Abstract
Background
Previous outbreaks of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV) have been associated with unfavourable pregnancy outcomes. SARS-CoV-2 belongs to the human coronavirus family, and since this infection shows a pandemic trend it will involve many pregnant women.
Aims
This systematic review and meta-analysis aimed to assess the impact of coronavirus disease 19 (COVID-19) on maternal and neonatal outcomes.
Sources
PubMed, EMBASE, MedRxiv, Scholar, Scopus, and Web of Science databases were searched up to 8th May 2020. Articles focusing on pregnancy and perinatal outcomes of COVID-19 were eligible. Participants were pregnant women with COVID-19.
Content
The meta-analysis was conducted following the PRISMA and MOOSE reporting guidelines. Bias risk was assessed using the Joanna Briggs Institute (JBI) manual. The protocol was registered with PROSPERO (CRD42042020184752). Twenty-four articles, including 1100 pregnancies, were selected. The pooled prevalence of pneumonia was 89% (95%CI 70–100), while the prevalence of women admitted to the intensive care unit was 8% (95%CI 1–20). Three stillbirths and five maternal deaths were reported. A pooled prevalence of 85% (95%CI 72–94) was observed for caesarean deliveries. There were three neonatal deaths. The prevalence of COVID-19-related admission to the neonatal intensive care unit was 2% (95%CI 0–6). Nineteen out of 444 neonates were positive for SARS-CoV-2 RNA at birth. Elevated levels of IgM and IgG Serum antibodies were reported in one case, but negative swab.
Implications
Although adverse outcomes such as ICU admission or patient death can occur, the clinical course of COVID-19 in most women is not severe, and the infection does not significantly influence the pregnancy. A high caesarean delivery rate is reported, but there is no clinical evidence supporting this mode of delivery. Indeed, in most cases the disease does not threaten the mother, and vertical transmission has not been clearly demonstrated. Therefore, COVID-19 should not be considered as an indication for elective caesarean section.
Keywords: Caesarean delivery, Coronavirus, COVID-19, Meta-analysis, Pregnancy, SARS-Cov-2, Systematic review, Vertical transmission
Introduction
The novel coronavirus disease (COVID-19) was first identified in December 2019 in Wuhan, China [1]. Preliminary reports have shown that older people are more exposed to the risk of COVID-19, with men more affected by the virus than women and more likely to have severe illness or die [2]. Severe forms, however, have also been described in young women [3]. It is known that pregnancy is a state of special immune tolerance that predisposes women to viral infection [[4], [5], [6]]. Extensive population-based cohort studies have shown that seasonal influenza epidemics place pregnant women at increased risk of severe complications [7,8].
Limited information is available on the consequences of previous coronavirus outbreaks—severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV)—on pregnancy [9,10]. COVID-19 shares several features with SARS-CoV-1 and MERS-CoV, and seems to have a similar pathogenic potential [11,12]. Two early meta-analyses showed that a high percentage of pregnant women affected by these infections experienced unfavourable pregnancy outcomes and that there was no evidence of vertical transmission [13,14]. However, both meta-analyses included small numbers of patients.
The aim of this systematic review and meta-analysis was to report maternal and neonatal outcomes related to COVID-19, including a large number of pregnant women.
Methods
Eligibility criteria
For the selection of the papers, the following inclusion criteria were defined: (a) articles focused on pregnancy and perinatal outcomes of COVID-19, and (b) articles with original data (case series, cohort, retrospective, case–control studies). We excluded from the review studies regarding other viruses in the coronavirus family (i.e. SARS-CoV-1, MERS-CoV) and case reports and case series with less than ten pregnancies.
Information sources
PubMed, EMBASE, MedRxiv, Scholar, Scopus, and Web of Science databases were searched up to May 8, 2020. No language limitation was applied to the search. The research strategy adopted included different combinations of the following terms: “SARS-CoV-2”, “COVID-19,” “pregnancy,” “pregnant,” “delivery,” “infant,” “childbirth,” “neonate” and “newborn”. The complete search strategies adopted by the electronic database are reported in the Supplementary Material Table S1.
Manual searches included scanning of reference lists of relevant papers and systematic reviews published during the analysis of the literature.
Study selection
All studies identified with the electronic and manual searches were listed by citation, title, authors and abstract. Duplicates were identified through an independent manual screening performed by two researchers and then removed.
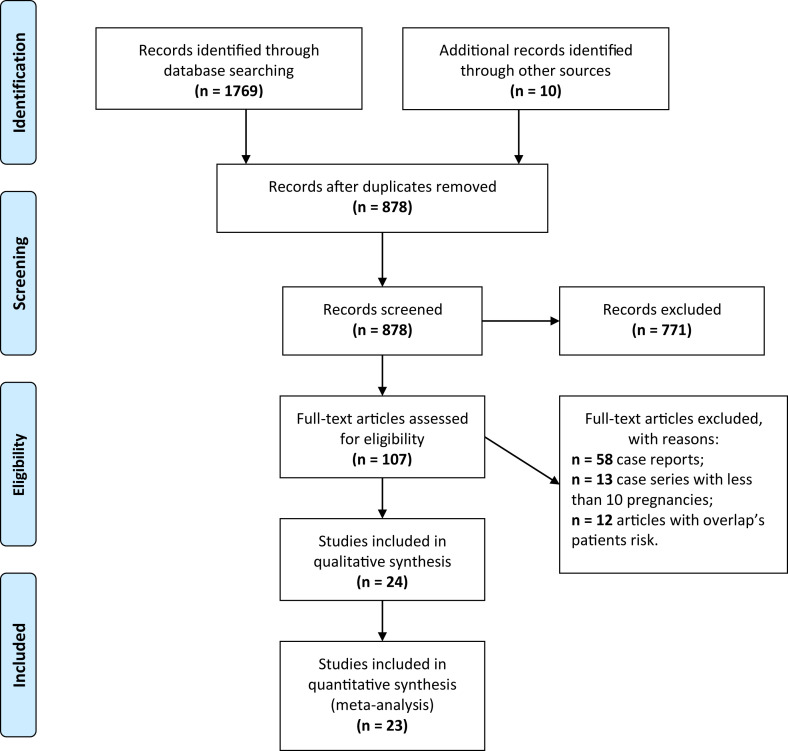
We have followed the PRISMA and MOOSE reporting guidelines [15,16]. The PRISMA flow diagram of the selection process and the MOOSE checklist are provided in Fig. 1 and Supplementary Material Fig. S1.
Fig. 1.
