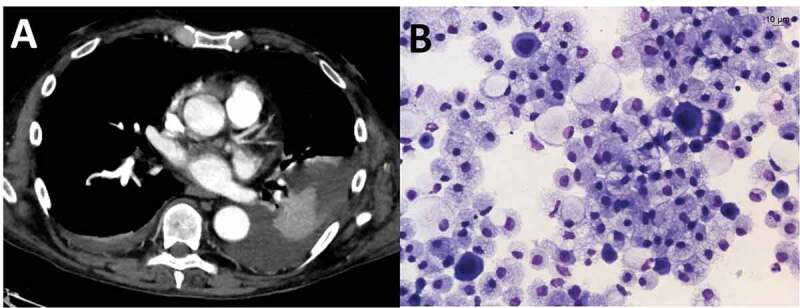
(a): Chest CT after intravenous administration of only 60 ml of contrast medium. Virtual monoenergetic reconstruction (55 KeV) of dual-energy CT data shows pleural effusion and atelectasis of the neighboring lung parenchyma and excludes active foci of bleeding. (b): Microvacuolated macrophages and scattered mesothelial cells with enlarged multiple nuclei suggesting viral infection
The laboratory characteristics of the fluid were compatible with transudative effusion (Table 2). Reverse transcriptase-polymerase chain reaction (RT-PCR) was positive for SARS-CoV-2 and cytology showed mesothelial cells with large multiple nuclei, consistent with a cytopathic effect of the virus (Figure 1(b)); microbiology was negative.
Table 2.
Features of pleural fluid
| Appearance | Clear |
|---|---|
| Colour | Yellow |
| Total protein | 2 g/dL |
| Lactate dehydrogenase | 79 U/L |
| White cell count | 25/mcl (80% mononuclear cells) |
The patient was treated with methylprednisolone 1.5 mg/kg/day for 5 days while the other immunosuppressants were suspended. Respiratory condition improved rapidly and the nasopharyngeal swab for SARS-CoV-2 became negative 8 days after admission. Unfortunately, renal function did not improve and long-term hemodialysis was begun. During hospitalization, the patient also developed a perforated diverticulum and left hemicolectomy was necessary. Despite the difficulties encountered during hospital stay, the patient recovered completely and was discharged 28 days after admission.
Discussion
SARS-CoV-2 infection may present with different symptoms that express direct or indirect involvement of various organs and systems [5]. In most patients, imaging of the lung shows ground glass opacities and crazy paving pattern in the early phases, and later larger consolidations in the basal or dependent lung regions, readily visible by CT [6]. Due to its safety, repeatability, absence of radiation, low cost and point of care use, ultrasound imaging of the lungs has shown good clinical value in COVID-19 patients [7]. Despite the high sensitivity of these techniques, pleural effusion has only occasionally been reported in COVID-19 [5]. In our experience, the incidence of pleural effusion in hospitalized COVID-19 patients is 7.5%; in most cases it was mild, not requiring drainage.
Mei et al. recently published a case report of a COVID-19 patient whose pleural fluid RT-PCR was positive for SARS-CoV-2 [8]. The present report is the first concerning pleural effusion in a kidney transplant patient with COVID-19. In our case, RT-PCR of pleural fluid was positive for SARS-CoV-2, and we also documented mesothelial cells with large multiple nuclei, consistent with a cytopathic effect of the virus. Unfortunately, we were unable to perform electron microscopy.
Dysregulated and/or exaggerated cytokine and chemokine responses in SARS-CoV-2 infection have been reported in many studies. Cytokine release syndrome is a systemic inflammatory response, that can be triggered by infection, certain drugs, and other factors. It has been demonstrated in COVID-19 patients [9]. In vitro experiments show that delayed release of cytokines and chemokines occurs in respiratory epithelial cells, dendritic cells, and macrophages in the early stage of SARS-CoV-2 infection, and that the cells secrete low levels of interferon antiviral factors and high levels of proinflammatory cytokines (interleukins IL-1β, IL-6, and tumor necrosis factor) and chemokines [10]. No specific antiviral therapy for SARS-CoV-2 infection has yet been found, although most reports suggest that immunomodulation therapy can play a positive role. Blockade of IL-6 and IL-1 has shown promising results and high doses of steroids prove to reduce mortality, moderating cytokine release [9,11].
The angiotensin-converting enzyme 2 (ACE2) receptor proves to play a crucial role in viral entry into cells and its reduced transmembrane expression is associated with increased risk of ARDS in infected subjects [12]. Drugs interfering with ACE2 receptor show promising positive effects, making the receptor a major focus in the search for new therapies [13].
