Abstract
Introduction
Studies have shown that both perioperative and anesthesia-related cardiac arrest (CA) and mortality rates are much higher in developing countries than in developed countries. This review aimed to compare the rates of perioperative and anesthesia-related CA and mortality during 2 time periods in Brazil.
Methods
A systematic review with meta-analysis of full-text Brazilian observational studies was conducted by searching the Medline, EMBASE, LILACS and SciELO databases up to January 29, 2020. The primary outcomes were perioperative CA and mortality rates and the secondary outcomes included anesthesia-related CA and mortality events rates up to 48 postoperative hours.
Results
Eleven studies including 719,273 anesthetic procedures, 962 perioperative CAs, 134 anesthesia-related CAs, 1,239 perioperative deaths and 29 anesthesia-related deaths were included. The event rates were evaluated in 2 time periods: pre-1990 and 1990–2020. Perioperative CA rates (per 10,000 anesthetics) decreased from 39.87 (95% confidence interval [CI]: 34.60–45.50) before 1990 to 17.61 (95% CI: 9.21–28.68) in 1990–2020 (P < 0.0001), while the perioperative mortality rate did not alter (from 19.25 [95% CI: 15.64–23.24] pre-1990 to 25.40 [95% CI: 13.01–41.86] in 1990–2020; P = 0.1984). Simultaneously, the anesthesia-related CA rate decreased from 14.39 (95% CI: 11.29–17.86) to 3.90 (95% CI: 2.93–5.01; P < 0.0001), while there was no significant difference in the anesthesia-related mortality rate (from 1.75 [95% CI: 0.76–3.11] to 0.67 [95% CI: 0.09–1.66; P = 0.5404).
Conclusions
This review demonstrates an important reduction in the perioperative CA rate over time in Brazil, with a large and consistent decrease in the anesthesia-related CA rate; however, there were no significant differences in perioperative and anesthesia-related mortality rates between the assessed time periods.
Introduction
The global surgical volume is increasing worldwide; however, the safety and quality of surgical care and anesthesia management remain poor in many regions, and outcome assessments are not often prioritized in developing countries [1]. Among the complications of surgery, cardiac arrest (CA) is one of the worst events, as it can result in sequelae, loss of function and death. The rates of perioperative CA and mortality can be used to explore the differences among facilities that perform surgical and anesthesia procedures in different centers and can serve as quality indicators to promote improvements in patient safety and reductions in unfavorable outcomes [2–4]. Brazil is a developing country that, over the years, has experienced great difficulty in offering adequate healthcare to its population [5].
Two systematic reviews with a proportion meta-analysis of worldwide studies have shown that the rates of both perioperative and anesthesia-related CA and mortality are much higher in developing countries than in developed countries [6, 7]. A review of the studies conducted in Brazil showed that there was a reduction in the rate of perioperative CA during the last 25 years, similar to the global trend [8].
This systematic review aimed to compare the rates of perioperative and anesthesia-related CA and mortality during 2 time periods in Brazil. We hypothesized that the rates of intraoperative and anesthesia-related CA and mortality have decreased over time.
Methods
The Cochrane Handbook for Intervention Reviews [9] guided our choice of methods. Our report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (S1 File) [10]. The protocol for this proportional meta-analysis was registered in a public registry (PROSPERO, #CRD42019141158).
Search strategy
We searched the medical literature to identify all Brazilian observational studies that reported perioperative and/or anesthesia-related CA or mortality rates. We searched the Medline (PubMed), EMBASE, Latin American and Caribbean Health Sciences Literature (LILACS), and Scientific Electronics Library Online (SciELO) databases from their inception to January 29, 2020. Search strategies were developed in consultation with a research librarian with systematic review expertise. The search was conducted using Index Terms (e.g., MeSH and Emtree) and text words and word variants for “an(a)esthesia”, “cardiac arrest” and “mortality”, as well as an exhaustive list of synonyms. The search strategy was adapted to each database to find relevant studies (S2 File). Five independent reviewers (L.G.B., M.P.M., R.S., M.P., and J.B.C.) performed the initial screening of the titles and abstracts of the studies to identify whether they contained relevant content. The relevant studies were downloaded and reviewed in full by 2 independent reviewers (L.G.B. and I.B.S.). These reviewers also reviewed the references of the included studies and added additional relevant studies. There were no restrictions on either year of publication or language.
Outcome definitions
The primary outcomes were perioperative CA and mortality events (regardless of triggering factors: patient’s disease/condition, surgery and anesthesia). The secondary outcomes were anesthesia-related CA and mortality events (entirely and partially anesthesia-related), and entirely anesthesia-related CA and mortality events, defined as CA or mortality deemed to be attributable only to anesthesia (e.g., depression of ventilation leading to hypoxemic CA after anesthesia induction in a stable patient without comorbidities). All perioperative, anesthesia-related, and entirely anesthesia-related CAs or mortality were defined by the authors of the studies included in this review.
Selection criteria
To ensure that the studies represented a mixed population, full-text studies were included if they fulfilled the following criteria: (1) observational studies; and (2) the study’s outcome measurements were perioperative and/or anesthesia-related CA or perioperative and/or anesthesia-related mortality up to 48 postoperative hours.
