Abstract
Background:
In view of the emerging coronavirus pandemic, the demand for knowledge about the impact of SARS-CoV-2 on people with Multiple Sclerosis (MS) continues to grow. Patients receiving disease modifying therapy (DMT) for MS have a higher background risk of infection-related health care utilization when compared to the general population. Therefore, there is a need of evidence-based recommendations to reduce the risk of infection and also managing MS patients with SARS-CoV-2.
Case Description:
We present three patients with history of Multiple Sclerosis (MS) on DMTs presenting with worsening MS symptoms likely pseudo exacerbation who were diagnosed with COVID-19.
Discussion:
An extensive review of 7 articles was performed, in addition to a brief review on DMTs use in MS patients with COVID-19. In our cases, all patients were on DMT and severe course of disease was noted in 2 cases. No fatality was observed.
Conclusions:
This review provides a base on the clinical characteristics, outcomes and the roles of DMTs in MS patients suffering from n-cov-2. Physicians need to be vigilant about considering COVID-19 infection related relapse in the MS patients, especially in this COVID-19 pandemic era and look for pseudo-exacerbation. As most cases are found to have mild course and full recovery on DMTs, further research is needed to formulate evidence-based guidelines. This review will particularly be helpful for the researchers and registries to collect future data on MS and COVID-19.
Keywords: COVID-19, Multiple sclerosis, SARS-CoV-2, Immunotherapies, Ocrelizumab, Novel coronavirus
Abbreviations: COVID-19, Coronavirus Disease 2019; MS, Multiple Sclerosis; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; n-Cov2, Novel coronavirus 2; ATS/IDSA, American Thoracic Society and Infectious Disease Society of America; RRMS, Relapsing Remitting Multiple Sclerosis; PPMS, Primary Progressive Multiple Sclerosis; EDSS, Expanded Disability Status Score; CT, Computed Tomography; RT PCR, Reverse Transcription Polymerase Chain Reaction; L, Liters; ul, Microliters; DMT, Disease Modifying Therapy; IV, Intravenous; AST, Aspartate Transaminase; ALT, Alanine Transaminase; BUN, Blood Urea Nitrogen; NK cells, Natural Killer Cells; CRP, C-Reactive Protein; JCV, John Cunnigham virus; CSF, Cerebrospinal fluid; Terminally differentiated late effector memory T cells, (TEMRA); Antigen presenting cells, (APCs); Progressive multifocal leukoencephalopathy, (PML).
Highlights
-
•
Our cases presented with MS pseudo-exacerbation, with confirmatory COVID-19
-
•
In our review, most MS patients had mild course of COVID-19
-
•
Most of them recovered and fatality was observed only in 9 patients
-
•
Use of DMTs in MS patients with moderate to severe COVID-19 is debatable
-
•
Studies are required to develop guidelines for managing MS with COVID-19
1. Introduction
The ongoing pandemic of SARS-CoV-2 has created the need to establish the effect of this novel infection of patients with pre-existing immune-mediated disorders. Data on the effect of COVID-19 on the clinical course and disease outcome of pre-existing immune mediated inflammatory disorders, such as multiple sclerosis (MS), is scant but gradually emerging. It remains unclear if people with MS have an increased risk of acquiring SARS-CoV-2 or developing severe COVID-19 illness [[1], [2], [3]]. However, older age population who receive disease modifying therapy (DMT) for MS have a higher background risk of infection-related health care utilization when compared to the general population [4]. Therefore, people with MS should follow the recommendations on preventative measures to reduce their risk of infection with SARS-CoV-2. While relapses in MS can occur, viral infections can also possibly trigger an exacerbation of their condition in a form known as pseudo exacerbation [5]. Physicians need to be vigilant about considering COVID-19 infection related relapse in the MS patients, especially in this COVID-19 pandemic era. Physicians should be aware of the neurotropic and neuro-invasive characteristics of SARS-CoV-2 that can interfere with the clinical course and disease outcome in MS patients. Emphasis should be given on formulating the guidelines for initiating, switching or cessation of DMT, considering that the antiviral response to SARS-CoV-2 is driven mainly by CD8+ cytotoxic T-cells, NK cells, and less so B-cells. Here, we review the existing data regarding SARS-CoV-2 infection in MS patients on DMT to describe the characteristics and outcomes of MS patients with COVID-19 and describe three additional such cases who presented to us and were diagnosed with COVID-19 [[6], [7], [8]].
