Highlights
Question: To explore the effect of COVID-19 infection on the types and severity of acute ischemic stroke in hospital admissions in multi-ethnic population in Qatar.
Findings: In this study from a large prospective database, we report that the number of ischemic stroke admissions decreased marginally during the COVID-19 pandemic. Patients with acute stroke and COVID-19 infection were more likely to have severe strokes with a higher frequency of cortical involvement. They also had a more prolonged length of stay in hospital and had poor outcome at discharge.
Meaning: The presence of COVID-19 infection is associated with more severe stroke symptoms, likely related to a pro-thrombotic state and more cortical involvement that results in slower recovery.
Key Words: Ischemic stroke, COVID-19, Outcome, Stroke types, Stroke severity, Bamford classification
Abstract
Objectives
The presence of COVID-19 infection may increase the risk of thrombotic events including ischemic strokes. Whilst a number of recent reports suggest that COVID-19 associated stroke tends to be severe, there is limited data on the effects of COVID-19 in prospective registries.
Material and Methods
To determine how COVID-19 infection may affect cerebrovascular disease, we evaluated the ischemic stroke sub-types, clinical course and outcomes prior to and during the pandemic in Qatar. The Hamad General Hospital (HGH) stroke database was interrogated for stroke admissions during the last 4 months of 2019 and January-May 2020.
Results
In Qatar the number of confirmed cases of COVID-19 increased from only 2 in February to 779 in March, 12,628 in April and 45,501 in May. Stroke admissions to HGH declined marginally from an average of 97/month for six pre-COVID months to 72/month in March–May. There were 32 strokes that were positive for COVID-19. When compared to non-COVID-19 stroke during the three months of the pandemic, COVID-19 patients were younger with significantly lower rates of hypertension, diabetes and dyslipidemia. COVID-19 positive patients had more cortical strokes (34.4% vs 5.6%; p = 0.001), severe disease (NIHSS >10: 34.4% vs 16.7%; p = 0.001) prolonged hospitalization and fewer with good recovery (mRS 0-2: 28.1% vs 51.9%; p = 0.001).
Conclusions
When compared to six pre-COVID-19 months, the number of ischemic stroke admissions during the three months of the pandemic declined marginally. COVID-19 positive patients were more likely to have a large cortical stroke with severe symptoms and poor outcome.
Introduction
The number of confirmed cases of COVID-19 worldwide has exceeded 31,000,000 with more than 901,000 confirmed deaths as of September 21, 2020.1 The virus mainly manifests through respiratory involvement with fever, cough, shortness of breath and other pulmonary symptoms.2 Neurological symptoms, including headaches, dizziness, myalgias, alteration in levels of consciousness and altered mental status are common symptoms and may occur in more than 50% of hospitalized patients.3 In one recent study, acute stroke has been reported in 2.5% of consecutive COVID-19 related hospital admissions.4 Whereas neuropathological examination reveals diffuse hypoxic injury in severe cases, likely related to the severe hypoxemia, there was no evidence for thrombosis, overt vasculitis or encephalitis in a series of 18 autopsy cases.5
Despite the lack of overt intracranial thrombosis in the autopsy study4 there is however evidence that COVID-19 infection has profound effects on the cardiovascular system including an increased risk of venous thrombosis and pulmonary embolism,6 myocardial injury7 and stroke.8, 9, 10, 11 In addition, an interesting report from New York suggests that during the COVID-19 pandemic, there was a higher likelihood for imaging-confirmed acute ischemic stroke to harbor the virus during ‘code-stroke’ activation.12
Another interesting observation from several centers across the globe have shown that transient ischemic stroke (TIA) and stroke admissions have decreased significantly during the COVID-19 pandemic.9 , 13, 14, 15 There was a decrease in the number of strokes in Piacenza (a city of 280,000 inhabitants), an important epicenter of the disease in Northern Italy.13 The monthly admissions decreased from an average of 51 cases to only 6 over a 5-week period.13 Large studies from China,8 Brazil,13 , 14 and Spain9 reported an approximately 25% decrease in ED admissions during the peak weeks of the pandemic. Additional reports suggest an increase in COVID-19-related severe stroke, especially in younger patients.10 This may be related to a possible pro-coagulant state seen with COVID-19 infection.16 A decrease in hospital admissions of cardiovascular disease and acute coronary syndromes has also been observed in EDs during the recent COVID-19 pandemic.17 The numbers of cardiac catheterization laboratory STEMI activations decreased by 38% in the USA18 and 40% in Spain19 as the pandemic spread in these countries. Although a decrease in minor ailments and trauma-related visits may be related to a fear of exposure to the virus during visits to the ED, the actual reason for significant decreases in ED visits for more serious illnesses remain unclear.