Studies were excluded if they met any of the following criteria: (1) the study focused on specific age groups (e.g., only geriatric patients); (2) the study included only a specific anesthetic technique (e.g., only neuraxial blockade); (3) the study included only a specific surgery type (e.g., only cardiac surgery); (4) the study included only a specific American Society of Anesthesiologists (ASA) physical status (e.g., only ASA I patients); and (5) the study included less than 3,000 patients because this number is the minimum required to enable estimation of the incidence of rare adverse events (≤ 1 per 1,000 anesthetics), according to the rule of three sample size approximations [11].
Assessment of methodological quality
Two independent reviewers (L.G.B. and J.R.C.B.) assessed the studies selected for retrieval for methodological validity prior to inclusion in the review using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for Prevalence Studies [12]. The nine domains in this tool were target population, sampling, sample size, description of participants and setting, coverage of identified sample, methods of identifying the outcome, reliability of the outcome measurement, appropriate statistical analysis and response rate. The cutoff for inclusion/exclusion was determined through consensus between the reviewers. The cutoff point for the inclusion of a study in the review was a “yes” answer to at least five of the nine questions (more than 50%) on the standardized critical appraisal instrument. Discrepancies were resolved after discussion between the two authors; if this was not possible, the discrepancies were resolved with a third author (M.G.B.).
Data extraction
We used a standard form to extract the data from the included studies (S1 Table). Two of the authors (L.G.B. and I.B.S.) independently identified the studies that were included in the review. Any discrepancies were resolved after discussion with a third reviewer (J.R.C.B.). When more than one study was published with the same population, data were extracted from the most recent and/or complete study. If eligible articles were missing data, their authors were contacted for clarification.
Statistical analysis
Proportion meta-analysis
Using StatsDirect software (StatsDirect Ltd., Altrincham, Cheshire, UK), a random-effects model with inverse Freeman-Tukey double arcsine transformation was applied to calculate the weighted event rates across all of the studies included in the proportion meta-analysis [13, 14]. For the purpose of this study, the data were dichotomized into 2 time periods. A similar method was used in three prior systematic reviews: two on perioperative CA [6, 15] and one on perioperative CA and mortality [7]. This stratification was based on many safety-improvement measures, such as monitoring for oxygenation and ventilation parameters, anesthesia workstations with modern ventilators, new anesthesia medications, supraglottic devices, anesthesia and surgery safety protocols, advances in postoperative pain management, and an increase in the number of postanesthesia and intensive care beds, which emerged towards the end of the 1980s in developed countries and later in some developing countries [16, 17].
We performed a sensitivity analysis to define the cutoff year between the 2 time periods (from 1988 to 1992). The studies were allocated to one of the two periods based on their median patient recruitment interval [6, 7, 15]. The event rate was defined as the number of CAs or deaths per 10,000 anesthetics. The data are reported with their 95% confidence intervals (CIs). To compare differences in the proportions of events, a model with a binomial distribution adjusted for overdispersion was generated using SAS for Windows® software, v.9.4 (SAS Institute, Cary, NC). The I2 statistic was also used as an alternative approach to quantify the degree of heterogeneity among studies [18]; values higher than 40% suggested significant heterogeneity among the studies [19]. A P value < 0.05 was considered significant.
Results
Selection of studies
The literature search identified 15,265 potentially eligible articles. After reviewing the titles and abstracts, we excluded 5,670 duplicate studies using the software EndNote® (X9.2/2019 version, Clarivate Analytics, PA) and 9,580 studies because of a lack of relevance. We retrieved 15 potentially relevant full-text papers for detailed evaluations. Of these articles, 11 studies met the inclusion criteria, including 6 studies reporting perioperative CA, 4 studies reporting anesthesia-related CA, 9 studies reporting perioperative mortality, and 7 studies reporting anesthesia-related mortality (Fig 1).
Fig 1. PRISMA flow diagram showing the study selection process.