2. Case presentation
2.1. Case 1
A 65-year old man with the history of RRMS (Relapsing Remitting Multiple Sclerosis) for 20 years, controlled by Glatiramer acetate for 5 years presented to the emergency department with fatigue, generalized weakness and shortness of breath, concerning for worsening MS symptoms. His significant past medical history included hypertension and hyperlipidemia. Prior to Glatiramer acetate the patient was on Interferon beta-1b. His last two major relapses dated back in 2013 and 2015. In year 2013, patient had an episode of right optic neuritis, whereas in 2015 he presented with lower extremity weakness. Both resolved following steroid therapy. MRI brain from July 2019 showed stable demyelinating plaques with no abnormal enhancing lesion. He was able to perform his routine activities independently and did not require any ambulatory aid. His EDSS was estimated to 4.0 for his current episode, his baseline EDSS was 2.0.
On examination, he was febrile, desaturating with pulse oximetry 85% at room air and required 2 L oxygen through the nasal canula. Complete blood count (CBC) showed normal leukocyte and lymphocyte count, CD 4+ and CD 8+ counts were within normal range (828 cells/uL and 442 cells/uL, range 382–1614 cells/uL and 157–813 cells/uL respectively) and the immunoglobulin subtypes (including IgG, IgM and IgA) were normal. C-reactive protein was elevated 181 mg/L (range < 8 mg/L), ferritin levels elevated 4462 ng/mL (range 24–336 ng/ml), AST was elevated 50 U/L (range 15–41 U/L), ALT was normal, BUN was elevated 25 mg/dL (range 6–20 mg/dL), and creatinine was 1.85 mg/dL (range 0.61–1.24 mg/dL).
Chest X-ray showed the right lower lobe infiltrates. CT chest showed patchy ground glass infiltrates at the lung bases bilaterally. Nasopharyngeal RT-PCR for SARS-CoV-2 was positive. He was admitted as a patient of a severe form of COVID-19, according to the ATS/IDSA severity index guidelines [9].
The patient was started on hydroxychloroquine and azithromycin for 5 days. His symptoms improved after four days of treatment and no additional oxygen supplementation was required. He likely had pseudo exacerbation of MS from ongoing COVID-19 infection. MRI imaging of the brain was stable from July 2019 (Fig. 1). His clinical course of COVID-19 lasted approximately two and half weeks after which he tested negative. He was discharged after 15 days of admission once he was completely back to baseline. His EDSS at discharge was 2.0, which was the same as his baseline. Patient continued on Glatiramer acetate following discharge.
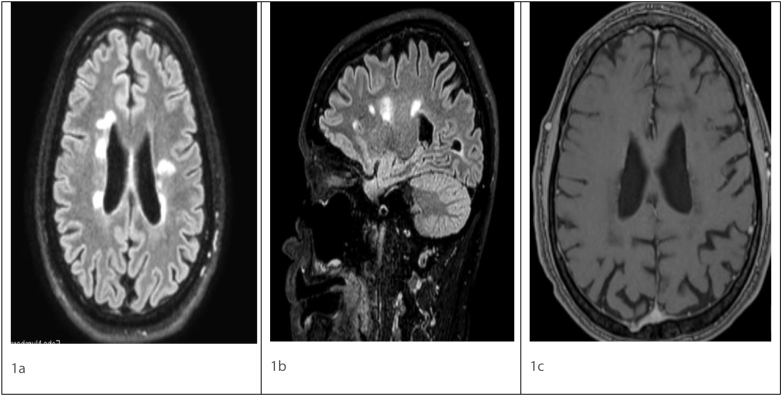
Fig. 1.