These reports unfortunately provide insufficient details on the relationship of COVID-19 infection and stroke. Prospective hospital-based or community registries are important to study the effects of pandemics on the types of vascular diseases.20 Registries may also provide important insight about time-sensitive healthcare delivery metrics such as door-to-intervention times, as was recently documented from treatment delays of myocardial infarctions in Hong Kong.15 Similarly, time-sensitive management of emergencies including thrombolysis in acute stroke may also be affected by the COVID-19 pandemic.
The main objective of the present study is to compare the types of ischemic strokes in patients with or without confirmed COVID-19 infection to a busy tertiary care hospital during the pandemic. We also determined if there were any differences in the rates of complications during hospitalization and short-term prognosis between acute stroke patients with COVID-19 infections and patients with acute stroke and no COVID-19 infection.
Methods
The Qatar Stroke Database prospectively collects information on all acute stroke patients admitted to the Hamad General Hospital (HGH) in Qatar since February 2013. The hospital has a dedicated stroke ward, and is the only center where treatment with intravenous thrombolysis and mechanical thrombectomy is offered. Almost 98% of acute stroke patients in Qatar are admitted in the HGH. The details of the database have previously been published.21 , 22 The present study was specifically designed to evaluate the effects of the recent COVID-19 pandemic on the trends of acute stroke care in Qatar. The Institutional Review Board, Hamad Medical Corporation at the Medical Research Centre (MRC-05-096) approved the study.
All acute stroke (AS) patients admitted to HGH between September-2019 to May-2020 were evaluated for the study. The clinical information including rates of admissions, ethnicity, risk factors, investigations during hospitalization, clinical presentation and course during hospitalization were recorded. The severity of symptoms at admission (NIHSS score), clinical diagnosis as defined by the TOAST classification23 and Bamford classification,24 and the length of stay in hospital are also recorded. Medical complications were documented. The modified Rankin Scale (mRS) was documented at discharge, and at 90-day follow-up.
For the present study, we evaluated the monthly rates of confirmed ischemic stroke admissions to the hospital for the last 4 months in 2019, prior to the onset of COVID-19 pandemic. We compared this to the first 5 months of 2020 as the pandemic was being documented in China and Europe (January-February) and as COVID-19 cases began to be diagnosed in Qatar (March–May).
We documented all patients with ischemic stroke who also were diagnosed with COVID-19. Any symptoms related to the viral infection (fever, cough, sore throat and severity of pulmonary illness) were documented. Particular attention was given to where the patient was admitted (ICU vs stroke ward), medical complications and treatment offered were all documented. Where available, we also documented changes in the laboratory markers of inflammation in COVID-19 subjects.
Statistical analysis
Descriptive statistics in the form of mean and standard deviations for continuous variables and frequency with percentages for categorical variables were performed. One-way ANOVAs were performed to see significant mean level differences for all continuous variables according to Pre-COVID-19 ischemic stroke, COVID-19 Negative ischemic stroke and COVID-19 positive categories. Student t tests were applied for continuous variables to see significant mean level differences between subcortical strokes or small vessel disease (SVD) vs cortical or large vessel disease (LVD), and “No evidence of pneumonia” on chest x-ray vs “Bilateral pneumonia” on chest X-ray. Chi-square tests were applied to see association of categorical variables according to Pre-COVID-19 ischemic stroke, COVID-19 negative ischemic stroke and COVID-19 positive cases, subcortical strokes or SVD vs cortical or LVD; and “No evidence of pneumonia” on chest x-ray vs “bilateral pneumonia” on chest X-ray respectively. Multivariate logistic regression analysis was used for the significant and important variables at univariate analysis to association of risk factors to COVID-19 positive group. Adjusted Odds Ratios with 95% C.I. and P values were presented in the tables. ROC curve with C-statistics was used to see discriminate power of the model for the COVID-19 positive cases. P value 0.05 (two tailed) was considered statistically significant level. The statistical tests were performed in IBM SPSS Statistics ver. 26 (IBM, Armonk, USA).
Results
During the 9 months of the study, there were 833 patients [age; 54.3 ± 13.5 male/female 675 (81%)/158 (19%)] admitted to HGH with a diagnosis of acute ischemic stroke. The higher percentage of males reflects the demographics of Qatar with a predominantly male expatriate population as has been previously reported.21 , 22 There were 585 admissions in the 6 months prior to when COVID-19 cases were confirmed in Qatar (average monthly admissions to HGH ED: 97. See Table 1 ). The first confirmed COVID-19 case in Qatar was reported on the 27th of February. In March, April and May, the monthly reported cases of COVID-19 were 779, 12,628 and 45,501 respectively. With the increase in confirmed COVID-19 cases in the country, there was a marginal decrease in the rates of acute stroke hospital admissions. The monthly rates of admission for the duration between March and May was 72 for the three months period.