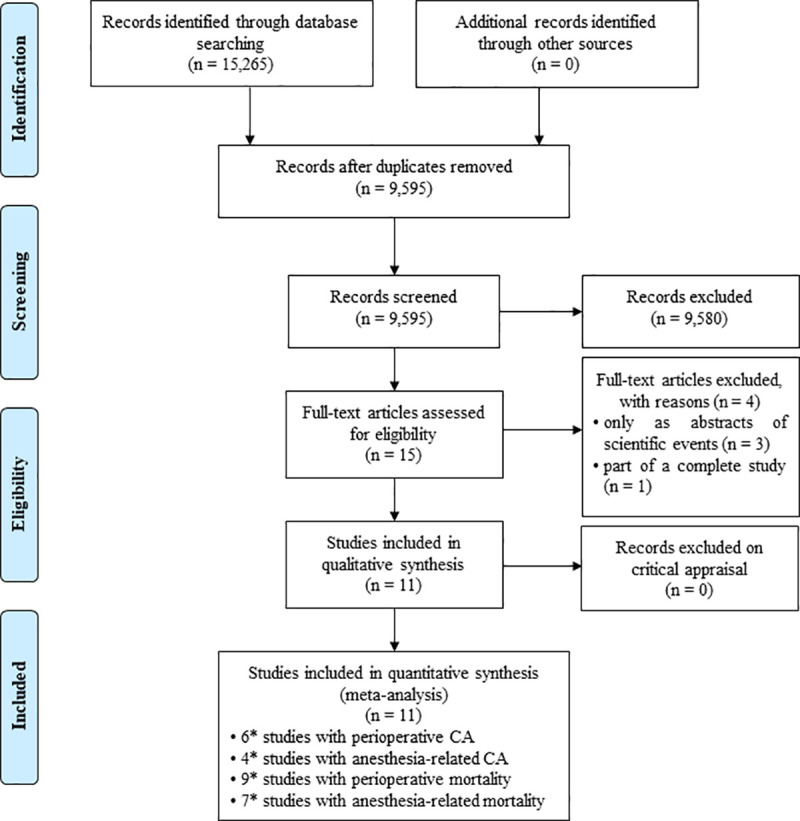
Abbreviations. CA: cardiac arrest; *some studies have been included in more than one category.
Study characteristics
The earliest study included was published in 1986 [20], and the most recent was published in 2019 [21]. The studies included 719,273 anesthetic procedures with 962 perioperative CA events, 134 anesthesia-related CA events, 1,239 perioperative deaths and 29 anesthesia-related deaths. The characteristics and designs of the studies are presented in Tables 1 and 2. The forest plots summarizing the data are presented in S3 File. As expected, there was significant heterogeneity among the studies, with a minimum I2 of 6.5% and a maximum of 98.3% for CA and a minimum I2 of 49.1% and a maximum of 99.0% for mortality (Tables 3 and 4).
Table 1. Description of included cardiac arrest studies.
| Investigators and year of publication | Data source, study period, and median year | Time period of CA occurrence | Excluded | Patients (n) | Perioperative CA (n) | Anesthesia-related CA (n) | Entirely anesthesia-related CA (n) |
|---|---|---|---|---|---|---|---|
| Ruiz Neto & Amaral, 1986 [20] | Tertiary university hospital Medical records 1982–1984 | OR | Cardiac surgery | 51,422 | 205 | 74 | 36 |
| 1983 | |||||||
| Braz et al., 1999 [22] | Tertiary university hospital—Database | OR and PACU | - | 58,553 | 184 | 21 | - |
| 1988–1996 | |||||||
| 1992 | |||||||
| Braz et al., 2006 [23] | Tertiary university hospital—Database | OR and PACU | - | 53,718 | - | 18 | 10 |
| 1996–2005 | |||||||
| 2001 | |||||||
| Sebbag et al., 2013 [24] | Tertiary university hospital—Database 2007 |
OR | Cardiac surgery | 40,379 | 52 | 21 | 0 |
| 2007 | |||||||
| Toledo et al., 2013 [25] | Tertiary university hospital—Database 2007–2009 |
OR | Cardiac surgery | 81,587 | 81 | - | - |
| < 18 years | |||||||
| 2008 | |||||||
| Carlucci et al., 2014 [26] | Tertiary university hospital—Database | OR and PACU | - | 90,909 | 280 | - | - |
| 1996–2009 | |||||||
| 2003 | |||||||
| Vane et al., 2019 [21] | Tertiary university hospital—Database 2007–2014 |
OR | Cardiac surgery | 167,574 | 160 | - | - |
| 2011 | < 18 years |
Abbreviations. CA: cardiac arrest; OR: operating room; PACU: postanesthesia care unit
Table 2. Description of included mortality studies.
| Investigators | Data source, study period, and median year | Time period in which death occurred | Excluded | Patients (n) | Perioperative mortality (n) | Anesthesia-related mortality (n) | Entirely anesthesia-related mortality (n) |
|---|---|---|---|---|---|---|---|
| Ruiz Neto & Amaral, 1986 [20] | Tertiary university hospital | OR | Cardiac surgery | 51,422 | 99 | 9 | - |
| Medical records | |||||||
| 1982–1984 | |||||||
| 1983 | |||||||
| Cicarelli et al., 1998 [27] | Tertiary university hospital—Database | Up to | Cardiac surgery | 25,926 | 129 | 2 | 2 |
| 24 hours postoperative | |||||||
| 1995 | |||||||
| 1995 | |||||||
| Braz et al., 1999 [22] | Tertiary university hospital—Database | OR and PACU | - | 58,553 | 124 | 5 | - |
| 1988–1996 | |||||||
| 1992 | |||||||
| Chan & Auler Jr, 2002 [28] | Tertiary university hospital—Database | Up to | - | 82,641 | 424 | 1 | 1 |
| 24 hours postoperative | |||||||
| 1998–1999 | |||||||
| 1999 | |||||||
| Braz et al., 2006 [23] | Tertiary university hospital—Database | OR and PACU | - | 53,718 | - | 6 | 3 |
| 1996–2005 | |||||||
| 2001 | |||||||
| Sebbag et al., 2013 [24] | Tertiary university hospital—Database | Up to | Cardiac surgery | 40,379 | 32 | - | - |
| 24 hours postoperative | |||||||
| 2007 | |||||||
| 2007 | |||||||
| Carlucci et al., 2014 [26] | Tertiary university hospital—Database | OR and PACU | - | 90,909 | 181 | - | - |
| 1996–2009 | |||||||
| 2003 | |||||||
| Pignaton et al., 2016 [29] | Tertiary university hospital—Database | OR and PACU | - | 55,002 | 88 | 0 | 0 |
| 2005–2012 | |||||||
| 2009 | |||||||
| Stefani et al., 2018 [30] | Quaternary university hospital -Database | Up to | - | 11,562 | 76 | 6 | 1 |
| 48 hours postoperative | |||||||
| 2012–2013 | |||||||
| 2013 | |||||||
| Vane et al., 2019 [21] | Tertiary university hospital—Database 2007–2014 |
Up to | Cardiac surgery | 167,574 | 86 | - | - |
| 24 hours postoperative | |||||||
| < 18 years | |||||||
| 2011 |
Abbreviations. OR: operating room; PACU: postanesthesia care unit
Table 3. Proportion meta-analysis of cardiac arrest rates in Brazilian studies by time period.