Axial FLAIR (1a), Sagittal FLAIR (1b), post contrast T1 (1c) magnetic resonance imaging showing ovoid hyperintense lesions in periventricular distribution in Fig. 1a and b. No enhancing lesion seen on post contrast image Fig. 1c.
FLAIR - Fluid attenuated inversion recovery.
2.2. Case 2
A 52-year old man with the medical history of RRMS since 2008, presented with complaints of constant nagging cough, generalized weakness, fever and increasing shortness of breath for the past six days. He was a chronic smoker with a past medical history of diabetes mellitus and hypertension. He had been taking dimethyl fumarate for 6 years and prior to that, he was on Interferon beta-1a. His last major relapse was in 2018 in form of right optic neuritis recovered after solumedrol treatment for 5 days. His last MRI imaging of the brain from November 2019 was stable as compared to August of 2018 with no abnormal enhancing lesion. He had been taking Dimethyl fumarate for 6 years and prior to that, he was on Interferon beta-1a. His last JCV antibody was tested negative from September of 2019. His baseline EDSS score was 3. He was found to have an EDSS score of 4.0 at admission. There were no symptoms or sign of meningitis, nor changes in mentation for other differentials such as encephalitis that could be have been considered as red flag, no lumbar puncture was warranted. PML was also excluded from the possibilities as he had no focal neurological deficits nor any suggestive clinical signs or symptoms. He improved back to baseline following COVID-19 management.
CBC with differential showed grade two lymphopenia of 0.7/ul (range 0.8–3.2^3/ul), CD-4+ count was 588 cells/ul, CD-8+ 210 cells/ul (range 382–1614 cells/ul and 157–813 cells/ul respectively), whereas IgG, IgM and IgA were normal. Inflammatory markers in serum such as CRP and ferritin were increased to 94 mg/L (range < 8 mg/L) and 3218 ng/ml (range 24–336 ng/ml) respectively. The chest x-ray showed the right basal infiltrates.
RT-PCR for SARS-CoV-2 was performed which tested positive. He improved back to baseline score of 3.0 after 4 weeks following COVID-19 management.
During hospitalization, he was treated with (Continuous Positive Airway Pressure) CPAP and hydroxychloroquine and anti-viral ritonavir, after which his symptoms resolved within a week. It was concluded that he likely had a pseudo exacerbation of MS. According to ATS/IDSA severity index criteria, this patient was categorized as having a severe form of COVID-19 [9]. His MRI imaging was stable, no active enhancing lesions were seen (Fig. 2). He was advised to resume Dimethyl fumarate He tested negative for COVID-19 on his follow-up visit to the outpatient MS clinic after four weeks.
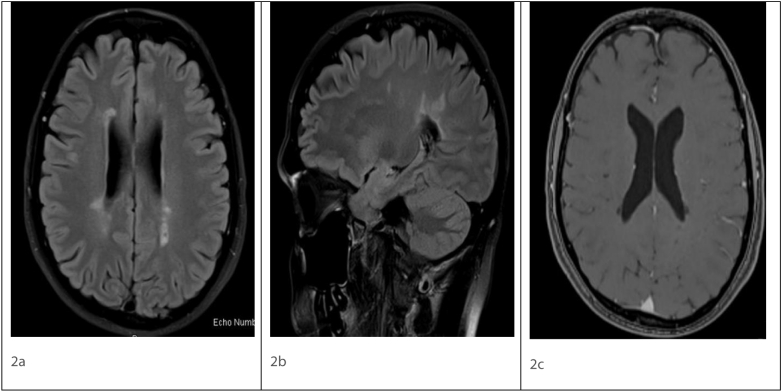
Fig. 2.
