Abstract
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is an emerging viral disease and dromedary camels are known to be the source of human spill over events. A cross-sectional epidemiological surveillance study was carried out in Kenya in 2017 to, 1) estimate MERS-CoV antibody seropositivity in the camel-dense counties of Turkana, Marsabit, Isiolo, Laikipia and Nakuru to identify, and 2) determine the risk factors associated with seropositivity in camels. Blood samples were collected from a total of 1421 camels selected using a multi-stage sampling method. Data were also collected from camel owners or herders using a pre-tested structured questionnaire. The sera from camel samples were tested for the presence of circulating antibodies to MERS-CoV using the anti-MERS-CoV IgG ELISA test. Univariate and multivariable statistical analysis were used to investigate factors potentially associated with MERS-CoV seropositivity in camels. The overall seropositivity in camel sera was 62.9 %, with the highest seropositivity recorded in Isiolo County (77.7 %), and the lowest seropositivity recorded in Nakuru County (14.0 %). When risk factors for seropositivity were assessed, the “Type of camel production system” {(aOR = 5.40(95 %CI: 1.67–17.49)}, “Age between 1–2 years, 2–3 years and above 3 years” {(aOR = 1.64 (95 %CI: 1.04–2.59}”, {(aOR = 3.27 (95 %CI: 3.66–5.61)}” and {(aOR = 6.12 (95 %CI: 4.04–9.30)} respectively and “Sex of camels” {(aOR = 1.75 (95 %CI: 1.27–2.41)} were identified as significant predictors of MERS-CoV seropositivity. Our studies indicate a high level of seropositivity to MERS-CoV in camels in the counties surveyed, and highlights the important risk factors associated with MERS-CoV seropositivity in camels. Given that MERS-CoV is a zoonosis, and Kenya possesses the fourth largest camel population in Africa, these findings are important to inform the development of efficient and risk-based prevention and mitigation strategies against MERS-CoV transmission to humans.
Keywords: Dromedary camels, Middle East respiratory syndrome coronavirus, Seropositivity, Risk factor, Pastoral
1. Introduction
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was first identified in humans in 2012 and the virus was subsequently detected in dromedary camel herds in the Arabian Peninsula, the Canary Islands, Central Asia and North Africa (World Health Organization, 2020). Dromedary camels are now known to be the animal reservoir and source of human MERS-CoV infection (Zaki et al., 2012)(Mohd et al., 2016). Human to human transmission have been described in healthcare and social settings (Abdullah et al., 2014) (Drosten et al., 2014). As of January 2020, there have been 2519 laboratory-confirmed human cases and 866 associated human deaths, representing a case fatality rate of 34 % (World Health Organization, 2020).
The World Health Organization’s Research and Development Blueprint has identified MERS-CoV as a high priority pathogen because of the high fatality rate in humans and the large geographic range of seropositive dromedary camel populations (Kelly-Cirino et al., 2019). Confirmation of an epidemiological link between camels and humans has been challenging to establish during outbreak investigations (Azhar et al., 2014). This can be attributed to the fact that camels show no or minor clinical signs if infected with MERS-CoV (Azhar et al., 2014) posing a major challenge in linking human cases to active camel infection. As such, detailed evidence is lacking on the disease epidemiology, viral prevalence and shedding patterns in dromedary camels.
Given the zoonotic nature of MERS-CoV, the health and welfare of camel-keeping communities in Kenya is endangered. This is attributed to the fact that camels show no or minor clinical signs if infected with MERS-CoV (Mohd et al., 2016)(Azhar et al., 2014). Studies available in Kenya indicate variations in MERS-CoV seroprevalence by production system and species surveyed. The seroprevalence of MERS-CoV in camels ranges from 12 % in the ranching production systems, to 72 % in the pastoral production system (Corman et al., 2014)(Deem et al., 2015) while the seroprevalence in high risk slaughter house workers is 10.8 % (Kiyong’a et al., 2020). The circulating MERS-CoV strain in Kenya belongs to clade C, while the full genome sequencing methods in yet another study placed circulating MERS-CoV strains in Kenya under sub-clade C2, in a cluster together with MERS-CoV isolated from Ethiopia and Egypt (Kiambi et al., 2018)(Ommeh et al., 2018).
In Kenya, risk factors associated with occurrence and distribution of MERS-CoV infection in dromedary camels have not yet been adequately investigated nor analysed comprehensively. Given that Kenya has the fourth largest camel population in Africa (3.1 million), behind Somalia (7 million), Chad (6 million) and Sudan (4.7 million), and that all camels in Kenya belong to the species, Camelus dromedaries (Food and Agriculture Organization of the United Nations, 2018), which are the putative reservoir for MERS-CoV transmission to humans, studies are warranted to determine the seroprevalence, geographical distribution and risk factors for MERS-CoV seropositivity in Kenya.
The current studies were commissioned by the Directorate of Veterinary Services of Kenya to determine the seroprevalence and geographic distribution of seropositive camels using ELISA tests and quantify the associations between MERS-CoV seropositivity in camels and host or environment variables. However it should be noted that the serologic evidence of MERS-CoV antibodies is an evidence of exposure and does not equate the measure of true prevalence of MERS-CoV infection in camels(Müller et al., 2014)(Degnah et al., 2020). Outputs of these studies were expected to inform the development of efficient and risk-based management strategies against the potential risk of MERS-CoV transmission to humans.