PRISMA 2009 flow diagram. From Moher et al. [15].
For the eligibility process, two authors (RG and DTF) independently screened the title and abstracts of all non-duplicated papers and excluded those not pertinent to the topic. The same two authors independently reviewed the full text of papers that passed the first screening and identified those to be included in the review. Discrepancies were resolved by consensus.
Data extraction
Two researchers (FDT and MG) performed data extraction using a predefined form including the following data: author, month and year, study location, period, design and setting, number of pregnancies included in the study, mean maternal age and list of the outcomes of interest.
During the data extraction, the outcomes of interest were classified into four groups: symptoms of COVID-19 during pregnancy, obstetric outcomes, maternal and neonatal treatment, and neonatal outcomes. We also contacted the authors of an article to obtain more precise data about the symptoms of the patients evaluated [17].
Assessment of risk of bias
The bias risk assessment was carried out using the forms included in the Joanna Briggs Institute (JBI) reviewers' manual [18].
The overlapping risk (inclusion of the same patients in different papers) was assessed through a manual search and led us to the exclusion of 12 articles (Supplementary Material Table S2).
Data analysis
The meta-analysis was performed with STATA (STATA Corp. LLC, version 14) using the ‘metan’ and ‘metaprop’ programs and led to pooled prevalences and means with 95% confidence intervals (95%CIs). The pooled estimates have been calculated after Freeman–Tukey double arcsine transformation to stabilize the variances [19].
The I2 and the Cochran Q test were used to assess heterogeneity. Low, moderate and high levels of heterogeneity were defined by I2 values of 25%, 50%, and 75%, respectively [20].
Random effects models were used to pool data, giving the moderate to high levels of heterogeneity found. According to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [21] a meta-regression analysis was conducted to determine the source of heterogeneity using the ‘metareg’ program (STATA Corp. LLC, version 14). The following factors were studied: geographic area (China, Europe, United States), study size (≤50 versus >50 patients), study quality (JBI incomplete versus JBI clear) and clinical severity (intubated versus non-intubated patients).
Potential publication bias was assessed using Egger's test and the creation of funnel plots for visual inspection (confunnel command in STATA) [22].
The outcomes with a limited amount of data are presented only in a descriptive manner. This study is registered with PROSPERO, number CRD42020184752.
Results
Study selection
The first screening of 1769 articles identified by title and abstracts through the database systematic search, in addition to the ten articles selected by manual search, led to the selection of 878 papers (Fig. 1). After evaluation of the full texts, 771 further papers were excluded (67 were reviews, 65 were guidelines, 42 did not include clinical or original data, 592 were not related to the subject of interest, and five were not valuable). Detailed reasons for exclusion are provided in the Supplementary Material Table S3).
The remaining 107 papers (the result of the 97 selected by informatic search and ten selected by manual search) were screened in order to exclude case reports (n = 58), case series with fewer than ten pregnancies (n = 13), and all articles which presented the risk of patients overlap (n = 12).
Study characteristics
The 24 articles identified included a total of 1104 pregnancies. One of those [23] had follow-up information about four patients included in another paper [24], and therefore the information in it was extracted together with the original paper (1100 pregnancies).
Seventeen were case series (n = 453 pregnancies) [23,[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]], five were cohort studies (n = 631 pregnancies) [17,[41], [42], [43], [44]], and one was a case–control study (n = 16 pregnancies) [45] (Supplementary Material Table S4).
Risk of bias of included study
The bias risk assessment showed that, among the included papers, seven respected all the JBI criteria for a good-quality study, 12 case series had unclear answers (nine regarding the consecutive and complete inclusion of the participants, three with only one unclear answer regarding either patient demographics or the presenting site/clinic information), three cohort studies didn't assess the presence of confounding factors and a strategy to deal with them, and the same situation occurred in one case–control study (Supplementary Material Figs S2–S4).
The evaluation of the funnel plots for some main outcomes highlighted the presence of an asymmetry in the distribution of the ES/seES ratios among the studies evaluated, although the analysis of the Egger's test p-values indicated no significant presence of publication bias or small size effect (p 0.146 for caesarean section, p 0.145 for COVID-related NICU, p 0.147 for NICU patients with respiratory distress, p 0.405 for neonatal deaths) (Supplementary Material Fig. S5).
Synthesis of results
The study population was composed of 511 Chinese women, 482 European women, 107 North American women (Supplementary Material Table S5). Of 1100 women, 512 were screened for COVID-19 using reverse transcription polymerase chain reaction (nasopharyngeal swab). Seventy-two of these had a negative result, but the local Control Disease Center (CDC) registered them as cases of COVID-19 because of the lack of other causes of fever, the typical CT, or clinical features. Five hundred eighty-eight women were registered as COVID-19 cases on clinical evaluation.
The pooled mean maternal age was 30.57 years (95%CI 29.06–32.08, 22 studies, 431 women) (Table 1 ).
Table 1.
Meta-analysis summary (mean values)
| Outcomes | Maternal and neonatal data |
|||||
|---|---|---|---|---|---|---|
| Number of studies | Number of patients | Overall ES (95%CI) | Heterogeneity test |
Egger's test |
||
| Iˆ2% | p | p | ||||
| Mean maternal age | 22 | 431 | 30.57 (29.06–32.082) | 0.00 | 1.000 | 0.803 |
| Mean gestational age at birth | 11 | 151 | 37.97 (37.59–38.35) | 0.00 | 0.957 | 0.217 |
| Preterm delivery mean gestational age (W) | 17 | 11 | 35.74 (35.55–35.93) | 76.6 | 0.019 | 0.185 |
| Birthweight (g) | 14 | 217 | 3144.71 (2894.95–3394.47) | 0.00 | 0.936 | 0.062 |
The most common symptoms presented by women and the laboratory results are listed in Fig. 2 . Maternal health deterioration (the onset of COVID-19-related symptoms that required hospitalization or prolonged the hospital stay after delivery) affects mainly the prenatal period (73% of patients; 95%CI 55–88; 16 studies; 525 women) as opposed to the postnatal period (22%; 95%CI 8–40; 13 studies, 462 women).