Axial FLAIR (2a), Sagittal FLAIR (2b), post contrast T1 (2c) magnetic resonance imaging showing ovoid hyperintense lesions in periventricular distribution in Fig. 2a and b. No enhancing lesion seen on post contrast image Fig. 2c.
FLAIR - Fluid attenuated inversion recovery.
2.3. Case 3
A 69-year female with a significant history of RRMS (Relapsing Remitting Multiple Sclerosis) for 20 years, presented to the hospital with the complaint of fever and cough. The patient was on Ocrelizumab since December 2018, with the last infusion in January 2020. Her prior DMTs include Betaserone, Glatiramer acetate and currently on Ocrelizumab. Her last JCV AB test was negative from August 2019. Her last MRI brain was stable from December 2019 compared to January of 2019. Last major relapse was in form of right lower extremity weakness in 2017 requiring solumedrol therapy. At baseline, she uses wheelchair for long distance ambulation and a walker at home. Her EDSS at baseline is 6.5 and has been stable on Ocrelizumab. She had a significant medical history of hypertension. Her MRI imaging of brain was stable with no enhancing lesion (Fig. 3). There was no neck rigidity, no headache no changes in mentation. Also, there was no focal neurological deficits in terms of any motor or sensory symptoms so CSF was not warranted.
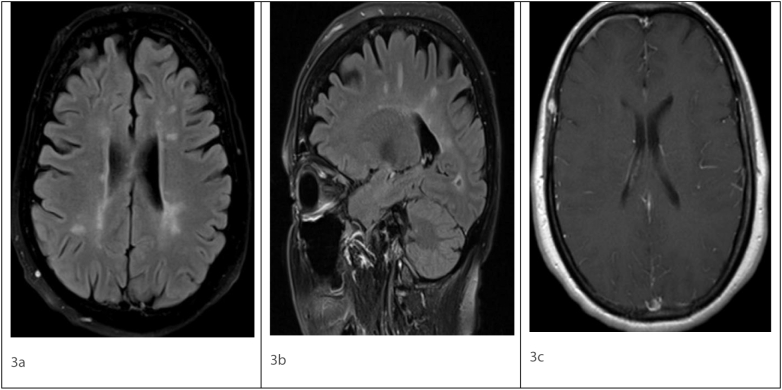
Fig. 3.
Axial FLAIR (3a), Sagittal FLAIR (3b), post contrast T1 (3c) magnetic resonance imaging showing ovoid hyperintense lesions in periventricular distribution in Fig. 3a and b. No enhancing lesion seen on post contrast image Fig. 3c.
FLAIR - Fluid attenuated inversion recovery.
CBC showed normal leukocyte and lymphocyte count, CD-19 and CD-20 B-cells absolute counts were 0 cells/mcL (range 56.6–417.4 cells/mcL and 74.4–441.1 cells/mcL respectively). Other inflammatory markers like CRP were 56 mg/L (range < 8 mg/L), IgG hypogammaglobulinemia (500 mg/dL, range 700–1500 mg/dL). Her liver enzymes were borderline elevated and chest x-ray was unremarkable.
RT-PCR for SARS-CoV-2 was performed which turned out to be positive. She was treated with non-steroidal anti-inflammatory agents for symptomatic fever, advised to quarantine herself, and follow-up in the outpatient MS clinic. Repeat COVID-19 RT-PCR is pending. According to the ATS/IDSA severity index criteria, this patient was categorized as having a mild form of COVID-19. She was recommended to follow up in the neurology clinic. Since last clinic visit in August 2020, she has been stable. Resuming Ocrelizumab was discussed with her and a plan to discontinue her medication and monitor her clinically was confirmed because of her age. She was also recommended MRI screening at regular interval. Table 1 highlights the demographics, comorbidities, DMT medications, EDSS and the time of diagnosis of COVID-19 in above reportd cases.