2. Materials and methods
2.1. Inclusion criteria and study sites
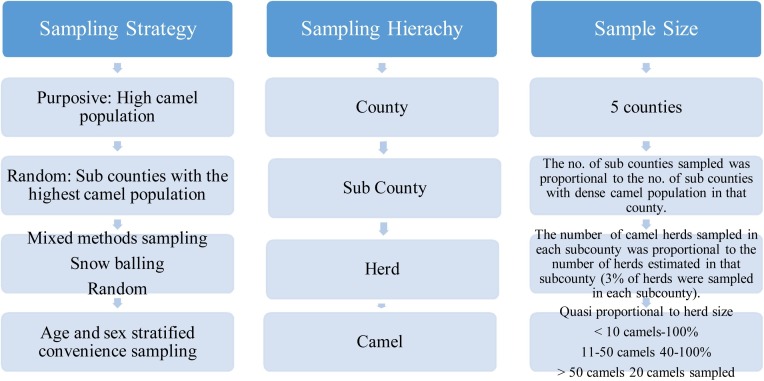
This cross-sectional study was conducted under the direction of the Directorate of Veterinary Services (DVS) in collaboration with the Food and Agriculture Organization of the United Nations (FAO) from June 2016 to July 2017. Five camel dense counties of Isiolo, Marsabit, Turkana, Nakuru and Laikipia were selected and sampled (Fig. 1 ). Criteria for inclusion in this study were: counties with high camel densities, accessibility, security and interface areas for wildlife-livestock interactions.
Fig. 1.
Sampling design used in the study.
Isiolo County is located in the lower eastern region of Kenya (0.3524 °N, 38.4850 °E)(Isiolo County Government, 2018) while Nakuru County is located in the Rift Valley (1.3665 °S, 35.3905 °E) (Nakuru County Government, 2018). Laikipia County is located in the central Rift Valley region (0.3606 °N, 36.7820 °E) (Laikipia County Government, 2018), Marsabit County (2.4426 °N, 37.9785 °E) (Marsabit County Government, 2018), is located in the extreme end of northern Kenya. Similarly, Turkana County (3.3122 °N, 35.5658 °E) is located in the North Western part of the country(Turkana County Government, 2013).
2.2. Study design and sample collection
A multi-stage sampling methodology was deployed using a mix of purposive, proportionate, random, and convenience sampling. The primary sampling unit for this study were counties in Kenya considered high risk for MERS-CoV. High risk counties were purposely selected by the Directorate of Veterinary Services based on the camel population and livestock movement as characterised by the traditional pastoral and semi-sedentary camel management systems. The secondary sampling units were sub counties, randomly selected from a list of sub–counties within each high risk county selected. Camel herds were selected in two ways. The first approach was snow balling where a county veterinarian was asked to recommend a camel herd for inclusion to participate in the study. In the snowballing approach, the owner of the first camel herd identified for MERS-CoV sampling would then recommend the next camel herd for sampling. This approach was used because there is no available database on camels to develop a sampling frame. The second approach was random selection of camel herds at village watering points. Communal village watering points, are aggregation points for camel herds migrating from various villages in search of pasture and water during the dry season. Camels to be sampled were also randomly picked from the known village herds at the communal watering points as depicted in Fig. 1.
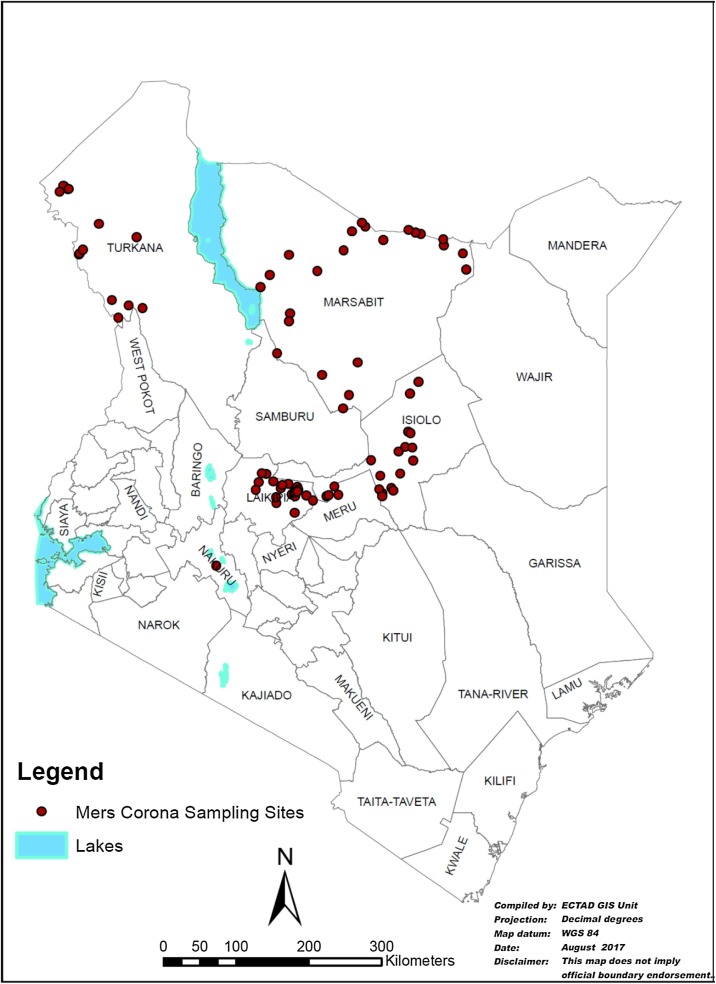
The number of herds selected in each county was proportional to the overall number of herds estimated to be in the county. Camels were conveniently sampled by stratification to cater for the inclusion of both sexes and age categories. Camels were categorized as juvenile (<2 years), young (2–3 years) and adult (>3 years). The number of camels sampled from the respective counties was Turkana (n = 417), Isiolo (n = 403), Marsabit (n = 370), Laikipia (n = 179) and Nakuru (n = 50) (Fig. 2 ).