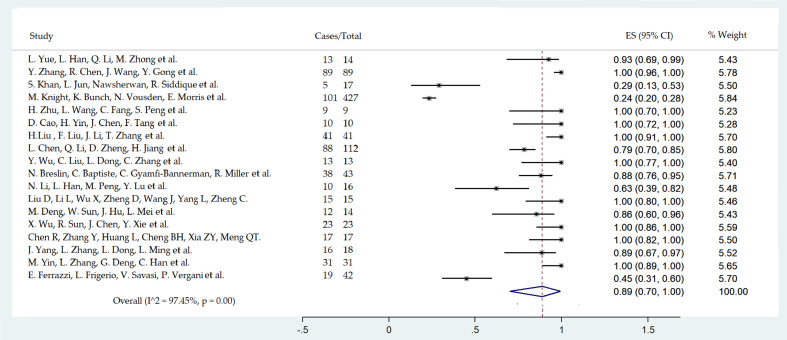
Fig. 2.
Forest plot of pneumonia prevalence among hospitalized women with a diagnosis of COVID-19: pneumonia pooled prevalence among mothers evaluated considering 18 studies including 951 women.
The pooled prevalence of intensive care unit (ICU) admission is reported in Fig. 3 .
Fig. 3.
Forest plot of maternal ICU admission due to COVID-19 evaluated considering 11 studies including 753 women.
Among the 1100 pregnant women, five cases of maternal death were reported. There were three stillbirths out of 779 pregnancies; in one case the death was not COVID-19-related, while in the other two cases it was unclear whether the infection had any influence [17].
The mean gestational age at birth was 37.97 weeks (95%CI 37.59–38.35; 12 studies, 151 women) (Table 1). The rate of preterm delivery (<37 weeks) was assessed in 17 studies resulting in a pooled prevalence of 23% (95%CI 12–37; 17 studies, 684 newborns) (Fig. 4 ). The pooled mean gestational age of preterm birth was 35.74 weeks (95%CI 35.55–35.94; three studies; 11 women).
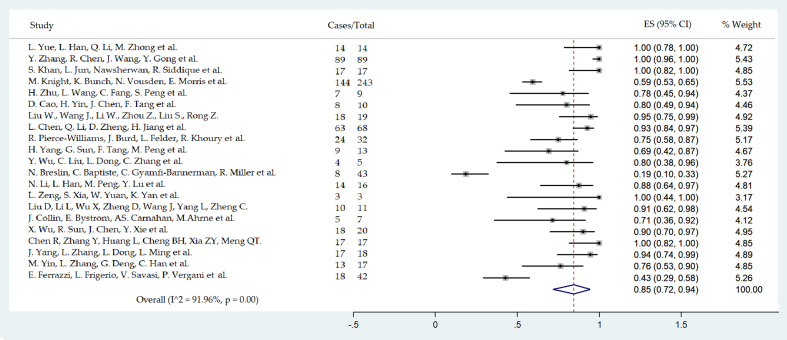
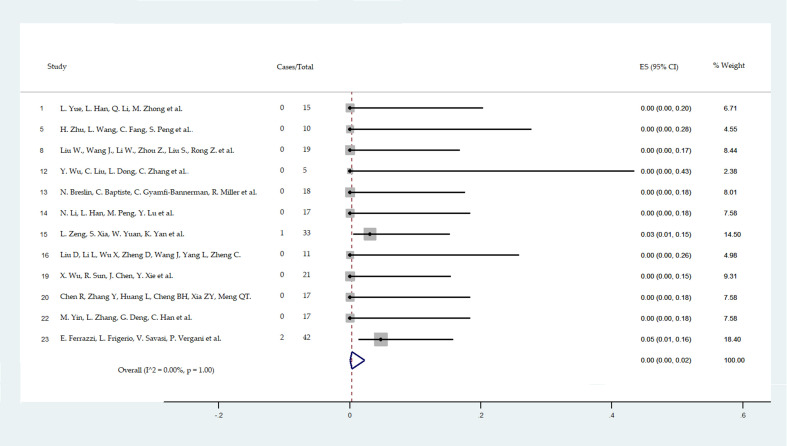
Fig. 4.
Forest plot of caesarean section delivery. Caesarean delivery pooled rate evaluated considering data from 21 studies including 713 patients.
The number of studies—considering the presence of preeclampsia, chorioamnionitis and premature preterm rupture of membranes as co-morbidities—was not sufficient to draw any conclusions.
A pooled prevalence of 85% (95%CI 72–94, 21 studies, 520 women) was observed for caesarean deliveries (Table 2 , Fig. 5 ). The pooled prevalences of emergency caesarean delivery for maternal and foetal indication are shown in Table 2. The mean birthweight was 3144.71 (95%CI 2894.95–3394.47, 14 studies, 217 newborns) (Table 1).
Table 2.
Meta-analysis summary
| Outcomes | Maternal data |
|||||
|---|---|---|---|---|---|---|
| Number of studies | Number of patients | Overall ES (95%CI) | Heterogeneity test |
Egger's test |
||
| Iˆ2 % | p | p | ||||
| Prenatal deterioration (COVID-19 symptoms) | 16 | 525 | 73% (55–88%) | 93.96 | 0.00 | 0.002 |
| Postnatal deterioration (COVID-19 symptoms) | 13 | 462 | 22% (8–40%) | 93.22 | 0.00 | 0.003 |
| Fever | 20 | 981 | 50% (40–61%) | 85.69 | 0.00 | 0.080 |
| Cough | 19 | 968 | 33% (23–43%) | 86.87 | 0.00 | 0.001 |
| Dyspnoea | 15 | 914 | 12% (6–20%) | 86.54 | 0.00 | 0.03 |
| Diarrhoea | 15 | 907 | 4% (2–6%) | 23.18 | 0.20 | 0.220 |
| Pneumonia | 18 | 951 | 89% (70–100%) | 97.45 | 0.00 | 0.001 |
| Other symptoms | 14 | 879 | 16% (4–32%) | 95.46 | 0.00 | 0.003 |
| Lymphocytopenia | 23 | 214 | 34% (19–50%) | 81.10 | 0.00 | 0.832 |
| CRP increase | 9 | 266 | 51% (30–72%) | 90.21 | 0.00 | 0.941 |
| Maternal ICU admission (Tot) | 11 | 753 | 8% (1–20%) | 91.81 | 0.00 | 0.644 |
| Obstetric data | ||||||
| Vaginal delivery | 18 | 686 | 14% (6–24%) | 87.41 | 0.00 | 0.251 |
| Caesarean delivery (CD) | 21 | 713 | 85% (72–94%) | 91.96 | 0.00 | 0.146 |
| Emergency CD for maternal condition | 10 | 457 | 19% (3–43%) | 94.35 | 0.00 | 0.779 |
| Emergency CD for foetal condition | 10 | 474 | 7% (1–16%) | 78.34 | 0.00 | 0.100 |
| Preterm birth | 17 | 684 | 23% (12–37%) | 91.11 | 0.00 | 0.515 |
| Preterm premature rupture membrane (pPROM) | 9 | 174 | 10% (3–19%) | 56.30 | 0.02 | 0.022 |
| Preeclampsia | 4 | 58 | 7% (0–20%) | 44.71 | 0.14 | 0.204 |
| Neonatal data | ||||||
| APGAR 5’ <7 | 12 | 225 | 0% (0–2%) | 0.00 | 1.00 | 0.004 |
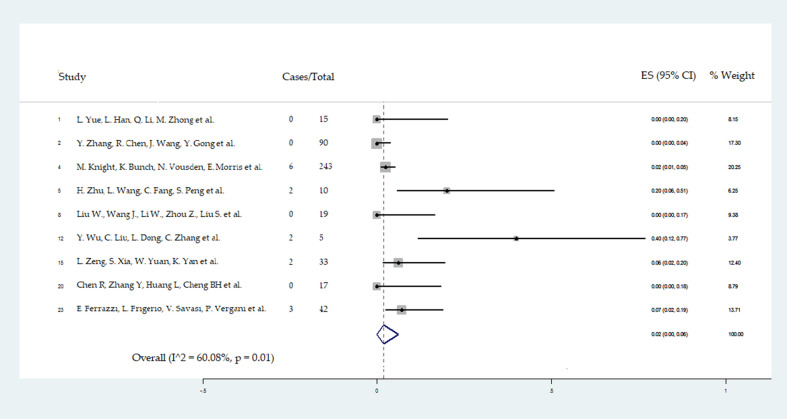
| NICU admission COVID-19-related | 9 | 474 | 2% (0–7%) | 65.20 | 0.01 | 0.145 |
| Respiratory distress | 10 | 272 | 4% (0–12%) | 76.97 | 0.00 | 0.147 |
| Maternal therapy | ||||||
| Oxygen support | 8 | 634 | 66% (27–96%) | 98.27 | 0.00 | 0.012 |
| Mechanical ventilation | 5 | 557 | 13% (2–30%) | 90.71 | 0.00 | 0.377 |
| Antiviral therapy | 2 | 623 | 34% (11–61%) | 96.02 | 0.00 | 0.010 |