Table 1.
Demographic and clinical characteristics of patients with COVID-19 stroke versus COVID-19 negative and historical ischemic stroke controls
| Characteristic or investigation | Total[n = 833] | Pre- COVID19 Ischemic Stroke(Sep 2019- Feb 2020)[n = 585, 70.2%] | Covid19 Negative Ischemic Stroke(Mar - May 2020)[n = 216, 25.9%] | Covid19 Positive Ischemic Stroke(Mar–May 2020)[n = 32, 3.8%] | P- Value |
|---|---|---|---|---|---|
| Demographics and risk factors | |||||
| Age- Mean, years | 54.3 ±13.5 | 54.3 ±13.3 | 54.9 ±14.1 | 48.9 ±11.5 | 0.06 |
| Sex – Males | 675 (81.0) | 479 (81.9) | 168 (77.8) | 28 (87.5) | 0.27 |
| Females | 158 (19.0) | 106 (18.1) | 48 (22.2) | 4 (12.5) | |
| Hypertension | 603 (72.4) | 443 (75.7) | 147 (68.1) | 13 (40.6) | 0.0001 |
| Diabetes | 464 (55.7) | 334 (57.1) | 120 (55.6) | 10 (31.3) | 0.02 |
| Dyslipidemia | 472 (56.7) | 345 (59.0) | 124 (57.4) | 3 (9.4) | 0.0001 |
| Coronary Artery Disease | 98 (11.8) | 70 (12.0) | 24 (11.1) | 4 (12.5) | 0.94 |
| Atrial Fibrillation on Admission | 83 (10.0) | 63 (10.8) | 18 (8.3) | 2 (6.3) | 0.46 |
| History of Stroke | 98 (11.8) | 72 (12.3) | 26 (12.0) | 0 | 0.11 |
| Active Smoking | 236 (28.3) | 181 (30.9) | 51 (23.6) | 4 (12.5) | 0.02 |
| Stroke Classification | |||||
| TOAST Classification | |||||
| Small Vessel Disease | 343 (41.2) | 258 (44.1) | 80 (37.0) | 5 (15.6) | 0.0001 |
| Large Vessel Disease | 149 (17.9) | 84 (14.4) | 52 (24.1) | 13 (40.6) | |
| Cardio-Embolic | 216 (25.9) | 151 (25.8) | 57 (26.4) | 8 (25.0) | |
| Stroke of Determined Origin | 42 (5.0) | 32 (5.5) | 10 (4.6) | 0 | |
| Stroke of Undetermined Origin | 83 (10.0) | 60 (10.3) | 17 (7.9) | 6 (18.8) | |
| Bamford Classification | |||||
| Total Anterior Circulation Stroke | 82 (9.8) | 59 (10.1) | 12 (5.6) | 11 (34.4) | 0.0001 |
| Partial Anterior Circulation Stroke | 237 (28.5) | 162 (27.7) | 67 (31.0) | 8 (25.0) | |
| Lacunar Strokes | 294 (35.3) | 206 (35.2) | 78 (36.1) | 10 (31.1) | |
| Posterior Circulation Stroke | 220 (26.4) | 158 (27.0) | 59 (27.3) | 3 (9.4) | |
| IV Thrombolysis Given | 74 (8.9) | 54 (9.2) | 17 (7.9) | 3 (9.4) | 0.83 |
| Thrombectomy Done | 24 (2.9) | 20 (3.4) | 3 (1.4) | 1 (3.1) | 0.31 |
| ICU care needed | 60 (7.2) | 41 (7.0) | 5 (2.3) | 14 (43.8) | 0.0001 |
| NIHSS Severity | |||||
| Mild Stroke (NIHSS 4 or less) | 533 (64.0) | 390 (66.7) | 133 (61.6) | 10 (31.3) | 0.001 |