Fig. 2.
Map showing sampling sites.
Source: FAO.
Protocol for preparation and preservation of the serum samples was done according to FAO guidelines, consistent with international best practices. Blood was collected into plain vacutainer tubes that were immediately inverted and allowed to settle to activate clotting. The tubes were then kept upright or slanted to allow serum to separate. Separated serum samples were frozen at -20 °C and transported to the Central Veterinary Investigation Laboratory after a maximum of 6 days of storage.
2.3. Data collection
Data were collected through a pretested, structured interviewer-administered questionnaire to camel keepers and/or owners on camel herd demographics (herd structure, sex, and age) and other variables of interest (prevailing production and farming systems, health history, wildlife interaction, county of production for camels, whether fencing was applied where the animals rest at night, water source for the camels, whether the camels were separated from other animals and housing status).The risk variables selected for evaluation were included because they have been evaluated for elsewhere – Age, sex and camel production systems (Reusken et al., 2013; Ali et al., 2017) or they constitute a potential, plausible and exploratory risk factors for introduction or transmission of MERS-CoV based on agro ecological practices (Counties of production for camels, fencing for camels, wildlife interactions, water source and separation from other livestock species).This is because previous study has requested studies to identify risk factors for exposures that might explain chains of transmission, zoonotic and environmental sources of human and animal infections (Omrani et al., 2015). High dromedary density area (a characteristic of the pastoral camel production system in Kenya); bringing dromedary camels to communal water points (resulting to interaction between camels and wildlife) were identified as risk factors for MERS-CoV infection between dromedary herds in an experts opinion elicitation exercise to obtain quantitative estimates on the transmission risks from dromedaries to humans(Funk et al., 2016).
Detailed bio-data of individual camel was recorded on the sampling form at the time of sample collection to capture information on county code, sub location name, village name, age and sex of the animal.
2.4. Sample testing
Serum samples were tested for the presence of IgG antibodies reacting with MERS-CoV using the Anti-MERS-CoV ELISA Camel (IgG) protocol (EUROIMMUN, Medizinsche Labordiagnostika AG, 2015) and according to the manufacturer’s instructions. Camel serum samples were classified based on serostatus as determined by ELISA optical density readings.
2.5. Data management and statistical analysis
All data were transferred into a Microsoft Excel spread sheet database, filtered for error and exported to the Statistical Package for Social Sciences (SPSS) for Windows® Version 22.0 (SPSS Inc., Chicago, Illinois) for statistical analysis. At univariate analysis, exploratory data techniques were performed to explore the distribution of the study variables and identify the outliers and descriptive statistics were used to describe patterns found within the data. At bivariate analysis, association between MERS-COV seropositivity and each specific variable at a time was assessed using Pearson’s Chi-square test (χ2) for categorical variables and logistic regression for continuous variables to identify the potential risk factors associated with ELISA seropositivity. With the Pearson’s Chi-square test (χ2) Crude Odds ratios (OR) and 95 % confidence intervals were used to estimate the strength of association between camel MERS-CoV seropositivity and a range of potential risk factors. Further, a multivariable conditional logistic regression model was used to assess all significant risk factors subsequent to the Pearson’s Chi-square test (χ2) bivariate analysis. The logistic regression procedure followed a backward elimination strategy, to determine significant risk factors for camel seropositivity (p < 0.05). Adjusted odds ratio (aOR) and 95 %CI were recorded for the strength of association resulting. Statistical significance was set at P < 0.05 at both bivariate and multivariable analyses.
3. Results
3.1. MERS-CoV seropositivity
A total of 1421 samples were collected from camels kept in pastoral (90.9 %) and ranching camel production systems (9.1 %).The distribution of camel samples assessed was as follows: Isiolo (- 28.4 %), Marsabit (26.0 %), Turkana (29.4 %), Laikipia (12.7 %) and Nakuru (3.5 %) (Table 1 ). Of the total samples, the majority were from female camels (79.5 %). Adults (> 3 years) represented 62.3 % of the age group included in the study. In terms of seropositivity per county, 77.7 % (95 %CI: 73.3 %–81.6 %), (313/403) of the samples from Isiolo were seroreactive while only 14.0 % (95 %CI: 5.8 %–26.7 %), (7/50) of the samples from Nakuru were seroreactive. Other counties presented with seropositivity ranging from 14.9 %–74.0 % (Table 1). The average seropositivity for all counties was 62.9 % (895/1421). Based on the production system, 69.0 % (95 %CI: 66.4 %–71.6 %), (892/1292) of the camels were reared in pastoral production system compared to those raised under ranching production system. For age, 72.6 % (95 %CI: 69.5 %–75.5 %), (643/885) camels aged above 3 years were seropositive for MERS-CoV while those aged below 1 year had a seropositivity of 38.2 % (95 %CI: 30.6 %–46.3 %), (60/157). Female camels had a higher seropositivity 66.6 % (95 %CI: 63.7 %–69.3 %), (752/1129) for MERS-CoV compared to the male camels with 52.3 % (95 %CI: 46.5 %–58.2 %), (153/292) (Table 2 ).