| Antibiotic therapy | 6 | 168 | 77% (37–100%) | 96.03 | 0.00 | 0.184 |
| Corticosteroid | 4 | 520 | 14% (6–24%) | 67.52 | 0.03 | 0.874 |
Fig. 5.
Forest plot of preterm delivery pooled rate evaluated considering data from 17 studies including 684 patients.
Viral RNA was sought in different specimens (Supplementary Material Table S6). Determination of anti-SARS-CoV-2 serum antibodies was never reported.
Different treatment regimens for pregnant women were adopted as shown in Table 2.
The pooled frequencies of NICU admission with respiratory symptoms possibly related to COVID-19 and 5-minute Apgar score <7 are reported in Fig. 6, Fig. 7 .
Fig. 6.
Forest plot of NICU admission caused by respiratory symptoms possibly COVID-19-related. NICU admission related to COVID-19 evaluated considering nine studies including 474 newborns.
Fig. 7.
Forest plot of newborns with APGAR <7 at 5′ evaluated considering data from 12 studies including 348 newborns.
Only one out of 54 newborns with reported data on respiratory symptoms and support was treated with nasal constant positive airway pressure (CPAP), and no one needed mechanical ventilation or oxygen supplementation. Three out of 537 newborns died; in two cases the death was definitely not related to COVID-19, and the third newborn was a preterm baby with a negative throat swab [17,27].
Data on breastfeeding was fully reported in only one article; 12 out of 42 newborns received maternal milk [40]. Breastfeeding was allowed by physicians in only ten women after vaginal delivery, with the patients using a surgical mask. Two other women received the diagnosis of COVID-19 infection during the postpartum period and breastfed without a surgical mask. Both newborns had a positive test for COVID-19 infection at days 1 and 3, respectively [40].
Nineteen out of 444 neonates were positive for SARS-CoV-2 RNA at birth [17,30,40,42,43]. Elevated levels of IgM and IgG serum antibodies were reported in one case [33]. Six neonates had a positive nasopharyngeal swab within the first 12 hours after birth, and one of these infants required NICU admission [17]. In 12 neonates the diagnosis of COVID-19 was late and based on virus RNA detection in respiratory samples collected 12–48 h after birth [17,40,42]. Of these, four had no clinical symptoms or signs, three presented with early-onset COVID-19, five were admitted to NICU, with no indication provided by the authors [17,33,40,43].
Among the three neonates with early-onset COVID-19, one experienced lethargy and fever; chest radiographic imaging showed pneumonia, but other laboratory tests (except procalcitonin) were normal [43]. The second neonate presented with lethargy, vomiting, and fever. Laboratory tests showed leukocytosis and lymphocytopenia. A chest radiographic image showed pneumonia. The third one required resuscitation due to neonatal respiratory distress syndrome [43]. He had a confirmed radiological pneumonia and also a suspected Enterobacter sepsis, with leukocytosis, thrombocytopenia and coagulopathy [43].
Another newborn developed gastrointestinal symptoms within a few hours after birth, and respiratory symptoms requiring NICU admission developed 3 days later; the first COVID-19 test was equivocal a few hours after delivery and positive 3 days later [40]. Only in one case was the presence of IgM and IgG in the neonate serum at 2 hours of age found; throat swab and blood sample were PCR-RT-negative and the newborn was asymptomatic [33].
Meta-regression analysis
In several outcomes through the meta-analysis (‘metaprop’ function) a high heterogeneity was observed (I 2 >75%). These values were reanalysed with the use of the ‘metareg’ program [46]. The results of this analysis showed that the weight of some single subclassification of the parameters (in particular, geographical area and sample size) was able to reduce significantly or cancel the residual heterogeneity (Table 3 ).
Table 3.