| Moderate Stroke (NIHSS 5-10) | 164 (19.7) | 106 (18.1) | 47 (21.8) | 11 (34.4) | |
| Severe Stroke (NIHSS > 10) | 136 (16.3) | 89 (15.2) | 36 (16.7) | 11 (34.4) | |
| NIHSS on admission | 5.5 ±6.4 | 5.2 ±6.3 | 5.6 ±6.1 | 9.8 ±7.9 | 0.0001 |
| NIHSS At Discharge | 4.3 ±5.8 | 3.8 ±5.3 | 4.7 ±5.9 | 11.3 ±8.7 | 0.0001 |
| HbA1c on Admission | 7.4 ±2.2 | 7.3 ±2.1 | 7.7 ±2.6 | 7.3 ±2.7 | 0.09 |
| Length of Stay | 5.5 ±5.8 | 5.5 ±5.9 | 4.6 ±4.0 | 10.9 ±10.8 | 0.0001 |
| Prognosis – At Discharge | |||||
| Good (mRS 0-2) | 469 (56.3) | 348 (59.5) | 112 (51.9) | 9 (28.1) | 0.001 |
| Poor (mRS 3-6) | 364 (43.7) | 237 (40.5) | 104 (48.1) | 23 (71.9) | 0.001 |
| Mortality – At Discharge | 16 (1.9) | 12 (2.1) | 3 (1.4) | 1 (3.1) | 0.73 |
NIHSS- National Institute of Heath Stroke Scale, mRS- Modified Rankin Score, IV- Intravenous, ICU- Intensive Care Unit
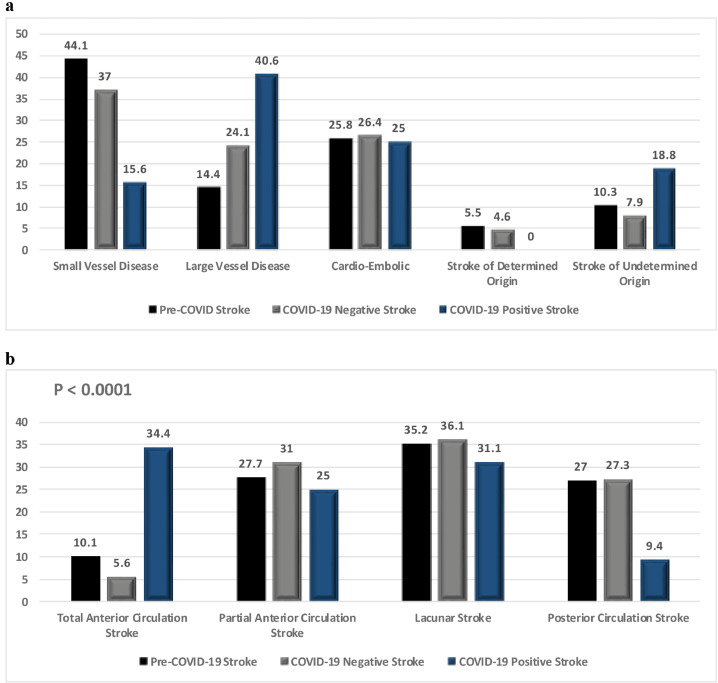
We used the Bamford classification25 for localization and the TOAST criteria for defining the suspected etiology of ischemic stroke in our patients as shown in Table 1 and Fig. 1a,b . Small vessel disease (SVD) is the most common type of stroke in the Qatari and expatriate population. This is likely due to the high prevalence of poorly controlled hypertension and diabetes.22 There was a relative decrease in the percentage of SVD in patients with confirmed ischemic stroke, decreasing from 44.1% in the preceding 6 months when compared to 37% in March–May. There was concomitant increase in the percentage of large vessel stroke, increasing from 14.4% in the pre-COVID 6 months to 24% in March–May (p=0.001) as shown in Table 1.
Fig. 1a.
Distribution of different types of ischemic strokes before and during COVID-19 pandemic according to TOAST classification (P < 0.0001) 1b. Distribution of different types of ischemic strokes before and during COVID-19 pandemic according to Bamford Classification (P < 0.0001).
Compared to the pre-COVID-19 6 months, there was no change in the severity of symptoms during the pandemic months in non-COVID-19 patients with acute ischemic stroke. The NIHSS on admission was 5.2 ± 6.3 in the initial pre-COVID six months and 5.6 ± 6.1 during March–May for non-COVID-19 patients. The NIHSS at admission for COVID-19 positive patients was however significantly higher 9.8 ± 7.9 (p= 0.001).