Table 1.
Patient Demographics, Outcomes and Severity.
| AGE | GENDER |
COMORID CONDITIONS |
TYPE OF MS | DURATION OF MS | DMT MEDICATION | EDSS AT BASELINE | OUTCOMES | SEVERITY OF COVID⁎ | |
|---|---|---|---|---|---|---|---|---|---|
| PT. 1 | 65 | M | HTN, HLD | RRMS | 20 YEARS | GLATIRAMER ACETATE | 2.0 | RECOVERED | SEVERE |
| PT. 2 | 52 | M | DM, HTN | RRMS | 12 YEARS | DIMETHYL FUMARATE | 3.0 | RECOVERED | SEVERE |
| PT. 3 | 69 | F | HTN | RRMS | 20 YEARS | OCRELIZUMAB | 6.5 | RECOVERED | MILD |
PT: Patient.
RRMS: Relapsing Remitting Multiple Sclerosis.
HTN: Hypertension.
HLD: Hyperlipidemia.
DM: Diabetes Mellitus.
RT PCR: Reverse Transcription Polymerase Chain Reaction.
DMT: Disease Modifying Therapy.
EDSS: Expanded Disability Status Scale.
: According to the American Thoracis Society and Infectious Disease Society of America Guidelines for Severity Index for COVID-19.
3. Discussion
Cases of Multiple Sclerosis and COVID-19 have been emerging recently. In order to increase the understandings of MS with COVID-19, we reviewed the articles currently available on this topic. It is already known that patients with MS are at a higher risk for infections than the general population. A large multi database study conducted to assess the risk of COVID-19 in MS patients reported a higher infection rates in the group with MS as compared to the non-MS group. However, risk of acquiring coronavirus infection was not separately evaluated [8]. While cases describing COVID-19 in MS patients have been reported, the information has not been sufficient to comment on whether DMT increases the risk of coronavirus infection [7,8].
Early experiences in the coronavirus pandemic led to pertinent questions regarding the course and outcome of COVID-19 in patients with MS, specifically those on DMT. A large multicenter study conducted in Italy, evaluated 232 MS patients with either suspected or confirmed SARS-CoV-2 infection. The wide majority of the patients under study (nearly 96%, n = 222/232) had a mild COVID-19 infection with only 4% of the patients suffering from a severe infection. 222 (96%) patients recovered from the infection. Of 10 severe cases, 5 (2%, n = 5/232) of them were fatal. However, only 24% (57/232) of the cases included could be confirmed to have COVID-19, and the remaining were suspected for n-Cov2 infection due to their symptoms [10] (refer Table-2). This preliminary result suggests outcomes of COVID-19 infections in MS patients similar to that in the general population. In the cases we described, 2 (66.7%) of them had severe courses with only 1 having a mild course of COVID-19. In all three cases, complete recovery was noted with symptomatic treatment for COVID-19. DMT was discontinued in case 3 and rest of the patients continued on DMT after 4–6 weeks of recovery from COVID-19 infection.
Table 2.
Clinical Presentations, Outcomes, Severity, DMT use and Imaging findings in COVID-19 with MS in Literature review.