Meta-regression for the analysis of heterogeneity in the main outcomes analysed: the statistical influence of confounding factors in the evaluation of residual heterogeneity
| Parameters | Independent variables | Coefficient | 95%CI | p |
|---|---|---|---|---|
| Pneumonia | Country | –0.4723522 | –0.9123816 –0.0323228 | 0.037 |
| Sample size | –0.1499616 | –0.4409635 0.1410404 | 0.286 | |
| Quality | 0.0485202 | –0.346751 0.4437913 | 0.795 | |
| Severity | 0.0729786 | –0.4541209 0.6000781 | 0.770 | |
| Cons | 0.9230959 | 0.7499989 1.096193 | 0.000 | |
| Fever | Country | 0.0541411 | –0.3493191 0.4576012 | 0.779 |
| Sample size | 0.1290172 | –0.1340649 0.3920992 | 0.312 | |
| Quality | 0.0475743 | –0.2548847 0.3500333 | 0.742 | |
| Severity | –0.0484275 | –0.5252193 0.4283644 | 0.832 | |
| Cons | 0.4504648 | 0.2882955 0.612634 | 0.000 | |
| Cough | Country | 0.1579024 | –0.2053284 0.5211332 | 0.367 |
| Sample size | 0.2302113 | 0.0068833 0.4535393 | 0.044 | |
| Quality | 0.0167079 | –0.2829139 0.3163296 | 0.906 | |
| Severity | –0.1139139 | –0.5578521 0.3300242 | 0.591 | |
| Cons | 0.2978404 | 0.1487898 0.446891 | 0.001 | |
| Dyspnea | Country | 0.1430722 | –0.238273 0.5244173 | 0.423 |
| Sample size | 0.0649849 | –0.1364552 0.2664251 | 0.489 | |
| Quality | 0.155048 | –0.1680703 0.4781663 | 0.310 | |
| Severity | –0.0536233 | –0.6091708 0.5019241 | 0.834 | |
| Cons | 0.047404 | –0.1217407 0.2165488 | 0.546 | |
| Increase of PCR | Country | –0.1116694 | –0.9635143 0.7401755 | 0.750 |
| Sample size | 0.0116586 | –0.790832 0.8141491 | 0.972 | |
| Severity | 0.0835687 | –1.01164 1.178778 | 0.852 | |
| Cons | 0.5164313 | 0.1559681 0.8768946 | 0.014 | |
| Caesarean section | Country | –0.4173112 | –0.7252412 –0.1093811 | 0.011 |
| Sample size | 0.1669119 | –0.014046 0.3478699 | 0.068 | |
| Quality | 0.0090592 | –0.2604085 0.2785268 | 0.944 | |
| Severity | –0.0281418 | –0.4186858 0.3624023 | 0.881 | |
| Cons | 0.8517038 | 0.7035435 0.9998641 | 0.000 | |
| Emergency for maternal condition | Country | 0.0774867 | –0.9992338 1.154207 | 0.861 |
| Sample size | 0.2479584 | –0.0772496 0.5731663 | 0.107 | |
| Quality | –0.3836545 | –0.9392774 0.1719683 | 0.136 | |
| Severity | –0.1836545 | –1.040787 0.6734775 | 0.605 | |
| Cons | 0.3836545 | 0.0689591 0.6983499 | 0.026 | |
| Emergency for foetal condition | Country | –0.1413118 | –1.242252 0.9596284 | 0.755 |
| Sample size | –0.0167328 | –0.3524948 0.3190291 | 0.903 | |
| Quality | –0.018563 | –0.5920985 0.5549725 | 0.937 | |
| Severity | 0.2490709 | –0.6179754 1.116117 | 0.493 | |
| Cons | 0.0509291 | –0.2989942 0.4008524 | 0.724 | |
| Respiratory distress | Country | –0.0449407 | –0.7638171 0.6739357 | 0.883 |
| Sample size | –0.0765406 | –0.4012812 0.2481999 | 0.585 | |
| Quality | 0.0239556 | –0.4741119 0.5220231 | 0.910 | |
| Cons | 0.0765406 | –0.157831 0.3109123 | 0.455 | |
| Maternal oxygen support | Country | –0.8110614 | –2.26178 0.639657 | 0.196 |
| Sample size | –0.0294046 | –1.264762 1.205953 | 0.950 | |
| Quality | 0.2881007 | –1.239171 1.815372 | 0.628 | |
| Cons | 0.9777281 | 0.1991738 1.756282 | 0.025 | |
| Maternal antiviral therapy | Country | –0.3157895 | –1.301622 0.6700427 | 0.448 |
| Sample size | 0.1185084 | –0.6136965 0.8507132 | 0.695 | |
| Quality | –0.2849887 | –1.134679 0.5647014 | 0.428 | |
| Cons | 0.6007782 | 0.2250636 0.9764929 | 0.009 | |
| Maternal antibiotic therapy | Country | –0.847815 | –1.475181 –0.220449 | 0.023 |
| Sample size | 0.4927326 | –0.1316467 1.117112 | 0.087 | |
| Cons | 0.9175824 | .5166319 1.318533 | 0.005 | |
| Maternal corticosteroid therapy | Country | 0.0389794 | –0.3737892 0.451748 | 0.833 |
| Quality | –0.0086396 | –0.3914519 0.3741728 | 0.960 | |
| Severity | –0.0389794 | –0.7867143 0.7087555 | 0.907 | |
| Cons | 0.0086396 | –0.2040965 0.2213756 | 0.928 | |
| Preterm birth | Country | 0.0551173 | –0.4160756 0.5263102 | 0.803 |
| Sample size | 0.0410649 | –0.2775296 0.3596593 | 0.784 | |
| Quality | 0.0549882 | –0.3914821 0.5014584 | 0.793 | |
| Severity | 0.0536936 | –0.5063904 0.6137776 | 0.838 | |
| Cons | 0.174901 | –0.0295406 0.3793426 | 0.087 | |
| Lymphocytopenia | Country | –0.2686917 | –0.7751601 0.2377767 | 0.242 |
| Quality | –0.2515488 | –0.84352 0.3404224 | 0.339 | |
| Severity | 0.1884512 | –0.6397138 1.016616 | 0.598 | |
| Cons | 0.4115488 | 0.1815874 0.6415102 | 0.005 | |
| Prenatal maternal deterioration (COVID-19 symptoms) | Country | 0.3357523 | –0.1332151 0.8047198 | 0.143 |
| Sample size | 0.3637611 | 0.0409476 0.6865746 | 0.031 | |
| Quality | 0.0000416 | –0.3957173 0.3958004 | 1.000 | |
| Severity | –0.2637575 | –0.8152511 0.287736 | 0.315 | |
| Cons | 0.5961997 | 0.3619205 0.8304788 | 0.000 | |
| Postnatal maternal deterioration (COVID-19 symptoms) | Country | –0.3040568 | –0.7362708 0.1281573 | 0.143 |
| Sample size | –0.3136545 | –0.6062192 –0.0210898 | 0.039 | |
| Quality | 0.1234261 | –0.3352324 0.5820845 | 0.552 | |
| Severity | –0.0076587 | –0.5524848 0.5371675 | 0.975 | |
| Cons | 0.385257 | .1361235 0.6343904 | 0.007 | |
| pPROM | Country | –0.3473107 | –1.392344 0.6977227 | 0.432 |
| Sample size | –0.0214393 | –0.5308038 0.4879251 | 0.918 | |
| Severity | 0.2880034 | –0.5453185 1.121325 | 0.415 | |
| Cons | 0.1119966 | –0.1431551 0.3671483 | 0.310 | |
| Vaginal delivery | Country | 0.3998788 | 0.0847798 0.7149778 | 0.017 |
| Sample size | –0.0630282 | –0.2545473 0.128491 | 0.490 | |
| Quality | –0.0085135 | –0.285622 0.2685949 | 0.948 | |
| Severity | –0.1475732 | –0.549827 0.2546805 | 0.442 | |
| Cons | 0.1137921 | –0.0465909 0.2741751 | 0.149 | |
| Maternal ICU admission | Country | 0.0576582 | –0.6317374 0.7470538 | 0.845 |
| Sample size | –0.1022978 | –0.5411524 0.3365567 | 0.589 | |
| Quality | –0.0375799 | –0.921696 0.8465362 | 0.921 | |
| Severity | 0.2516312 | –0.9263089 1.429571 | 0.620 | |
| Cons | 0.0375799 | –0.3149941 0.390154 | 0.803 | |