During the three months (March–May 2020), there were 32 acute stroke patients admitted to HGH who were positive for COVID-19 infection. Patients with COVID-19 infection were younger. The mean age of non-COVID-19 patients was 54.9 ±14.1 and COVID-19 positive patients was 48.9 ± 11.5 (p= 0.02). There were significantly fewer patients with hypertension, diabetes and hyperlipidemia in COVID-19 patients. The COVID-19 patients had higher frequency of cerebral cortical involvement. Patients with COVID-19 were sicker with more admissions to the ICU (43.8% vs 2.3%), more frequent intubation (31.3% vs 2.3%) and longer length of hospitalization [LOC] (10.9 ± 10.8 vs 4.6 ± 4.0 days). There were fewer COVID-19 patients with a good outcome at discharge when compared to non-COVID-19 patients as measured with mRS of 0-2. The percentage of patients with mRS of 0-2 decreased from a mean value of 59.5% in the pre-COVID months to 51.9 in March–May in the non-COVID-19 patients. Only 28.1% of COVID-19 positive patients had a mRS of 0-2 at discharge (p = 0.001). COVID-19 positive patients also have higher frequency of laboratory abnormalities as shown in Table 2 .
Table 2.
Characteristics of patients with COVID-19 infection and Ischemic Stroke with bilateral Pneumonia on Chest X-ray.
| Characteristic or Investigation |
Total (n = 32) |
No Bilateral Pneumonia on Chest X-Ray (n = 19) | Bilateral Pneumonia on Chest X-Ray (n = 13) | P- Value |
|---|---|---|---|---|
| Mean Age | 48.9 ±11.5 | 46.9 ±9.4 | 51.7 ±14.0 | 0.26 |
| Prognosis at Discharge | ||||
| Good (mRS 0-2) | 9 (28.1) | 8 (42.1) | 1 (7.7) | 0.03 |
| Poor (mRS 3-6) | 23 (71.9) | 11 (57.9) | 12 (92.3) | |
| Diabetes on Admission | 10 (31.3) | 7 (36.8) | 3 (23.1) | 0.41 |
| Hypertensive on Admission | 13 (40.6) | 8 (42.1) | 5 (38.5) | 0.84 |
| Coronary Artery Disease | 4 12.5) | 1 (6.7) | 3 (17.6) | 0.35 |
| ICU care needed | 14 (43.8) | 4 (21.1) | 10 (76.9) | 0.002 |
| Intubated | 10 (31.3) | 2 (10.5) | 8 (61.5) | 0.002 |
| NIHSS on Admission | 9.8 ±7.9 | 6.2 ±5.6 | 14.9 ±8.1 | 0.001 |
| NIHSS on Discharge | 11.3 ±8.7 | 7.8 ±7.4 | 16.8 ±7.9 | 0.003 |
| Length of Stay | 10.9 ±10.9 | 7.4 ±10.9 | 15.9 ±8.9 | 0.03 |
| D-Dimer levels (n=20) | 4.1 ±5.9 | 1.0 ±0.7 | 6.7 ±7.2 | 0.03 |
| Fibrinogen Levels (n=13) | 5.1 ±2.1 | 6.6 ±1.5 | 4.7 ±2.1 | 0.20 |
| CRP Levels (n=26) | 58.1 ±72.9 | 33.6 ±51.4 | 82.7 ±84.4 | 0.09 |
| LDH Levels (n=11) | 566.3 ±445.2 | 351.6 ±163.8 | 745.2 ±538.9 | 0.15 |
| Procalcitonin Levels (n=16) | 5.3 ±16.5 | 1.9 ±4.0 | 6.8 ±19.9 | 0.60 |
| Ferritin levels (n=22) | 666.8 ±693.2 | 307.3 ±217.9 | 915.6 ±803.3 | 0.04 |
Abnormal Chest X-ray represents pulmonary infiltrates, consolidation (uni or bilateral), and/or patchy ground glass appearance. ICU- Intensive care Unit, CRP- C Reactive Protein, LDH- Lactate Dehydrogenase