Table 1.
MERS-CoV Seropositivity burden among camels in selected counties in Kenya.
| Variable | No. of samples | No. Positive |
|---|---|---|
| n (%) | n (%), 95 %CI | |
| County | ||
| Isiolo | 403 (28.4 %) | 313 (77.7 %), (95 %CI: 73.3 %–81.6 %) |
| Marsabit | 370 (26.0 %) | 274 (74.0 %), (95 %CI: 69.2 %–78.4 %) |
| Turkana | 417 (29.4 %) | 284 (68.1 %), (95 %CI: 63.4 %–72.6 %) |
| Laikipia | 181 (12.7 %) | 27 (14.9 %), (95 %CI: 10.0 %–20.9 %) |
| Nakuru | 50 (3.5 %) | 7 (14.0 %), (95 %CI: 5.8 %–26.7 %) |
Table 2.
Distribution and association between MERS-CoV sero-positivity and selected factors in five counties.
| Total | Seropositive | |||
|---|---|---|---|---|
| Indicators | n | Mean/P (95 %CI) | cOR (95 %CI) | P-Value |
| Herd size | 1421 | 136¥ (127–145) | 1.0022(1.0014–1.0029) | <0.001 |
| Production system | ||||
| Pastoralism | 1292 | 69.0 % (66.5 %–71.6 %) | 5.11 (1.62–16.16) | 0.005 |
| Ranching | 129 | 10.1 % (5.5 %–16.6 %) | Reference | |
| Perimeter wall | ||||
| No | 1318 | 67.8 % (65.2 %–70.3 %) | 3.42 (1.02–11.49) | 0.047 |
| Yes | 103 | 10.7 % (5.5 %–18.3 %) | Reference | |
| Interaction | ||||
| No | 103 | 10.7 % (5.5 %–18.3 %) | Reference | |
| Yes | 1318 | 67.8 % (65.2 %–70.3 %) | 3.42 (1.02–11.49) | 0.047 |
| Water source | ||||
| Communal source | 1304 | 68.4 % (65.8 %–70.9 %) | 3.37 (1.01–11.32) | 0.049 |
| Ranch source | 117 | 11.1 % (6.1 %–18.2 %) | Reference | |
| Confined separately from other species | ||||
| Yes | 1401 | 63.5 % (60.9 %–66.1 %) | Reference | |
| No | 20 | 75.0% (50.9 %–91.3 %) | 1.84 (0.63–5.42) | 0.267 |
| Age in years | ||||
| <1 year | 157 | 38.2 % (30.6 %–46.3 %) | Reference | |
| 1−2 years | 223 | 48.0% (41.2 %–54.7 %) | 1.56 (0.99–2.45) | 0.053 |
| 2−3 years | 156 | 60.9 % (52.8 %–68.6 %) | 3.32 (1.94–5.69) | <0.001 |
| >3 years | 885 | 72.7 % (69.6 %–75.6 %) | 6.87 (4.57–10.34) | <0.001 |
| Sex | ||||
| Female | 1129 | 66.6 % (63.8 %–69.4 %) | 2.76 (2.05–3.70) | <0.001 |
| Male | 292 | 52.4 % (46.5 %–58.2 %) | Reference | |
P = Prevalence,¥ =Mean, cOR = Crude Odds Ratio, CI = Confidence Interval, P-Value = Probability Value.
3.2. Factors associated with MERS – CoV seropositivity amongst camels
Association between MERS-CoV seropositivity and each independent risk factors (herd size, production systems, fencing, interaction with wildlife, water source, and separation from other species, age and sex of the animals) were tested accounting for variation between county, sub-county and each individual camel. P-values less than 0.05 (P ≤ 0.05) were considered as significant association as shown in Table 2.
Majority 69.0 %, (95 %CI; 66.5 %–71.6 %) of the camels whose production system was pastoralism were seropositive for MERS-CoV compared to those in the ranching production system 10.1 %, (95 %CI; 5.5 %–16.6 %). Camels whose production system was pastoralism were 5.11[95 %CI = 1.62–16.16, P = 0.005] times likely to be seropositive for MERS-CoV. Higher proportions of seropositive for MERS-CoV were observed among camels not confined within a perimeter wall 67.8 %, (95 %CI; 65.2 %–70.3 %), that interacted with wild/other domestic animals 67.8 %, (95 %CI; 65.2 %–70.3 %) and those whose water source was communal 68.4 % (95 %CI; 65.8 %–70.9 %).