| Maternal death | Country | 0.0018102 | –0.3562318 0.3598522 | 0.992 |
| Sample size | 0.0034593 | –0.1715208 0.1784395 | 0.967 | |
| Quality | 0.0018102 | –0.2985012 0.3021215 | 0.990 | |
| Severity | 0.0042078 | –0.5058023 0.5142179 | 0.986 | |
| Cons | –0.0018102 | –0.1430766 0.1394562 | 0.978 |
Statistical heterogeneity in pneumonia and cough was explained respectively by the geographical area (p 0.037) and the size of the study (p 0.044). Heterogeneity in caesarean section rate was explained by the geographical area of the studies (p 0.011). Heterogeneity in prenatal and postnatal maternal deterioration (COVID-19-related) were both explained by the number of patients recruited in the study (p 0.031 and p 0.039, respectively).
Pneumonia and cough were more frequently reported in Chinese patients and in small studies. The caesarean rate was higher in the series from China. Prenatal and postnatal maternal deterioration appeared to be greater in small studies.
The heterogeneity in other outcomes was not totally explained by our analysed confounders, although there was a notable reduction in residual heterogeneity in most of these (with an . Three remaining outcomes showed a reduction of the I2 just above the limit set: the increase in PCR rate COVID-19-related (from an initial value of 90.21%), the presence of maternal antiviral therapy ( from an initial value of 96.02%) as well as the maternal ICU admission ( from an initial value of 91.81%); none of these was fully explained by our selected confounders.
The maternal oxygen support was not explained by our analysed confounders (= 90.28% from an initial value of 98.27%). For a complete description, see Table 3.
Discussion
Principal findings
Our large systematic review and meta-analysis involving 1100 patients from 24 studies represents a comprehensive overview of the impact of COVID-19 on pregnancy. The results show that in the majority of cases the clinical course of infection in pregnant women was not complicated. The most common symptoms in COVID-19-positive pregnant women were those typically related to the virus, i.e., fever and cough followed by symptoms of anosmia, ageusia, myalgia, fatigue, sore throat, malaise, rigor, headache, and poor appetite [47].
The most frequent laboratory abnormalities in COVID-19-positive pregnant women were elevated C-reactive protein and reduced lymphocyte count, whereas leukocytosis was rarely found. These findings are generally consistent with those observed in non-pregnant adults with COVID-19 [47].
The pooled prevalences of ICU admission and maternal death were comparable with those reported in non-pregnant women. Indeed, a preliminary analysis of cases in the United States showed that among adults aged 20–44 ICU admission and the case fatality rate (CFR) were 2.0–4.2% and 0.1–0.2%, respectively, likewise reported in our analysis [48].
A similar CFR for non-pregnant subjects aged 20–49 has also been reported from China [49]. However, there is a possibility that criteria for ICU admission were different among the studies.
Overall, deterioration in maternal condition requiring hospitalization or extension of the hospital stay due to the onset of COVID-19-related symptoms occurred more often before delivery.
The results of the meta-analysis showed that caesarean section was the preferred mode of delivery in pregnant women with COVID-19 despite the guidelines and recommendation of experts which suggest opting for vaginal delivery whenever possible [[50], [51], [52]]. The rate of caesarean delivery is not completely explained by the severity of maternal disease or foetal compromise. Data showed that only a few studies reported COVID-19-related maternal complications or non-reassuring foetal status as indications for caesarean delivery. Consequently, other clinical and non-clinical factors must be taken into account. The presence of obstetric comorbidities (i.e. preeclampsia), the management of the mother's respiratory disease, the prevention of vertical transmission, and the COVID-19 itself might have led the caregiver to decide for caesarean delivery [22].
The pooled prevalence of preterm delivery was 23%. This rate seems to be higher than that observed in the general obstetric population, where it ranges from about 5% in several European countries to 18% in some African countries [53]. The data show that higher frequency was due to worsening maternal and foetal conditions with the need to deliver prematurely, but also to rupture of membranes and spontaneous delivery. It is unclear, however, whether COVID-19 might be the direct cause of preterm delivery; viral infection during pregnancy can induce an abnormal response to an opportunistic bacterial infection that might lead to preterm labour and delivery [54].
The heterogeneity observed among the studies considered in this meta-analysis, the lack of knowledge of the disease and therapies, and the preliminary nature of the results of many studies suggest caution when drawing definitive conclusions on these topics. Further research is needed, related to the stage of the COVID-19, in order to assess whether the mode and timing of delivery may affect the course of maternal and foetal outcomes differently.
Viral RNA was detected in patient stool samples in a low percentage of case, but was absent in amniotic fluid, placenta, vaginal secretion and blood, suggesting that intrauterine/intrapartum transmission is unlikely. The presence of IgG and IgM in maternal serum was not investigated in the studies we considered. Further efforts are needed to better understand the target organs and dynamics of the immune response.
Only one of 225 newborns had an Apgar score <7 at 1 minute and in three cases at 5 minutes, confirming that the infection does not have a significant impact on foetal health (Table 2).