| Author (Year) | Type of article/ Total number of cases | Type of DMT | Presented with worsening MS symptoms | Imaging findings | Severity of COVID-19 | Outcomes |
|---|---|---|---|---|---|---|
| Chaudhry F. et al. (2020) |
Original Research | Not reported | Presented with predominantly viral prodromal symptoms – cough, fever, headache, shortness of breath | Not reported | 19/40(47.5%) had mild course, 15/40(37.5%) had moderate course and 6/40(15%) had severe course | 4/40(10%) of patients were fatal, while all other patients recovered 36/40(90%). |
| Novi G. et al. (2020) |
Case report 1 case |
Ocrelizumab | Only viral symptoms - cough, fever, no neurological worsening symptoms | none | Mild | Recovered |
| Thornton J. R. et al. (2020) |
Case series 2 cases |
Both cases on Ocrelizumab | Only viral symptoms - cough, fever, no neurological worsening symptoms | none | Mild | Recovered |
| Sormani M. P. et al. | Pilot study 232 cases | Not reported | none | Mild 222 (96%), 10 (4%) severe | 5 (2%) fatal | |
| Barzegar, M. et al. | Case report 1 case |
Fingolimod | Gradually worsening sensory and motor symptoms with concern of relapse v/s pseudo exacerbation | none | Mild | Recovered |
| ⁎Domingues R.B. et al. | Case report 1 case | Not reported | Due to paresthesias of the left upper limb, later progressing to left hemithorax, and hemiface. | MRI brain normal, MRI cervical spine C6 ventral cord lesion | Mild | Recovered |
| Montero-Escribano P.et al. | Letter to editor | 54 patients Rituximab, 6 Ocrelizumab | Fever, gastrointestinal alterations, dyspnea, ageusia, fatigue, odynophagia, myalgias, anosmia | Not reported | No severe cases | Recovered |
Patient had Clinical Isolated Syndrome (CIS) not definitive Multiple Sclerosis.
Another study from Chaudhry et al. studied 40 patients with MS in three centers who were confirmed to have a SARS-CoV-2 infection [11]. Similar results were reported with only 15% (6/40) patients having a severe infection, while the remaining 47.5% (19/40) and 37.5% (15/40) had mild and moderate courses respectively. Four patients (80%) were found to be mechanically ventilated out of 5 which were admitted in the ICU. They also reported differences in the severity based on the type of MS with Primary Progressive MS showing the highest frequency (33%). Moreover, they reported that most of the severe infections were in people who were older [11] (refer Table-2). In our cases series, 2 (66.7%) patients required ICU care. Table 2 describes the summary of Clinical Presentations, Outcomes, Severity, DMT use and Imaging findings in COVID-19 with MS in cases reported in literature so far. (See Table 2.)
A large cohort study by Montero-Escribano P. et al., observed the effect of anti-CD20 monoclonal antibody therapies in 60 patients with MS (54 on rituximab and 6 on ocrelizumab). Only 11% (7/60) cases were confirmed to have COVID-19 and recovery was observed in all of them without any severe complication [12]. Barzegar M. et al. described a case report of COVID-19 in a MS patient treated with fingolimod. She had a favorable outcome after discontinuation on fingolimod and was discharged after resolution of symptoms [13]. Another case report by Novi G. et al. described SARS-CoV-2 infection in an MS patient on Ocrelizumab and explored a possible protective role of immunosuppressive therapy for COVID-19 infection. The case described had depleted B-cell reserve and low levels of proinflammatory cytokine detection during the infection leading to a mild clinical course which supports this hypothesis [14]. Thornton J. R. et al. also described two cases of COVID-19 in MS patients on Ocrelizumab. Both cases had a mild course and complete recovery further adding to the above hypothesis [15]. There is one case reported of clinical isolated syndrome in patient with COVID-19 presented with hemisensory numbness with non-confirmatory clinical definitive MS diagnosis [16] (refer Table-2).
It is also important to recognize the manifestation of COVID-19 in patients with MS. Our cases represent a cohort of patients with Multiple Sclerosis on disease modifying therapy (DMT). They presented with worsening of MS symptoms with complaints of weakness, fatigue, shortness of breath, likely a pseudo exacerbation due to COVID-19. Of all the cases, the severity index in two patients was severe; while one was mild (according to the ATS/IDSA severity criteria) [9]. They were all later found to be SARS-CoV-2 positive with RT-PCR nasopharyngeal swab test. (refer to Table 1).