| Time period | Studies n |
I2% | Events n | Patients n | Proportion meta-analysis per 10,000 anesthetics (95% CI) | P value for subgroup |
|---|---|---|---|---|---|---|
| Pre-1990 versus 1990–2020 | ||||||
| Perioperative cardiac arrest | ||||||
| Pre-1990 | 1 | NA | 205 | 51,422 | 39.87 (34.60–45.50) | < 0.0001 |
| 1990–2020 | 5 | 98.3 | 757 | 439,002 | 17.61 (9.21–28.68) | |
| Anesthesia-related cardiac arrest | ||||||
| Pre-1990 | 1 | NA | 74 | 51,422 | 14.39 (11.29–17.86) | < 0.0001 |
| 1990–2020 | 3 | 6.5 | 60 | 152,650 | 3.90 (2.93–5.01) | |
| Entirely anesthesia-related cardiac arrest | ||||||
| Pre-1990 | 1 | NA | 36 | 51,422 | 7.00 (4.89–9.49) | < 0.0001 |
| 1990–2020 | 2 | 91.8 | 10 | 94,097 | 0.58 (0.00–3.72) | |
Abbreviations. I2: indicates heterogeneity among studies; CI = confidence interval; NA = not available
Table 4. Proportion meta-analysis of mortality rates in Brazilian studies by time period.
| Time period | Studies n |
I2% | Events n | Patients n | Proportion meta-analysis per 10,000 anesthetics (95% CI) | P value for subgroup |
|---|---|---|---|---|---|---|
| Pre-1990 versus 1990–2020 | ||||||
| Perioperative mortality | ||||||
| Pre-1990 | 1 | NA | 99 | 51,422 | 19.25 (15.64–23.24) | 0.1984 |
| 1990–2020 | 8 | 99.0 | 1,054 | 532,546 | 25.40 (13.01–41.86) | |
| Anesthesia-related mortality | ||||||
| Pre-1990 | 1 | NA | 9 | 51,422 | 1.75 (0.76–3.11) | 0.5404 |
| 1990–2020 | 6 | 81.0 | 20 | 287,402 | 0.67 (0.09–1.66) | |
| Entirely anesthesia-related mortality | ||||||
| Pre-1990 | - | - | - | - | - | NA |
| 1990–2020 | 5 | 49.1 | 7 | 228,849 | 0.22 (0.05–0.68) | |
Abbreviations. I2: indicates heterogeneity among studies; CI = confidence interval; NA = not available
Methodological quality
Eleven studies meeting the inclusion criteria were assessed for methodological quality. All of them obtained more than five “yes” answers, indicating that they were of good quality (S2 Table).
Proportion meta-analysis of CA rate
The sensitivity analysis revealed no substantial change in the event rates during the time period from 1988 to 1992, justifying the establishment of 1990 as the temporal cutoff point. Thus, the event rates during 2 time periods (pre-1990 versus 1990–2020) were analyzed.
The rate of perioperative CA decreased (2.26-fold) from 39.87 (95% CI: 34.60 to 45.50) before 1990 to 17.61 (95% CI: 9.21 to 28.68) per 10,000 anesthetics in 1990–2020 (P < 0.0001) (Table 3). At the same time, the rates of both anesthesia-related and entirely anesthesia-related CA decreased consistently (by 3.7- and 12-fold, respectively) from 14.39 (95% CI: 11.29 to 17.86) and 7.00 (95% CI: 4.89 to 9.49) before 1990 to 3.90 (95% CI: 2.93 to 5.01) and 0.58 (95% CI: 0.00 to 3.72) per 10,000 anesthetics in 1990–2020, respectively (P < 0.0001 and P < 0.0001, respectively) (Table 3).