Camels aged 2–3 years 60.9 % (95 %CI: 52.8 %–68.6 %) and above 3 years 72.7 % (95 %CI: 69.6 %–75.6 %) were 3.32[95 %CI = 1.94–5.69, P < 0.001] and 6.87[95 %CI = 4.57–10.34, P < 0.001] respectively, times more likely to be seropositive for MERS-CoV compared to camels aged less than one year, 38.2 % (95 %CI: 30.6 %–46.3 %). Female camels 66.6 % (95 %CI: 63.8 %–69.4 %) were 2.76[95 %CI = 2.05–3.70, P < 0.001] times likely to be seropositive for MERS-CoV compared to male camels, 52.4 % (95 %CI: 46.5 %–58.2 %).
Highly significant associations were found between camel production system and confinement within a perimeter wall, camel production system and interaction with wildlife, camel production system and water source for the camels. Consequently, in order to reduce multi-collinearity, only camel production system, sex of the camel and camel’s age in years were included in the multivariable regression model.
Multilevel logistic regression was conducted using generalized linear mixed model to determine a reduced model of factors associated with MERS-CoV seropositivity treating herd size, camel production system, camel’s age in years and sex of the camels as fixed effects whereas county, sub-county and individual camel were treated as random effects. Four factors were retained as possible predictors at 5% level of significance as shown in Table 3 .
Table 3.
Multivariable mixed effects logistic regression of factors associated with MERS-CoV Seropositivity.
| Reduced Model | ||
|---|---|---|
| Indicators | aOR (95 %CI) | P-Value |
| Herd Size | 1.0014 (1.00023−1.00271) | 0.021 |
| Production system | ||
| Pastoralist | 5.40 (1.67–17.49) | 0.005 |
| Ranching | Reference | |
| Age in years | ||
| <1 year | Reference | |
| 1−2 years | 1.64 (1.04–2.59) | 0.033 |
| 2−3 years | 3.27 (1.91–5.61) | <0.001 |
| >3 years | 6.12 (4.04–9.30) | <0.001 |
| Sex | ||
| Female | 1.75 (1.27–2.41) | 0.001 |
| Male | Reference | |
aOR = Adjusted Odds Ratio, CI = Confidence Interval, P-Value = Probability value.
Adjusting for camel production system, age and sex, a unit increase of the herd size would increase the likelihood of seropositive for MERS-CoV by 0.14 % [95 %CI = 0.02 % – 0.27 %, P = 0.021]. Adjusting for herd size, age and sex, camels whose production system was pastoralism were 5.40 times likely to be seropositive for MERS-CoV compared to camels in a ranching production system 5.40[95 %CI = 1.41–14.03, P = 0.011].
Similarly, adjusting for herd size, camel production system and sex, camels aged 1–2 years, 2–3 years and more than 3 years were 1.64, 3.29 and 6.13 respectively, times more likely to be seropositive for MERS-CoV compared to camels aged less than 1 year, 1.64 [95 %CI = 1.04–2.59, P = 0.033], 3.27[95 %CI = 1.91–5.61, P < 0.001], 6.12[95 %CI = 4.04–9.30, P < 0.001]. Adjusting for herd size, camel production system and age, female camels were 1.75 times more likely to be seropositive for MERS-CoV compared to male camels, 1.75[95 %CI = 1.27–2.41, P = 0.001].
4. Discussion
In this study, we assessed camels from various production systems for MERS-CoV IgG antibodies and identified factors associated with MERS-CoV seropositivity. The high rate of seropositivity by ELISA observed in this study confirms previous exposure of camel populations in Kenya to virus. Because the antibody ELISA used detects only IgGs, we can conjecture that camel exposure to MERS-CoV did not occur recently; IgG antibodies are thought to be long lived but exact antibody profiles are not known.
This study reported higher (69.0 %) seropositivity rates in pastoral production systems when compared to ranching system (9.9 %). This is consistent with observations in previous studies in the country, an indicator that freedom of movement is associated with higher seropositivity to MERS CoV antibodies in camel populations. These results tally observations in a study in the Kingdom of Saudi Arabia that recorded lower rates for MERS-CoV seropositivity (12.1 %) in dromedary camels in slaughterhouses, farms and wholesale markets (Sabir et al., 2016) compared to MERS-CoV seropositivity rates in camel farms (100 %) (Paden et al., 2018).
Various countries have also reported high percentage of MERS-CoV antibodies in dromedary camels. In farms across Israel, MERS-CoV seropositivity rates ranging from 61.8%–71.8 % have been documented (David et al., 2018)(Harcourt et al., 2018). For instance, results from Egypt indicated a seropositivity of between 79.1 % and 84.5 % in the pastoral camel production systems (Ali et al., 2017) while those from Ethiopia confirmed a seropositivity of 93 % from adult dromedaries and 97 % in juvenile dromedaries from pastoral production system (Reusken et al., 2013). In Somalia, MERS seropositivity was 87.5 % while in Sudan, a seropositivity of 81.7 % was reported (Corman et al., 2014). Previous MERS-CoV seroprevalence studies on retrospective samples from 1992, in Kenyan dromedary camels populations have established the presence of MERS-CoV antibodies (Corman et al., 2014). In these earlier analyses, Marsabit County presented with a seropositivity rate of 90 % (Munyua et al., 2017) while the lowest value of 46.9 % was recorded in Laikipia County with ranching and pastoral camel production systems (Deem et al., 2015). Dromedary camels have been implicated in the emergence and transmission of MERS-CoV to humans as a result of isolation of near–identical MERS-CoV strains from human and dromedary camels in the same epidemiological location (Omrani et al., 2015). In another case, a covert MERS-CoV infection was detected in two men in a screening centre located at the Oman and United Arab Emirates border who had contact with an infected herd of camels and tested positive for MERS by RT-PCR (Al Hammadi et al., 2015). Presence of MERS-CoV RNA had also been confirmed in a 43-year-old male patient admitted at the King Abdulaziz University Hospital in Jeddah who have had history of contact with infected camels (Memish et al., 2014).