Glatiramer acetate (GA) is an immunomodulatory agent often used in MS. It does not cause any T-cell depletion and rather induce CD-8 T-cell response in patients with MS [17]. Dimethyl fumarate causes reduction in circulating memory B-cells as well as in T-helper cells (CD4) and cytotoxic T-cells (CD8). It also activates nuclear related factor 2 involved in antioxidative response pathways leading to additional cytoprotective effects [18]. Ocrelizumab is anti-CD-20 monoclonal antibody therapy in RRMS and PPMS. Predominantly, it has an effect on B-cell lineage has minimal effects on the T-cell population mainly on a subset of T-cells and is not associated with severe viral infections [19]. Glatiramer acetate, Dimethyl fumarate and Ocrelizumab are considered to be very low risk, low risk and intermediate risk categories of disease modifying agents respectively, to attribute to the risk of novel coronavirus infection [3,20]. Assuming the antiviral response to SARS-CoV-2 is driven mainly by CD8+ cytotoxic T-cells, NK cells, and less so B cells, we should be aware of the risk associated with different classes of DMT in case a patient with MS develops COVID-19.
Though all of our patients were on immunotherapies, all of them recovered from the novel coronavirus infection and all cases except case 3 continued to be on disease modifying therapy. There has been much concern regarding the continuation of DMT in MS patients which induces an immunosuppressed state and predisposes to infections. Some studies have recommended tapering the dose of DMT in MS patients during the pandemic era to decrease the risk of contracting the disease. It is also suggested that patients who are older and with other risk factors for a severe disease may benefit from complete stoppage of DMT. Aging may lead to reduced number of naïve T cells and increased terminally differentiated late effector memory T cells (T EMRA). As seen with aging, DMTs also reduce number and functionality of the lymphocytes, which can predispose to the higher risk of PML secondary to lymphopenia [21].
Naïve T cells are deficit in IL-2 which can affect differentiation of the effector cells and have a high threshold for productive T-cell receptor signaling which leads to less activation, and collectively cause hampering of viral clearance. DMTs may further impair immune response in older patients which make them vulnerable to chronic infections. Loss of CD28 in auto-immune diseases suggests premature aging of immune system [22]. Reduced T-cell priming and activation by antigen presenting cells (APCs) is mostly observed. Studies have reported impaired dendritic cells maturation, which leads to reduced antigen uptake and presentation capacity [23]. Loss of costimulatory signal with APCs can also be the cause of impaired T-cell activation seen with aging. Therefore, the mechanism by which DMTs impair the functions of APCs more likely to have implications for immunosurveillance, irrespective of the absolute numbers of T cells [21].
DMT should be temporarily discontinued on MS patients who developed severe COVID-19 for at least 4 weeks [20]. More data and studies are needed to determine the continuation of the disease modifying therapies in patients who are already on them and have overlying novel coronavirus like symptoms that need further investigations. Additionally, as with other viral infections, people with MS can experience worsening of their symptoms due to COVID-19, and thus can manifest as a pseudo-exacerbation [20]. Physicians should be vigilant when they encounter MS patients with relapse and should reconsider the possibility of pseudo-relapse, especially in this COVID-19 pandemic era. Moreover, as intravenous steroids are mainly the primary indication for acute exacerbation of multiple sclerosis, its use in MS patients with pseudo-exacerbation is not warranted. The utilization of steroids in the patients infected with SARS-CoV-2 is now being extensively studied in patients with a severe infection. There is an ongoing clinical trial development which identifies the efficacy for the use of IV methylprednisolone, in patients suffering from acute respiratory distress syndrome due to COVID-19 infection (ClinicalTrials.gov, Identifier: NCT04323592). In summary, as most MS patients with COVID-19 had mild course and recovered on DMTs, continuation of immunotherapy treatment is generally considered by physicians during mild viral infections. Consideration of this recommendation with moderate to severe overlying COVID-19 infection is still debatable.