The pooled mean birthweight was 3144.71 g. As COVID-19 presents as an acute infection, if developed close to delivery it is unlikely to have an impact on birth weight. However, for infections that occur early in pregnancy, and for women who suffer from chronic hypoxia, a growth scan is recommended to assess the risk of intrauterine growth retardation [55].
Only one study reported the number of newborns receiving breast milk, showing a low rate of lactation (28.6%) [40]; breastfeeding was allowed by physicians in only ten women after vaginal delivery, with the women using a surgical mask, and no cases of virus transmission were reported. The authors described two other cases in which skin-to-skin contact after birth and breastfeeding was allowed without a mask because the infection was not known. Both neonates tested positive at days 1 and 3 after birth, respectively. However, viral testing was not carried out immediately after birth, so that vertical transmission cannot be excluded [40].
Another study reported that mothers were “encouraged to breastfeed with the use of hand hygiene and maternal masking”, even though no clear data on the number of mothers who actually breastfed were provided [34].
Considering that virus RNA was rarely detected in maternal milk, breastfeeding during maternal COVID-19 should not be contraindicated, according to WHO guidelines, although further studies on more extensive series are needed [56]. When considering NICU COVID-related hospitalization, the number of symptomatic infants appears to be very small and only one newborn needed non-invasive ventilation [41]. The low rate and lack of non-invasive and invasive ventilation could be due to the absence of reported data. Indeed, some authors did not report ventilatory support, even though they reported NICU hospitalization for respiratory symptoms. Overall respiratory distress occurred with a lower frequency than in the general population of non COVID-19 term births [57].
These findings suggest that the majority of NICU hospitalizations were done pre-cautiously to closely monitor neonatal conditions or with the aim of isolating the newborns from their mothers to prevent infection transmission. Furthermore, the NICU admission criteria could differ between hospitals.
In our study population there were three stillbirths and three neonatal deaths, but in none of these was a clear correlation with the infection reported [17,27]. These results suggest that the foetal and neonatal mortality risk is extremely low.
In our meta-analysis different types of treatments were used, but their efficacy was not compared. Randomized controlled trials are needed to identify an effective therapeutic protocol for pregnant women.
Although SARS-CoV-2 RNA was not detected in the peripheral blood of pregnant women included in this review, its presence was reported in 1% of infected non-pregnant adults [58]. Therefore, theoretically, mother-to-child in utero transmission could not be excluded. In this meta-analysis, 19 newborns had a positive nasopharyngeal swab. However, the swabs were not collected immediately after birth. Additionally, viral RNA has never been detected in cord or neonatal blood. Therefore, the possibility of newborn contamination from the mother or from healthcare staff members, or from other sources of infection, could not be ruled out. For example, SARS-CoV-1 was found in peritoneal fluid collected during caesarean delivery [59]. In one newborn, elevated serum concentrations of IgM and IgG were detected even though there were no RT-PCR-positive specimens, and he was asymptomatic [33]. IgM antibodies do not cross the placenta because they are too large, and they do not appear until 3–7 days after infection. Therefore, the detection of IgM in neonatal blood suggests an intrauterine infection. Great caution in interpreting these results has been advocated [60]. The specificity of IgM tests is not 100%, so false positives could not be excluded. Furthermore, the reported rapid decline in IgM concentrations was unusual when compared with other vertically transmitted virus infections [60]. Finally, it could not be ruled out that an infected placenta could allow the passive transfer of IgM.
Our heterogeneity assessments using meta-regression analysis showed that the variation in some estimates was significantly influenced by the geographical area and the size of the study. The majority of Chinese studies reported data from patients observed in the early stages of the outbreak. These patients probably presented with more severe disease [49]. Moreover, the standard of care of the disease has evolved over the course of the COVID-19 pandemic, resulting in better clinical outcomes [49]. Most of the small studies were published earlier and likewise included higher-risk patients.
The high heterogeneity in other outcomes were not or not totally resolved by the meta-regression analysis, although there was a notable reduction in residual heterogeneity in most of these. This might be due to residual confounders not accounted for in this analysis.
Strengths and limitations
A large number of pregnant women were included from studies carried out in China, North America and Europe, so that the results of this meta-analysis provide a broad overview of COVID-19 in pregnancy. Studies with fewer than ten cases were excluded due to a high potential for bias. All the studies selected during the Eligibility Phase (according to the PRISMA guidelines) have been further evaluated by manual comparison of populations, study settings and authors to exclude overlapping cases. Some studies are from MedRxiv and are not yet peer-reviewed. However, JBI evaluation indicated that these studies are of good quality and so they have been included in the meta-analysis.
There are also some limitations to our systematic review and meta-analysis. First, the data extracted from all studies were collected retrospectively. Second, in many studies patient information regarding some secondary outcomes were missing or unavailable. Third, for many outcomes a substantial heterogeneity between studies was found. In some outcomes the high degree of heterogeneity was not fully explained by the potential confounders analysed in the metaregression analysis. Finally, the risk of caesarean delivery and neonatal NICU admission would be overestimated due to concern for mother-to-newborn virus transmission.
Conclusions and implications
Clinical characteristics of COVID-19 in pregnant patients seem to be similar to those in non-pregnant infected adults. This meta-analysis shows a high frequency of preterm births and caesarean deliveries and a low rate of breastfeeding, not fully explained by the severity of maternal disease or foetal compromise. Indeed, maternal and perinatal outcomes of COVID-19 during pregnancy are generally good and are not characterized by a severe clinical course. There is currently no clear evidence of vertical transmission of COVID-19. Therefore, COVID-19 itself should not be considered as an indication for elective caesarean delivery. Prospective studies are needed to clarify the real risk of COVID-19 in pregnancy and to define its optimal management.
Author contributions
FDT made substantial contributions to conception and design of the study, analysis and interpretation of data, and to the drafting of the manuscript. MG made substantial contributions to the analysis and interpretation of the data and to the drafting of the manuscript. GDL made substantial contributions to the conception and design of the study, analysis and interpretation of the data, and to the drafting of the manuscript. DDS made substantial contributions to analysis and interpretation of the data and to the revision of the manuscript. FDS made substantial contributions to the analysis and interpretation of the data and to the drafting of the manuscript. GM made substantial contributions to analysis and interpretation of the data and to the drafting of the manuscript. FMR made substantial contributions to the analysis and interpretation of the data and to the drafting of the manuscript. FR made substantial contributions to analysis and interpretation of the data and to the drafting of the manuscript. UW made substantial contributions to the analysis and interpretation of data and to the revision of the manuscript. RLD’A made substantial contributions to the analysis and interpretation of the data and to the revision of the manuscript. LR made substantial contributions to the analysis and interpretation of the data and to the revision of the manuscript. GR made substantial contributions to the conception and design of the study, and to drafting and revising the manuscript. All authors have given final approval of the version of the manuscript to be published.