Specifically, we evaluated eight plausible risk factors for association with MERS-CoV IgG; three were determined to be significant predictors for camel MERS-CoV seropositivity (Production system, Camel age and Sex) after adjusting for all the risk factors. The findings in this study increase our understanding of the predictors of MERS-CoV and plausible dynamics of MERS CoV infection in the Kenyan camel populations. This information can be used to target early risk-based surveillance and devise response strategies against circulating MERS-CoV strains. These identified predictors were identified earlier as risk factors for MERS-CoV exposure in Burkina Faso, Ethiopia and Morocco (Miguel et al., 2017).
Isiolo, Turkana and Marsabit Counties have higher seropositivities for MERS CoV IgG in this study. This finding is unsurprising for a number of reasons: 1) the three counties share contiguous borders; 2) the counties have similar agro ecologies and are characterized by large camel herds under the pastoral production system, and 3) two of these counties border neighbouring countries and are characterized by seasonal movement over vast ranges of land in search of pasture and water and for trade. Seasonal movement across county lines and national borders in search of water and pasture is an important factor in MERS-CoV epidemiology, because of the aggregation of camel herds from different regions at common watering points during the dry season (Gikonyo et al., 2018). The high camel density at the pasture and watering points and in the markets results in closer interaction between these camel herds, creating an opportunity for MERS-CoV infections and spill over among herds from the three counties and neighbouring countries and this epidemiological feature has previously demonstrated with other camel diseases (Wasonga et al., 2016). The effect of seasonal migration in MERS-CoV transmission can further be exacerbated by inter-clan feuds and insecurity.
The higher rates of MERS-CoV seropositivity in camels in our study contradict the findings of studies in Burkina Faso, Ethiopia and Morocco, where production system was not a significant determinant of MERS-CoV seropositivity. Our findings also contradict results from a study conducted in Saudi Arabia, where high rates of seropositivity were observed in camel dense zero-grazed units and semi open grazing system (Elfadil et al., 2018). Studies to elucidate the dynamics of camel production systems and their influence on the rate of infection are warranted.
In this study, camels aged above 3 years were 6.87 times more likely to be MERS-CoV seropositive compared to juvenile camels. This study findings are similar to those from studies conducted in Saudi Arabia (Elfadil et al., 2018) (Alagaili et al., 2014). The waning of passive immunity in animals aged 6 months and older may produce a state of immunological naivety, resulting a cycle of infection and re-infection, possibly explaining the high seroprevalence rate observed among adult camels. Females were 2.76 times more likely to be seropositive compared to males, and this was similar to previous findings (Deem et al., 2015) (Munyua et al., 2017). The pastoral community in Kenya keeps camels mainly for milk production because female camels are known to withstand the harsh climatic conditions (Onono et al., 2010). This may explain the high rate of seropositivity among female camels due to the longevity of females in the herd.
We utilised a cross-sectional study design, which was not ideal for investigation of risk factors associated with MERS-CoV seropositivity. In addition, we measured permanent exposures (sex, production system, and other factors related to production system), missing out the risk of reverse-association. A cross-sectional study design does not permit investigators to determine when a seropositive camel first entered the herd. This may contribute to misclassification of the factors associated with seropositivity. However, since the study aimed to identify associations and not cause-effect relationships, we deemed that the design was suitable determine camel seropositive status, and describe possible factors associated with MERS-CoV infection in this region.
5. Conclusions and recommendations
Despite the study limitations, we were able to demonstrate seropositivity of MERS-CoV IgG antibodies in various camel production systems, with highest seropositivity reported in pastoral production systems. The study also identified independent factors associated with MERS seropositivity to include county of production, production system, camel age and sex. This study identified and described potential factors associated with MERS-CoV seropositivity in the country from a larger geographical range, examining defined production and management systems. The association of these factors, in addition to camel characteristics like age and sex, with MERS-CoV seropositivity has been explored. Knowledge of risk factors associated with MERS-CoV seropositivity is key in advising on appropriate management of the emerging zoonotic infection that may represent a global public health threat.
The strain of MERS-CoV detected in Isiolo County in Kenya clustered within the proposed Clade C African MERS-CoV strains in phylogenetic analysis (Kiambi et al., 2018). More research is needed to understand if these strains have zoonotic potential. Zoonotic transmission of MERS-CoV from camels to humans in Kenya cannot be ruled out. Studies are currently being undertaken to determine if there has been any infection of humans who have had close contact with camels. We therefore recommend advanced genetic characterization of any MERS-CoV strain isolated from camels or other livestock species in Kenya and investigation of seropositivity in humans who are in frequent contact with camels.