4. Conclusion
It is still questionable to assume that disease modifying therapies are the risk factor for acquiring COVID-19 infection in MS patients. Further studies are needed to identify evidence-based guidelines for the management of the patients with MS and COVID-19. Most of the patients with MS had mild course of COVID-19, whereas only handful of them were found to have severe course out of which nearly half of them suffered fatality. The individual risk of morbidity and outcome of COVID-19 in these patients is most likely multifactorial and depends on age, weight, underlying medical conditions and ambulatory status.
The patients with Multiple Sclerosis can experience worsening of their symptoms due to COVID-19 infection and they can present possibly as pseudo exacerbation. It is paramount for the physicians to determine if the patient has an underlying acute exacerbation of the disease or a pseudo-relapse.
There also clearly exists a dilemma for physicians in deciding the continuation of immunotherapies or DMTs when managing patients with immune mediated inflammatory disorders such as MS, as few of the DMTs are thought to cause increase in infection risks. In order to develop standardized guidelines for the physicians caring for immune-mediated disorders such as multiple sclerosis, the urging demand for additional studies and data will be quintessential.
We believe this review will be essential in aiding to the future studies on MS and COVID-19. It will also provide a base on the clinical characteristics, outcomes and the roles of DMTs in MS patients suffering from n-Cov2. This review will particularly be helpful for the researchers and registries to collect future data on MS and COVID-19.
Declaration of conflict of interest
None of the authors have any conflict of interest. All the authors had access to the data and a role in writing the manuscript.
Acknowledgements
None.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Berger, J.R., R. Brandstadter, and A. Bar-Or, COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm, 2020. 7(4). [DOI] [PMC free article] [PubMed]
- 2.Zheng C. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020:1–18. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannoni G. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39:102073. doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills E.A., Mao-Draayer Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult Scler. 2018;24(8):1014–1022. doi: 10.1177/1352458518775550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez de Antonio L.A. Non-inflammatory causes of emergency consultation in patients with multiple sclerosis. Neurologia. 2018 doi: 10.1016/j.nrleng.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen J.D. COVID-19 in MS: initial observations from the Pacific northwest. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis M.D., Robertson N.P. Multiple sclerosis and the risk of infection: considerations in the threat of the novel coronavirus, COVID-19/SARS-CoV-2. J Neurol. 2020;267(5):1567–1569. doi: 10.1007/s00415-020-09822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metlay J.P. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry, F., et al., COVID-19 in Multiple Sclerosis Patients and Risk Factors for Severe Infection. medRxiv, 2020: p. 2020.05.27.20114827. [DOI] [PMC free article] [PubMed]
- 12.Montero-Escribano P. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid. Spain Mult Scler Relat Disord. 2020;42:102185. doi: 10.1016/j.msard.2020.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barzegar M. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurology - Neuroimmunology Neuroinflammation. 2020;7(4):e753. doi: 10.1212/NXI.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novi G. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020;42:102120. doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult Scler Relat Disord. 2020;44:102341. doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingues R.B. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020:1–3. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karandikar N.J. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109(5):641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longbrake E.E. Dimethyl fumarate induces changes in B- and T-lymphocyte function independent of the effects on absolute lymphocyte count. Mult Scler. 2018;24(6):728–738. doi: 10.1177/1352458517707069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingele S. Ocrelizumab depletes CD20(+) T cells in multiple sclerosis patients. Cells. 2018;8(1) doi: 10.3390/cells8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownlee W. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- 21.Mills, E.A. and Y. Mao-Draayer, Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Multiple sclerosis (Houndmills, Basingstoke, England), 2018. 24(8): p. 1014–1022. [DOI] [PMC free article] [PubMed]
- 22.Aiello A. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10(2247):2019. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longbrake, E.E., et al., Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Multiple Sclerosis Journal–Experimental, Translational and Clinical, 2015. 1: p. 2055217315596994. [DOI] [PMC free article] [PubMed]