Multivariate analysis
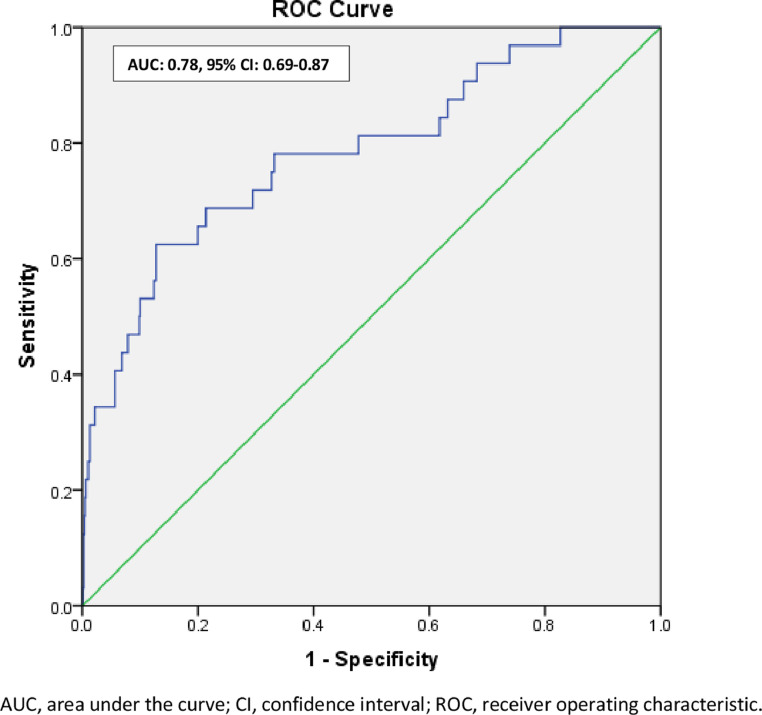
Significant and important independent variables at univariate analysis were included for multivariate logistic regression analysis to determine associated factors for COVID -19 positive cases. After adjusting for age, NIHSS at admission, DM at admission, HTN at admission in the model, we found that poor prognosis at discharge (Adjusted OR: 3.0, 95% C.I.: 1.2–7.8, p = 0.02) in comparison to good prognosis, Bamford TACS (Adjusted OR: 3.0, 95% C.I.: 1.2–7.6, p = 0.03) in comparison to its other categories and length of stay in hospital measured in days (Adjusted OR: 1.05, 95% C.I.: 1.01 – 1.10, p = 0.03) were found to be associated with COVID-19 positive cases (Table 3 ). C-statistics was 0.78 with 95% C.I.: 0.69 – 0.87, suggesting the model's good ability to discriminate for COVID cases (Fig. 2 ).
Table 3.
Multivariate logistic regression analysis for showing factors associated with COVID-19 positivity compared with COVID-19 negative and pre-COVID ischemic stroke.
| Variable | P-Value | Adjusted Hazard Ratio | 95% CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Poor prognosis at discharge | 0.02 | 3.023 | 1.17 | 7.81 |
| Age of the patient | 0.26 | 0.98 | 0.95 | 1.01 |
| NIHSS score on admission | 0.79 | 0.99 | 0.93 | 1.05 |
| Diabetic on admission | 0.06 | 0.45 | 0.19 | 1.05 |
| Hypertensive on admission | 0.02 | 0.36 | 0.16 | 0.83 |
| Length of stay | 0.03 | 1.05 | 1.01 | 1.10 |
| TACS on Bamford classification | 0.02 | 2.99 | 1.18 | 7.56 |
TACS- Total Anterior Circulation Stroke, NIHSS- National Institute of Heath Stroke Scale
Fig. 2.
ROC curve to discriminate COVID-19 cases in the study AUC.
Discussion
To our knowledge, this is the first study that compares COVID-19 patients to non-COVID-19 patients within a prospectively collected stroke database. Similar to previous case reports and case-controlled studies, from USA,10 Iran,26 Dubai,27 France28 and China,8 a third of our COVID-19 positive patients had severe disease, required ICU admissions, stayed longer in hospital and had fewer subjects with good outcome. These patients likely represent a subset of stroke patients in whom the viral infection likely contributed to a prothrombotic state resulting in vascular occlusions and large strokes. In the remainder of patients, especially those presenting with small vessel disease, the viral infection was perhaps coincidental and did not influence the clinical course and outcome of the illness. We did not observe any delays in times from onset to hospital admission, or any differences in the rates of thrombolysis or thrombectomies in patients with or without COVID-19 infection. The higher rates of admission to ICU in COVID-19 positive patients is likely related to the severity of illness at presentation.