Availability of data
The datasets used and/or analysed during the current study are available on request.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was funded by the United States Agency for International Development (USAID) through the MERS-CoV applied research activities in Middle East and North East Africa under the USAID’s Emerging Pandemic Threats Program. We thank the staff of the DVS, State Department of Livestock, Ministry of Agriculture, Livestock and Fisheries. We acknowledge the contributions of the relevant Directors of Veterinary Services, Directorates of Veterinary Services, Kenya. We thank the numerous participants and camel owners. The views and opinions expressed in this paper are those of the authors and are not necessarily the views of USAID or FAO.
Footnotes
Risk Factors for MERS-CoV seropositivity in Kenya, a cross-sectional study of Nakuru, Marsabit, Isiolo, Turkana and Laikipia Counties in 2017.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prevetmed.2020.105197.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abdullah Assiri, McGeer Allison, Perl Trish M., Price Connie S., Al Rabeeah Abdullah A., Cummings Derek A.T. 基因的改变NIH public access. Bone. 2014;23:1–7. doi: 10.1038/jid.2014.371. [DOI] [Google Scholar]
- Al Hammadi Z.M., Chu D.K.W., Eltahir Y.M., Al Hosani F., Al Mulla M., Tarnini W., Hall A.J., Perera R.A.P.M., Abdelkhalek M.M., Peiris J.S.M., Al Muhairi S.S., Poon L.L.M. Asymptomatic MERS-CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, May 2015. Emerg. Infect. Dis. 2015;21:2197–2200. doi: 10.3201/eid2112.151132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., El-Shesheny R., Kandeil A., Shehata M., Elsokary B., Gomaa M., Hassan N., El Sayed A., El-Taweel A., Sobhy H., Fasina F.O.3, Dauphin G., Masry El, Wolde A.W., Daszak P., Miller M., VonDobschuetz S., Morzaria S., Lubroth J., M.Y Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Eurosurveillance Mar. 2017;16(22):1560–7917. doi: 10.2807/1560-7917.ES.2017.22.11.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y., Gluecks I., Lattwein E., Bosch B., Drexler J.F., Bornstein S., Drosten C., Müller M.A. Antibodies against MERS Coronavirus in Dromedary Camels, Kenya 1992-2013. Emerg. Infect. Dis. Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Rotenberg D., Khinich E., Erster O., Bardenstein S., van Straten M., Okba N.M.A., Raj S.V., Haagmans B.L., Miculitzki M., Davidson I. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Heal. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem S.L., Fèvre E.M., Kinnaird M., Browne A.S., Muloi D., Godeke G.J., Koopmans M., Reusken C.B. Serological evidence of MERS-CoV antibodies in dromedary camels (camelus dromedaries) in laikipia county, Kenya. PLoS One. 2015;10:11–15. doi: 10.1371/journal.pone.0140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnah A.A., Al-amri S.S., Hassan A.M., Almasoud A.S., Mousa M., Almahboub S.A., Alhabbab R.Y., Mirza A.A., Hindawi S.I., Alharbi N.K., Azhar E.I., Hashem A.M. Seroprevalence of MERS-CoV in healthy adults in western Saudi Arabia, 2011–2016. J. Infect. Public Health. 2020;13:697–703. doi: 10.1016/j.jiph.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Müller M.A., Corman V.M., Al-Masri M., Hossain R., Madani H., Sieberg A., Bosch B.J., Lattwein E., Alhakeem R.F., Assiri A.M., Hajomar W., Albarrak A.M., Al-Tawfiq J.A., Zumla A.I., Memish Z.A. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2018. FAOSTAT, Live Animals data, 2017 [WWW Document] URL http://www.fao.org/faostat/en/#data/QA (Accessed 23 August 2019) [Google Scholar]
- Funk A.L., Goutard F.L., Miguel E., Bourgarel M., Chevalier V., Faye B., Malik Peiris J.S., van Kerkhove M.D., Roger F.L. MERS-CoV at the animal-human interface: inputs on exposure pathways from an expert-opinion elicitation. Front. Vet. Sci. 2016;3:1–12. doi: 10.3389/fvets.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J.L., Rudoler N., Tamin A., Leshem E., Rasis M., Giladi M., Haynes L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health. 2018;65:749–754. doi: 10.1111/zph.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiolo County Government . 2018. Isiolo County Annual Development Plan 2018/19 [WWW Document] URL https://isiolo.go.ke/wp-content/uploads/2018/12/ANNUAL-DEVELOPMENT-PLAN-2018-19.pdf (Accessed 23 August 2019) [Google Scholar]