Proportion meta-analysis of mortality rate
There was no significant alteration in the rate of either perioperative mortality (from 19.25 [95% CI: 15.64 to 23.24] before 1990 to 25.40 [95% CI: 13.01 to 41.86] per 10,000 anesthetics in 1990–2020; P = 0.1984) or anesthesia-related mortality (from 1.75 [95% CI: 0.76 to 3.11] before 1990 to 0.67 [95% CI: 0.09 to 1.66] per 10,000 anesthetics in 1990–2020; P = 0.5404; Table 4). None of the Brazilian studies evaluated entirely anesthesia-related mortality rates before the 1990s, making a subanalysis of data between periods impossible; this rate in 1990–2020 was 0.22 (95% CI: 0.05 to 0.68) per 10,000 anesthetics (Table 4).
Discussion
To the best of our knowledge, this study is the first comprehensive synthesis showing the temporal trends in perioperative CA and mortality rates in a developing country. When comparing the pre-1990 and 1990–2020 periods in Brazilian studies, a reduction in the perioperative CA rates was found, with a relatively large decrease in the rate of anesthesia-related CA; however, there were no significant differences in the rates of perioperative and anesthesia-related mortality.
A systematic review with a proportion meta-analysis of the worldwide studies from developed and developing countries during 2 time periods (pre-1990 versus 1990–2014) showed a significant increase in the rate of perioperative CA and no differences in the rates of anesthesia-related and entirely anesthesia-related CA in developing countries, while in developed countries, there was a significant decrease in all CA rates [6], similar to the findings of the present study. Comparing the CA rates after 1990, we verified that the rates of both perioperative and anesthesia-related CA in Brazilian studies were similar to the rates in developing countries (19.9 and 4.5 per 10,000 anesthetics, respectively) but were 2.8- and 5.6-fold higher, respectively, than the rates in developed countries (6.2 and 0.7 per 10,000 anesthetics, respectively); the rate of entirely anesthesia-related CA in Brazilian studies was 2.7-fold lower than the rate in developing countries (1.6 per 10,000 anesthetics) and similar to the rate in developed countries (0.5 per 10,000 anesthetics) in the aforementioned review.
Contrary to the findings of Brazilian studies, a systematic review with a meta-analysis of studies from developed and developing countries showed a significant reduction in the rate of perioperative mortality over 3 time periods (pre-1970s, 1970s-1980s and 1990–2009), despite the increasing baseline ASA risk status of the patients [7]. The authors of this review suggested that their results indicated improved perioperative and anesthesia safety, particularly in developed countries. Comparing the mortality rates between the aforementioned review and Brazilian studies published after 1990, we verified that the rate of perioperative mortality in Brazil was similar to the rate in developing countries (24.45 per 10,000 anesthetics) and 2.3-fold higher than the rate in developed countries (10.95 per 10,000 anesthetics), while the rate of entirely anesthesia-related mortality in Brazil was 6.4-fold lower than the rate in developing countries (1.41 per 10,000 anesthetics) and similar to the rate in developed countries (0.25 per 10,000 anesthetics). The rate of anesthesia-related mortality was 2.4-fold higher than the rate (0.28 per 10,000 anesthetics) reported in a recent study in the USA [31].
There is no consensus about the time frame for perioperative CA or mortality events [32]. Thus, perioperative CA and mortality rates depend on how the perioperative period is defined: only intraoperative, intraoperative and recovery from anesthesia, up to 24 h postoperative or up to 48 h postoperative. It must be highlighted that all CA events reported in the studies occurred in only the intraoperative period (operating room or operating room plus postanesthesia care unit), while in the mortality studies, the events occurred in the intraoperative (55.6%) or 24–48 h postoperative (44.4%) periods. A Brazilian study reported a postoperative 24 h mortality rate (7.9 per 10,000 anesthetics) that was 2-fold higher than the intraoperative mortality rate (3.9 per 10,000 anesthetics) [24], while a Korean study reported a postoperative 24 h mortality rate (1.25 per 10,000 anesthetics) that was 8-fold higher than the intraoperative mortality rate (0.15 per 10,000 anesthetics) [33]. Thus, the time frame for the events may have interfered with the results of the current review.
Poor ASA physical status (III-V) has been reported to be the most important predictor of perioperative CA and mortality events in Brazilian studies [23, 24, 28, 29] and in studies from developed countries [31, 34, 35]. Two Brazilian studies demonstrated that many patients present for surgery with poor health at baseline and without the optimization of disease management [29, 36]. Thus, patient disease/condition remained the major triggering factor for perioperative CA and mortality, followed by surgery and anesthesia in low proportions [23, 28, 29].
The most influential cause of perioperative CA and mortality events in Brazilian studies was sepsis, followed by trauma [23, 29, 30, 37]. Therefore, comorbid conditions seem to be major contributors to the high perioperative CA and mortality rates in Brazil [5]. Thus, preanesthetic management of comorbidities plays a major role in minimizing perioperative complications and adverse effects. These findings demonstrate that there is a need to improve the quality and quantity of resources that can be used as well as access to healthcare, both of which are inadequate, in developing countries [15].