In patients with COVID-19 infection, there is increasing evidence for activation of inflammatory and thrombosis pathways. Case series suggest a high incidence of venous thrombosis despite anticoagulation treatment,6 myocarditis7 and stroke.8, 9, 10 Similar to our patients, COVID-19 positive patients from other reports with acute stroke were younger, have fewer vascular risk factors and many had recurrent thrombi in the large cranial arteries.8, 9, 10 The longer hospitalization in our COVID-19 positive patients was also likely related to more severe disease as evidenced by higher NIHSS scores and more cortical involvement. Such patients were also less likely to have a favorable outcome on discharge despite longer length of hospitalization. In a recent study from Europe, patients with COVID-19 associated ischemic strokes were more severe with worse functional outcome and higher mortality than non-COVID-19 ischemic strokes.29
An intriguing observation during the COVID-19 pandemic is the decreasing number of stroke admissions reported from a number of countries.9 , 13, 14, 15 The reason for the decrease in rates of cardiovascular disease remains unclear. Perhaps the most important reason may be the fear of exposure to COVID-19 in hospitals and thus opting for staying home to minimize the risk. This may explain the decrease in the number of “stroke mimics” admission to hospital in our study and fewer hospitalization of patients with TIAs and milder strokes in the study from Brazil.14 A recent report captured the rates of utilizing RAPID software platform (iSchemaView, California USA) in the months preceding the pandemic and during the initial weeks of the disease. RAPID is a popular diagnostic tool used in cranial imaging of patients with a suspected stroke. Data on 231,753 patients undergoing acute stroke CT scans in 856 hospitals across the USA was reviewed. There was a 39% decrease (1.18 patients per day per hospital in the pre-pandemic epoch to 0.72 per day in the early-pandemic epoch). Unlike our experience however, this decrease was seen across all ages, in both sexes and all stroke severities. It was evident across all regions of the country irrespective of the severity of the local COVID-19 infection.30
The decreased emergency department visits are also evident with other acute medical illnesses. The number of reported cases evaluated in emergency departments in England decreased by 25% in the week following declaration of the lockdown.17 Similarly, there was a dramatic decline in the number of surgical emergencies being treated in Italy during the peak weeks of the pandemic. In the city of Pesaro-Fano (population 360,000) an epicenter in the pandemic, the number of serious surgical emergencies declined from 82 in the month preceding the lockdown to only 12 during the first month of the lockdown.31 An increase in telephone consultation by family doctors may explain the decrease in emergency department visits for milder complaints. It is however difficult to understand why the number of serious surgical emergencies decreased during the pandemic in cities with high rates of infections.
There are strengths to our study. The Qatar Stroke Database is very robust and has prospectively recorded stroke trends in the country for more than 7 years. We did not document major changes in the admission of ischemic stroke sub-types during the months preceding and during the three months of the COVID-19 pandemic. Whereas other studies comprised of case reports or case series, we compare profiles of non-COVID-19 acute stroke patients before and during the pandemic with acute stroke patients who were COVID-19 positive. Our study reveals that COVID-19 positive patients were more likely to be sicker, had more cortical involvement and had prolonged hospitalization. In addition, fewer patients had a good recovery at discharge as measured with mRS.
The study has some limitations. The study period was only three months and may not be sufficient to determine if these were related to COVID-19 infections. We did not document the relationship of the illness to the severity of COVID-19-related laboratory abnormalities. We also do not have enough follow-up data on the patients seen during the pandemic to adequately document the changes in outcomes.
Summary
The onset of COVID-19 pandemic has been associated with a decrease in non-COVID-19 associated admissions to hospitals in Asia, Europe and North America. We present data from a prospective stroke database showing that ischemic stroke decreased marginally during the COVID-19 pandemic. We also review the presenting features and clinical course of 32 COVID-19 positive patients. Our data suggests that in a third of acute stroke patients, the viral infection results in a more severe disease, whereas in the remainder, the COVID-19 illness has very little effect on the course of the illness. In summary, we believe that the spectrum of acute stroke in the COVID-19 includes three presentations; stroke and no COVID-19 infection, stroke with incidental COVID-19 infection and COVID-19 induced prothrombotic ischemic stroke.
Declaration of Competing Interests
None
Acknowledgments
Funding
None
Patient Consent and ethical approval
No patient consent was required due to the study design. The study was approved by the Institutional Review Board, Hamad Medical Corporation at the Medical Research Centre (MRC-05-096).
Guarantor
Dr. Ashfaq Shuaib, also the corresponding author.
Contributorship
Concept, design and draft: N Akhtar, A Shuaib
Acquisition, analysis, interpretation of data, technical and administrative support: N Akhtar, S Kamran, Y Imam, S Al-Jerdi, Muna Al-Muslamani
Critical review: Saadat Kamran, S Al-Jerdi, Fatma Abid
Statistical analysis: N Akhtar, R Singh
Acknowledgments
We acknowledge the assistance of all involved physicians, nurses, and staff of the Stroke Team in HMC. We also thank Ms. Reny Francis for her editorial assistance and supportive care.