In solid organ transplant recipients, such as our patient, the risk of pneumonia and development of ARDS is expected to be higher, although a number of reports have indicated a similar incidence to that of the general population [14,15]. The hypothesis that post-transplant immunosuppression can somehow protect patients against the hyperinflammatory syndrome resulting from the cytokine storm induced by SARS‐CoV‐2 is intriguing and needs further confirmation [9,16]. In the present case, despite the setbacks our patient had to face (severe kidney failure and intestinal perforation requiring dialysis and surgery, respectively), the outcome was positive and the patient was eventually discharged.
Diagnostic and therapeutic procedures, such as thoracentesis, must only be performed in COVID-19 patients if there are strict clinical indications. All safety criteria for operators performing collection and analysis of samples must comply with international standards. In our case, pleural fluid was drawn at the patient’s bedside in the COVID-19 isolation ward. The pleural fluid samples were treated according to current national and international regulations. All diagnostic laboratories in our hospital operate at biosecurity levels 2, 3, and 4 and are able to handle biological samples potentially infected with SARS-CoV 2 [17].
Conclusions
Although pleural involvement is not common in COVID-19, patients should be checked for the presence of effusion. Signs of a direct pathological effect on pleural cells can even be observed in cases with mild/moderate pneumonia, as we report in this case, suggesting that the pulmonary and pleural compartments may behave distinctly and that participation of the pleura is not always a result of pulmonary spread. However, we do not have reliable data to support this hypothesis, verification of which will require further studies. RT-PCR for SARS-CoV-2 should be part of routine examination of pleural effusion, even in patients with mild respiratory symptoms or with comorbidities that seem to explain the cause of effusion. How to treat SARS-CoV-2 infection is still debated. Immune modulation has shown promising results and several trials are underway [11]. Solid organ transplant patients offer a unique in vivo model of biological responses to this new virus and can be useful to help understand response to therapy. Pleural drainage should nevertheless be an aspect of non-pharmacological therapy in selected COVID-19 patients to improve respiratory dynamics and prognosis.
Funding Statement
There was no funding received for this article.
Declaration of financial/other relationships
The contents of the paper and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication.
Declaration of interest
The authors have no conflicts of interest to declare.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contribution
DB performed the literature search, data collection, data analysis and interpretation and wrote the manuscript. FF, EDV, MAM, LV, MGD, GG, AG, MGC, EB, SS, SV, RG and BF performed data analysis and interpretation. All authors contributed equally to clinical management of the patient during his hospital stay. All authors are guarantors of the paper, taking responsibility for the integrity of the work as a whole.
All authors read and approved the final version of the manuscript.
References
- 1.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020. July;92(7):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. February 15;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Coppola S, Cressoni M, et al. 19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020. May 15;201(10):1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remuzzi A, Remuzzi G.. COVID-19 and Italy: what next? Lancet. 2020. April 11;395(10231):1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020. June;55(6):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020. June;295(3):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MJ, Hayward SA, Innes SM, et al. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020. April 10;80: 607–613. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei F, Bonifazi M, Menzo S, et al. First detection of SARS-CoV-2 by real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay in pleural fluid. CHEST. 2020;158:e143-e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capecchi PL, Lazzerini PE, Volterrani L, et al. Antirheumatic agents in covid-19: is IL-6 the right target? Ann Rheum Dis. 2020. April 16:annrheumdis–2020–217523. [DOI] [PubMed] [Google Scholar]
- 10.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine storm’ in COVID-19. J Infect. 2020. June;80(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020. June 16;582:469. [DOI] [PubMed] [Google Scholar]
- 12.Alfano G, Guaraldi G, Fontana F, et al.; for the Modena Covid-19 Working Group (MoCo19) . The role of the renin-angiotensin system in severe acute respiratory syndrome-CoV-2 infection. Blood Purif. 2020. April 28:1–5. DOI: 10.1159/000507914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoufaly A, Poglitsch M, Aberle JH, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020. September 24. DOI: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant. 2020. April 16;20:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett D, De Vita E, Ventura V, et al. Impact of SARS-CoV-2 outbreak on heart and lung transplant: A patient-perspective survey. Transpl Infect Dis. 2020. August;2:e13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conticini E, Bargagli E, Bardelli M, et al. COVID-19 pneumonia in a large cohort of patients treated with biological and targeted synthetic antirheumatic drugs. Ann Rheum Dis. 2020. May 15:annrheumdis–2020–217681. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.National Research Council (US) Committee on Prudent Practices in the Laboratory . Prudent practices in the laboratory: handling and management of chemical hazards: updated version. Washington (DC): National Academies Press (US); 2011. 4, Evaluating Hazards and Assessing Risks in the Laboratory. [PubMed] [Google Scholar]