In Brazil and other developing countries, the combination of poverty and precarious healthcare with increasing and aging populations has increased both the number of patients in poor physical condition and the demand for surgical procedures in recent decades. However, the surgical volume in Brazil is approximately 2.5-fold lower [38, 39] than the international target set to achieve universal access to surgical care, which is 5 surgeries per 100 inhabitants per year [4]. In addition, in 2014, the density of the health professionals (surgeons, anesthesiologists and obstetricians) was 34.7 per 100,000 inhabitants in Brazil [38], while developed countries have an average of 56.9 of these professionals per 100,000 inhabitants [40]. These factors, combined with limited resources and numbers of surgical beds and operating rooms and increasing costs of surgery and anesthesia, seem to result in an important consequence: high perioperative CA and mortality rates in developing countries [41].
Governmental and nongovernmental organizations should prioritize and increase healthcare investments in Brazil and other developing countries [42]. Policy makers and healthcare professionals must address practices that have demonstrable effectiveness in improving perioperative outcomes. Human resources are pivotal; the number and the education and training of both anesthesiologists and surgeons must be increased [41, 42]. Preoperative management must be optimized; multidisciplinary discussions of adverse effects must be prompted; the provision of new monitoring techniques (e.g., pulse oximetry, capnography, echocardiography), modern anesthetic drugs, anesthesia equipment and workstations must be addressed universally; and practice guidelines and checklists in surgery and anesthesia as well as a structured approach to reducing errors must be adopted [41–45]. The mandatory period in the postanesthesia care unit should also be expanded, and the number of intensive care beds for critical patients should be increased to minimize the occurrence of adverse events. Global efforts should be directed towards ensuring that the increase in the surgical volume is accompanied by the implementation of basic safety measures in developing countries. Feedback regarding the implementation of such measures should be required from both developed and developing countries to ensure that the existing gap between health care systems is effectively reduced [1].
The limitations of this review must be acknowledged. This review is limited by a paucity of studies, particularly before 1990, which could have influenced the findings. Thus, we were not able to analyze publication bias because there were fewer than ten eligible studies addressing each outcome in a proportion meta-analysis; otherwise, the findings from the assessment of risk of bias would not be reliable. Despite the heterogeneity and small number of studies, the meta-analyses showed consistent results that strongly reinforce the relevance of our systematic review. The studies differed greatly in their design; differences in the time frames of the events (e.g., intraoperative or up to 24–48 h postoperative) and in the types of surgery (e.g., whether cardiac surgeries were included) accounted for most of the heterogeneity. A random-effects model with inverse Freeman-Tukey double arcsine transformation was applied to minimize this heterogeneity when assessing the trends between the two time periods. All the studies were conducted at only governmental tertiary or quaternary university hospitals. Some studies included a small sample size, and all included studies presented data from a single center; there were no multicenter studies. We included only studies with > 3,000 patients and calculated weighted event rates across all studies to minimize possible bias. The case mix, the type of anesthesia and surgery, and ASA physical status classification were not included in this review. Considering that the current review covered a 32-year period with recruitment of patients from 1982 to 2014, it must be highlighted that significant advances in resuscitation, such as, Advanced Trauma Life Support (ATLS) and Advanced Cardiovascular Life Support (ACLS), and operative protocols have occurred, which may have influenced the results of the current review.
This review demonstrates that the rates of perioperative CA have decreased over time in Brazil, and the proportional decrease has been large and consistent in the rates of anesthesia-related and entirely anesthesia-related CA. However, the rates of both perioperative and anesthesia-related mortality did not show significant differences between the time periods. Further reviews of perioperative and anesthesia-related CA and mortality must be periodically performed to continue to monitor the rates in surgical patients in Brazil.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