- Kelly-Cirino C., Mazzola L.T., Chua A., Oxenford C.J., Van Kerkhove M.D. An updated roadmap for MERS-CoV research and product development: focus on diagnostics. BMJ Glob. Heal. 2019;4:e001105. doi: 10.1136/bmjgh-2018-001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiambi S., Corman V.M., Sitawa R., Githinji J., Ngoci J., Ozomata A.S., Gardner E., von Dobschuetz S., Morzaria S., Kimutai J., Schroeder S., Njagi O., Simpkin P., Rugalema G., Tadesse Z., Lubroth J., Makonnen Y., Drosten C., Müller M.A., Fasina F.O. Detection of distinct MERS-Coronavirus strains in dromedary camels from Kenya, 2017. Emerg. Microbes Infect. 2018;7 doi: 10.1038/s41426-018-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyong’a A.N., Cook E.A.J., Okba N.M.A., Kivali V., Reusken C., Haagmans B.L., Fèvre E.M. Middle East respiratory syndrome coronavirus (MERS-CoV) seropositive camel handlers in Kenya. Viruses. 2020;12:10–16. doi: 10.3390/v12040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laikipia County Government . 2018. County Integrated Development Plan 2013-1018 [WWW Document]. Cty. Integr. Dev. Plan 2013-1018. URL https://roggkenya.org/wp-content/uploads/Laikipia_CIDP_2018-2022_County-Integrated-Development-Plan.pdf (Accessed 23 August 2019) [Google Scholar]
- Marsabit County Government . 2018. Marsabit County Integrated Development Plan 2018-2022 [WWW Document] URL www.google.com/search?q=marsabit+county+integrated+plan (Accessed 23 August 2019) [Google Scholar]
- Memish Z.A., Cotten M., Meyer B., Watson S.J., Alsahafi A.J., Al Rabeeah A.A., Corman V.M., Sieberg A., Makhdoom H.Q., Assiri A., Al Masri M., Aldabbagh S., Bosch B.J., Beer M., Müller M.A., Kellam P., Drosten C. Human Infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 2014;20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E., Chevalier V., Ayelet G., Ben Bencheikh M.N., Boussini H., Chu D.K., El Berbri I., Fassi-Fihri O., Faye B., Fekadu G., Grosbois V., Ng B.C., Perera R.A., So T., Traore A., Roger F., Peiris M. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Eurosurveillance. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir Susanna Lau. Virol. J. 2016;13:1–7. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Bosch B.J., Lattwein E., Hilali M., Musa B.E., Bornstein S., Drosten C. Mers coronavirus neutralizing antibodies in camels, eastern Africa, 1983–1997. Emerg. Infect. Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyua P., Corman V.M., Bitek A., Osoro E., Meyer B., Müller M.A., Lattwein E., Thumbi S.M., Murithi R., Widdowson M.A., Drosten C., Njenga M.K. No serologic evidence of middle east respiratory syndrome coronavirus infection among camel farmers exposed to highly seropositive camel herds: a household linked study, Kenya, 2013. Am. J. Trop. Med. Hyg. 2017;96:1318–1324. doi: 10.4269/ajtmh.16-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakuru County Government . 2018. County Government of Nakuru Nakuru County Integrated Development Plan [WWW Document] URL www.nakuru.go.ke. (Accessed 23 August 2019) [Google Scholar]
- Ommeh S., Zhang W., Zohaib A., Chen J., Zhang H., Hu B., Ge X.Y., Yang X.Lou, Masika M., Obanda V., Luo Y., Li S., Waruhiu C., Li B., Zhu Y., Ouma D., Odendo V., Wang L.F., Anderson D.E., Lichoti J., Mungube E., Gakuya F., Zhou P., Ngeiywa K.J., Yan B., Agwanda B., Shi Z.L. Genetic Evidence of Middle East Respiratory Syndrome Coronavirus (MERS-Cov) and Widespread Seroprevalence among Camels in Kenya. Virol. Sin. 2018;33:484–492. doi: 10.1007/s12250-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle east respiratory syndrome coronavirus (Mers-coV): animal to human interaction. Pathog. Glob. Health. 2015;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C.R., Yusof M.F.B.M., Al Hammadi Z.M., Queen K., Tao Y., Eltahir Y.M., Elsayed E.A., Marzoug B.A., Bensalah O.K.A., Khalafalla A.I., Al Mulla M., Khudhair A., Elkheir K.A., Issa Z.B., Pradeep K., Elsaleh F.N., Imambaccus H., Sasse J., Weber S., Shi M., Zhang J., Li Y., Pham H., Kim L., Hall A.J., Gerber S.I., Al Hosani F.I., Tong S., Al Muhairi S.S.M. Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Public Health. 2018;65:322–333. doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.F., Smits S.L., El Tahir Y.E., De Sousa R., van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.J., Rottier P., Osterhaus A., Gort K.M. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir J.S.M., Lam T.T.Y., Ahmed M.M.M., Li L., Shen Y., Abo-Aba S.E.M., Qureshi M.I., Abu-Zeid M., Zhang Y., Khiyami M.A., Alharbi N.S., Hajrah N.H., Sabir M.J., Mutwakil M.H.Z., Kabli S.A., Alsulaimany F.A.S., Obaid A.Y., Zhou B., Smith D.K., Holmes E.C., Zhu H., Guan Y. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science (80-.) 2016;351:81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Turkana County Government . 2013. Turkana County Integrated Development Plan, 2013/14-2017/18 [WWW Document]. Null.https://turkana.go.ke/wp-content/uploads/2016/10/Turkana-CIDP-Final-1.pdf URL. [Google Scholar]
- World Health Organization . World Health Organization; 2020. WHO MERS Global Summary and Assessment of Risk. [WWW Document] URLhttps://www.who.int/csr/don/24-february-2020-mers-saudi-arabia/en/(Accessed 22 August 2019) [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available on request.