Transparency declaration
The authors report no conflicts of interest. This work was supported by a grant from the Institute for Maternal and Child Health ‘IRCCS Burlo Garofolo’, Trieste, Italy (RC 8/20). The funder had no role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, the writing of the manuscript or the decision to submit the manuscript for publication.
Acknowledgements
Authors would like to acknowledge Dr Martina Bradaschia for English revision of the manuscript.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.10.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonney E.A. Alternative theories: pregnancy and immune tolerance. J Reprod Immunol. 2017;123:65–71. doi: 10.1016/j.jri.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127:1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rac H., Gould A.P., Eiland L.S., Griffin B., McLaughlin M., Stover K.R. Common bacterial and viral infections: review of management in the pregnant patient. Ann Pharmacother. 2019;53:639–651. doi: 10.1177/1060028018817935. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207:S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 8.Regan A.K., Moore H.C., Sullivan S.G., DE Klerk N., Effler P.V. Epidemiology of seasonal influenza infection in pregnant women and its impact on birth outcomes. Epidemiol Infect. 2017;145:2930–2939. doi: 10.1017/S0950268817001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases and review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Wang L.-L., Zhao S.-J., Kwak-Kim J., Mor G., Liao A.-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favre G., Pomar L., Musso D., Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395:e40. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasraeian M., Zare M., Vafaei H., Asadi N., Faraji A., Bazrafshan K. COVID-19 pneumonia and pregnancy; a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;19:1–8. doi: 10.1080/14767058.2020.1763952. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Stroup D.F. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R. The Joanna Briggs Institute; 2017. Joanna Briggs Institute reviewer’s manual. [Google Scholar]
- 19.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks J.J., Higgins J.P.T., Altman D.G. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., editors. Cochrane Handbook for systematic reviews of Interventions version 6.1 Cochrane. 2020. www.training.cochrane.org/handbook (updated September 2020).Available from: [Google Scholar]
- 22.Sterne J.A.C., Harbord R.M. Funnel plots in meta-analysis. Stata J Promot Commun Stat Stata. 2004;4:127–141. [Google Scholar]
- 23.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 24.Li L., Liu D., Yang L. Follow-up information about the four pregnant patients with coronavirus disease (COVID-19) pneumonia who were still in the hospital at the end of our study. AJR Am J Roentgenol. 2020;215:W1–W2. doi: 10.2214/AJR.20.23247. [DOI] [PubMed] [Google Scholar]
- 25.Yue L., Han L., Li Q., Zhong M., Wang J., Wan Z. Anesthesia and infection control in cesarean section of pregnant women with COVID-19 infection: a descriptive study. J Clin Anesth. 2020;66:109908. doi: 10.1016/j.jclinane.2020.109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan S., Peng L., Siddique R., Nabi G., Nawsherwan, Xue M. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020;41:748–750. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao D., Yin H., Chen J., Tang F., Peng M., Li R. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. Int J Infect Dis. 2020;95:294–300. doi: 10.1016/j.ijid.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14:193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y., Liu C., Dong L., Zhang C., Chen Y., Liu J. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG Int J Obstet Gynaecol. 2020;127:1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collin J., Byström E., Carnahan A., Ahrne M. Pregnant and postpartum women with SARS-CoV-2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng M., Sun W., Hu J., Mei L., Weng D., Bo Liu B. 2020. Radiological features on HRCT and RT-PCR testing for the diagnosis of coronavirus disease 2019 (COVID-19) in China: a comparative study of 78 cases in pregnant women. [DOI] [Google Scholar]
- 37.Wu X., Sun R., Chen J., Xie Y., Zhang S., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynecol Obstet. 2020;150:58–63. doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen R., Zhang Y., Huang L., Cheng B.-H., Xia Z.-Y., Meng Q.-T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67:655–663. doi: 10.1007/s12630-020-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin M., Zhang L., Deng G., Han C., Shen M., Sun H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy in China: a retrospective cohort study. http://medrxiv.org/lookup/doi/10.1101/2020.04.07.20053744 Available at:
- 40.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Chen R., Wang J., Gong Y., Zhou Q., Cheng H. medRxiv; 2020. Anaesthetic management and clinical outcomes of parturients with COVID-19: a multicentre, retrospective, propensity score matched cohort study.https://www.medrxiv.org/content/10.1101/2020.03.24.20042176v1 Available at: [Google Scholar]
- 42.Pierce-Williams R.A.M., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020;2:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth. 2020;20:394. doi: 10.1186/s12884-020-03026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case–control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harbord R.M., Higgins J.P.T. Meta-regression in stata. Stata J Promot Commun Stat Stata. 2008;8:493–519. [Google Scholar]
- 47.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 50.Favre G., Pomar L., Qi X., Nielsen-Saines K., Musso D., Baud D. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.COVID-19 FAQs for obstetrician-gynecologists, obstetrics. https://www.acog.org/en/Clinical Information/Physician FAQs/COVID 19 FAQs for Ob Gyns Obstetrics Available from:
- 52.Coronavirus (COVID-19) infection and pregnancy. Royal college of obstetricians & gynaecologists. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/ Available from:
- 53.Blencowe H., Cousens S., Oestergaard M.Z., Chou D., Moller A.-B., Narwal R. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 54.Mendz G.L., Kaakoush N.O., Quinlivan J.A. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol. 2020;3:58. doi: 10.3389/fcimb.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poon L.C., Yang H., Kapur A., Melamed N., Dao B., Divakar H. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynecol Obstet. 2020;149:273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO EMRO Breastfeeding advice during the COVID-19 outbreak. Nutrition-infocus. Nutrition. http://www.emro.who.int/nutrition/nutrition-infocus/breastfeeding-advice-during-covid-19-outbreak.html [cited 2020 Jun 10]. Available from:
- 57.Edwards M.O., Kotecha S.J., Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29–37. doi: 10.1016/j.prrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shek C.C., Ng P.C., Fung G.P.G., Cheng F.W.T., Chan P.K.S., Peiris M.J.S. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 60.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection be acquired in utero?: more definitive evidence is needed. JAMA. 2020;323:1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.