R.S., M.P. and J.B.C. received a scholarship from The National Council for Scientific and Technological Development (CNPq; 125054/2016-5, 149852/2019-3 and 144230/2019-4, respectively) and L.G.B. received a fellowship from CNPq (304174/2018-1). M.P.M. received a scholarship from Coordination of Improvement for Higher Academic Staff (CAPES). Both CNPq and CAPES supported our study by financial support. We did not receive funding from our institution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94: 201–209F. 10.2471/BLT.15.159293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watters DA, Hollands MJ, Gruen RL, Maoate K, Perndt H, McDougall RJ, et al. Perioperative mortality rate (POMR): a global indicator of access to safe surgery and anaesthesia. World J Surg. 2015;39: 856–864. 10.1007/s00268-014-2638-4 [DOI] [PubMed] [Google Scholar]
- 3.Hinkelbein J, Andres J, Thies KC, DE Robertis E. Perioperative cardiac arrest in the operating room environment: a review of the literature. Minerva Anestesiol. 2017;83: 1190–1198. 10.23736/S0375-9393.17.11802-X [DOI] [PubMed] [Google Scholar]
- 4.Meara JG, Hagander L, Leather AJM. Surgery and global health: a Lancet Commission. Lancet. 2014; 383:12–13. 10.1016/S0140-6736(13)62345-4 [DOI] [PubMed] [Google Scholar]
- 5.Braz LG, Morais AC, Sanchez R, Porto DSM, Pacchioni M, Serafim WDS, et al. Epidemiology of perioperative cardiac arrest and mortality in Brazil: a systematic review. Rev Bras Anestesiol. 2020;70: 82–89. 10.1016/j.bjan.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga FA, El Dib R, Wakasugui W, Roça CT, Corrente JE, Braz MG, et al. Anesthesia-related and perioperative cardiac arrest in low- and high-income countries: a systematic review with meta-regression and proportional meta-analysis. Medicine (Baltimore). 2015;94: e1465 10.1097/MD.0000000000001465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge D, Martin J, Arango M, Cheng D; Evidence-based Peri-operative Clinical Outcomes Research (EPiCOR) Group. Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet. 2012;380: 1075–1081. 10.1016/S0140-6736(12)60990-8 [DOI] [PubMed] [Google Scholar]
- 8.Vane MF, do Prado Nuzzi RX, Aranha GF, da Luz VF, Sá Malbouisson LM, Gonzalez MM, et al. Perioperative cardiac arrest: an evolutionary analysis of the intra-operative cardiac arrest incidence in tertiary centers in Brazil. Rev Bras Anestesiol. 2016;66: 176–182. 10.1016/j.bjan.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6, 2019. Available from: https://training.cochrane.org/handbook/current [DOI] [PMC free article] [PubMed]
- 10.Liberati A, Altman DG, Tetzlaff J Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6: e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311: 619–620. 10.1136/bmj.311.7005.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13: 147–153. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 13.Gurgel SJ, El Dib R, do Nascimento P Jr. Enhanced recovery after elective open surgical repair of abdominal aortic aneurysm: a complementary overview through a pooled analysis of proportions from case series studies. PLoS One. 2014;9: e98006 10.1371/journal.pone.0098006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 15.Braghiroli KS, Braz JRC, Rocha B, El Dib R, Corrente JE, Braz MG, et al. Perioperative and anesthesia-related cardiac arrests in geriatric patients: a systematic review using meta-regression analysis. Sci Rep. 2017;7: 2622 10.1038/s41598-017-02745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper JB, Gaba D. No myth: anesthesia is a model for addressing patient safety. Anesthesiology. 2002;97: 1335–1337. 10.1097/00000542-200212000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Eichhorn JH. Review article: practical current issues in perioperative patient safety. Can J Anaesth. 2013;60: 111–118. 10.1007/s12630-012-9852-z [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SW, Lam DM. Heterogeneity in meta-analyses. Comparing apples and oranges? Anaesthesia. 2017;72: 532–534. 10.1111/anae.13832 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz Neto PP, Amaral RVG. Cardiac arrest during anesthesia in a multicenter hospital. A descriptive study. Rev Bras Anestesiol.1986;36, 149–158. [Google Scholar]
- 21.Vane MF, Carmona MJC, Pereira SM, Kern KB, Timerman S, Perez G, et al. Predictors and their prognostic value for no ROSC and mortality after a non-cardiac surgery intraoperative cardiac arrest: a retrospective cohort study. Sci Rep. 2019;9: 14975 10.1038/s41598-019-51557-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braz JRC, Silva ACM, Carlos E, do Nascimento P Jr., Vianna PTG, Castiglia YMM, et al. Cardiac arrest during anesthesia at a tertiary teaching hospital (1988 to 1996). Rev Bras Anestesiol.1999;49: 257–262. [Google Scholar]
- 23.Braz LG, Módolo NS, do Nascimento P Jr, Bruschi BA, Castiglia YM, Ganem EM, et al. Perioperative cardiac arrest: a study of 53718 anaesthetics over 9 yr from a Brazilian teaching hospital. Br J Anaesth. 2006;96: 569–575. 10.1093/bja/ael065 [DOI] [PubMed] [Google Scholar]