References
- 1.J. Hopkins Coronavirus Recourse Center https/coronavirus.jhu.edu/map.html)
- 2.Zhou F, Du R, Fan G. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Sanchez CM, Diaz-Maroto I, Fernandez E. Neurological manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodigiani C, Iapichino I, Carenzo L. Venous and arterial thrombotic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon IH, Normindin E, Bhattacharyya S. Neuropathological features of COVID-19. New Eng Medical J. 2020 doi: 10.1056/NEJMc2019373. First on line June 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichmann D, Sperhake J-P, Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Internal Med. 2020 doi: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 7.Clerkin KJ, Fried JA, Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Li H, Knung D. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51 doi: 10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montaner J, Barragan-Prieto A, Perex-Sanchez S. Break in the stroke chain of survivial due to COVID-19. Stroke. 2020;51 doi: 10.1161/STROKEAHA.120.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxley TJ, Mocco J, Majidi S. Large-vessel stroke as a presenting feature of COVID_19 in the young. New Eng J Med. 2020 doi: 10.1056/NEJMc2009787. published on-line April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghi S, Ishida K, Torres J. SARS2-CoV-2 and stroke in New York healthcare system. Stroke. 2020;51:00. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PubMed] [Google Scholar]
- 12.Belani P, Schefflein J, Rigney B, et al. COVID-19 is an independent risk factor for acute ischemic stroke. AJNR 2020. 10.3174/ajnr.A6650 [DOI] [PMC free article] [PubMed]
- 13.Morelli N, Rota E, Terracciano C. The baffling case of ischemic stroke disappearance from casualty department in the COVID-19 era. Eur Neurol. 2020 doi: 10.1159/000507666. Published on-line April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diegoli H, Magalhaes PSC, Martins SCO et al. Decrease in hospital admissions for transient ischemic attacks, mild and moderate stroke during COVID-19 era. Stroke: 20205; 51: 00-00. 10.1161/STROKEAHA.120.030481. [DOI] [PMC free article] [PubMed]
- 15.Siegler JE, Heslin MS, Thau L, Smith A, Jovin TG. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowles L, Platton S, Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. New Engl J Med. 2020:2020. doi: 10.1056/NEJMc2013656. online 5 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton J. Covid-19: A&E visits in England fall by 25% in week after lockdown. BMJ. 2020;369 doi: 10.1136/bmj.m.1401. [DOI] [PubMed] [Google Scholar]
- 18.Garcia S, Albaghdadi MS, Meraj PM et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. JACC; (accepted April7). [DOI] [PMC free article] [PubMed]
- 19.Rodríguez-Leor O, Cid-Alvarez B, Ojeda S. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología ntervencionista en España. REC Interv Cardiol. 2020 [Google Scholar]
- 20.Alger HM, Williams JH, Walchok JG, Bolles M, Fonarow GC, Rutan C. Role of data registries in the time of COVID-19. Circul: Cardiovasc Q Outcomes. 2020;13:eoo6766. doi: 10.1161/CIRCOUTCOMES.120.006766. [DOI] [PubMed] [Google Scholar]
- 21.Tam CF, Cheung KS, Lam S, Wong AL. Impact of Coronavirus 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Cir. Cardiovasc Q Outcomes. 2020;13 doi: 10.1016/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhtar N, Kamran S, Singh R. Beneficial effects of implementing stroke protocols require establishment of a geographically distinct unit. Stroke. 2015;46:3494–3501. doi: 10.1161/STROKEAHA.115.010552. [DOI] [PubMed] [Google Scholar]
- 23.Akhtar N, Salam A, Kamran S. Ethnic variation in acute cerebrovascular disease: analyses from the Qatar stroke registry. Eur Stroke J. 2016;1:231–241. doi: 10.1177/2396987316663776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam HP, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Bamford J, Sandercock P, Dennis M. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 26.Mehrpour M, Shuaib A, Farahani M. Coronavirus disease 2019 and stroke in Iran: a case series and effects on stroke admissions. Int J Stroke. June 2020 doi: 10.1177/1747493020937397. 2020 Accepted 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan M, Ibrahim RHM, Siddiqi SA. COVID-19 and acute stroke–a case series from Dubai, UAE. Int J Stroke. 2020:2020. doi: 10.1177/1747493020938285. accepted 8 June. [DOI] [PubMed] [Google Scholar]
- 28.Esclard S, Maier B, Redjem H. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020;51:00. doi: 10.1161/STROKEAHA.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ntaios G, Michel P, Georgiopoulos G, Guo Y, et al. Stroke. 2020;51:00–00. 10.1161/STROKEAHA.120.031208 [DOI]
- 30.Ap K., MS G., Hamiton S, Alberts GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Eng J Med. 2020 doi: 10.1056/NEJMc2014816. published online 8 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patriti A, Eugeni E, Guerra F. What happened to surgical emergencies in the era of COVID-19 outbreak? Considerations of surgeons working in an Italian red zone. Update Surg. 2020 doi: 10.1007/s13304-020-00779-6. Published online 23 April. [DOI] [PMC free article] [PubMed] [Google Scholar]