- 24.Sebbag I, Carmona MJ, Gonzalez MM, Alcântara HM, Lelis RG, Toledo Fde O, et al. Frequency of intraoperative cardiac arrest and medium-term survival. Sao Paulo Med J. 2013;131: 309–314. 10.1590/1516-3180.2013.1315507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toledo FO, Gonzalez MM, Sebbag I, Lelis RG, Aranha GF, Timerman S, et al. Outcomes of patients with trauma and intraoperative cardiac arrest. Resuscitation. 2013;84:635–638. 10.1016/j.resuscitation.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 26.Carlucci MT, Braz JR, do Nascimento P Jr, de Carvalho LR, Castiglia YM, Braz LG. Intraoperative cardiac arrest and mortality in trauma patients. A 14-yr survey from a Brazilian tertiary teaching hospital. PLoS One. 2014;9: e90125 10.1371/journal.pone.0090125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cicarelli DA, Gotardo AO, Auler JOC Jr, Olivetti GT, Oliveira FS. Incidence of deaths during the 24-hour period following anesthesia. A review of Hospital das Clínicas–FMUSP records in 1995. Rev Bras Anestesiol. 1998;48: 289–294. [Google Scholar]
- 28.Chan RPC, Auler JOC Jr. Retrospective study of anesthetic deaths in the first 24 hours. Review of 82,641 anesthetics. Rev Bras Anestesiol. 2002;52, 719–727. [PubMed] [Google Scholar]
- 29.Pignaton W, Braz JR, Kusano PS, Módolo MP, de Carvalho LR, Braz MG, et al. Perioperative and anesthesia-related mortality: an 8-year observational survey from a tertiary teaching hospital. Medicine (Baltimore). 2016;95: e2208 10.1097/MD.0000000000002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefani LC, Gamermann PW, Backof A, Guollo F, Borges RMJ, Martin A, et al. Perioperative mortality related to anesthesia within 48 h and up to 30 days following surgery: A retrospective cohort study of 11,562 anesthetic procedures. J Clin Anesth. 2018;49: 79–86. 10.1016/j.jclinane.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 31.Pollard RJ, Hopkins T, Smith CT, May BV, Doyle J, Chambers CL, et al. Perianesthetic and anesthesia-related mortality in a southeastern United States population: a longitudinal review of a prospectively collected quality assurance data base. Anesth Analg. 2018;127: 730–735. 10.1213/ANE.0000000000003483 [DOI] [PubMed] [Google Scholar]
- 32.Morray JP, Posner K. Pediatric perioperative cardiac arrest: in search of definition(s). Anesthesiology. 2007;106: 207–208. 10.1097/00000542-200702000-00004 [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Kil HK, Kim HJ, Koo BN. Risk assessment of mortality following intraoperative cardiac arrest using POSSUM and P-POSSUM in adults undergoing non-cardiac surgery. Yonsei Med J. 2015;56: 1401–1407. 10.3349/ymj.2015.56.5.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newland MC, Ellis SJ, Lydiatt CA, Peters KR, Tinker JH, Romberger DJ, et al. Anesthetic-related cardiac arrest and its mortality: a report covering 72,959 anesthetics over 10 years from a US teaching hospital. Anesthesiology. 2002;97: 108–115. 10.1097/00000542-200207000-00016 [DOI] [PubMed] [Google Scholar]
- 35.Nunnally ME, O'Connor MF, Kordylewski H, Westlake B, Dutton RP. The incidence and risk factors for perioperative cardiac arrest observed in the National Anesthesia Clinical Outcomes Registry. Anesth Analg. 2015;120: 364–370. 10.1213/ANE.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 36.Nunes JC, Braz JR, Oliveira TS, de Carvalho LR, Castiglia YM, Braz LG. Intraoperative and anesthesia-related cardiac arrest and its mortality in older patients: a 15-year survey in a tertiary teaching hospital. PLoS One. 2014;9: e104041 10.1371/journal.pone.0104041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braz LG, Carlucci MTO, Braz JRC, Módolo NSP, do Nascimento P Jr, Braz MG. Perioperative cardiac arrest and mortality in trauma patients: A systematic review of observational studies. J Clin Anesth. 2020;64: 109813 10.1016/j.jclinane.2020.109813 [DOI] [PubMed] [Google Scholar]
- 38.Covre ER, Melo WA, Tostes MFDP, Fernandes CAM. Trend of hospitalizations and mortality from surgical causes in Brazil, 2008 to 2016. Rev Col Bras Cir. 2019;46: e1979 10.1590/0100-6991e-20191979 [DOI] [PubMed] [Google Scholar]
- 39.Massenburg BB, Saluja S, Jenny HE, Raykar NP, Ng-Kamstra J, Guilloux AGA, et al. Assessing the Brazilian surgical system with six surgical indicators: a descriptive and modelling study. BMJ Glob Health. 2017;2: e000226 10.1136/bmjgh-2016-000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmer H, Lantz A, Kunjumen T, Finlayson S, Hoyler M, Siyam A, et al. Global distribution of surgeons, anaesthesiologists, and obstetricians. Lancet Glob Health. 2015;3: S9–11. 10.1016/S2214-109X(14)70349-3 [DOI] [PubMed] [Google Scholar]
- 41.Bharati SJ, Chowdhury T, Gupta N, Schaller B, Cappellani RB, Maguire D. Anaesthesia in underdeveloped world: Present scenario and future challenges. Niger Med J. 2014;55: 1–8. 10.4103/0300-1652.128146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan FA, Merry AF. Improving anesthesia safety in low-resource settings. Anesth Analg. 2018;126: 1312–1320. 10.1213/ANE.0000000000002728 [DOI] [PubMed] [Google Scholar]
- 43.Ivani G, Walker I, Enright A, Davidson A. Safe perioperative pediatric care around the world. Paediatr Anaesth. 2012;22: 947–951. 10.1111/pan.12009 [DOI] [PubMed] [Google Scholar]
- 44.Pearse RM, Holt PJ, Grocott MP. Managing perioperative risk in patients undergoing elective non-cardiac surgery. BMJ. 2011;343: d5759 10.1136/bmj.d5759 [DOI] [PubMed] [Google Scholar]
- 45.Wacker J, Staender S. The role of the anesthesiologist in perioperative patient safety. Curr Opin Anaesthesiol. 2014;27: 649–656. 10.1097/ACO.0000000000000124 [DOI] [PMC free article] [PubMed] [Google Scholar]


