Abstract
Osteonecrosis of the femoral head (ONFH) is a debilitating disease that can cause deformity and collapse of the femoral head, thus leading to the development of degenerative joint disease that can incapacitate the patient with pain and reduction in hip mobility. This study aims to determine the safety and efficacy of tantalum rod insertion in the treatment of ONFH with a minimum follow-up period of 1 year. A multi-database search was performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Data from studies assessing the clinical and radiological outcomes as well as complications of tantalum rod insertion in the treatment of ONFH with a minimum follow-up period of 1 year were extracted and analyzed. Ten studies were included in this meta-analysis, consisting of 550 hips. There was a statistically significant increase in HHS (MD = 30.35, 95% CI: 20.60–40.10, P < 0.001) at final follow-up versus pre-operative scores. The weighted pooled proportion (PP) of radiographic progression of ONFH was 0.221 (95% CI: 0.148–0.316), while that of progression into femoral head collapse was 0.102 (95% CI: 0.062–0.162). Conversion to total hip arthroplasty (THA) had a PP of 0.158 (95% CI: 0.107–0.227) with a mean weighted period of 32.4 months (95% CI: 24.9–39.9 months). Subgroup analysis of conversion to THA when tantalum rods were used in conjunction with bone grafting (PP = 0.150, 95% CI: 0.092–0.235) showed a marginal risk reduction than when compared with subgroup analysis of tantalum rods being used alone (PP = 0.154, 95% CI: 0.078–0.282). Tantalum rod is a safe alternative option to the current joint-preserving procedures available in the treatment of ONFH. However, more studies are needed to investigate and identify the most appropriate patients who would benefit most and the synergistic effect brought on by the use of complementary biological augmentation of bone grafting or stem cells with tantalum rods.
INTRODUCTION
The femoral head is a pre-dilected site at risk for the development of osteonecrosis [1]. It can occur when blood supply to the femoral head is disrupted, resulting in infarction and avascular necrosis. If left untreated, most cases will continue to progress leading to deformity and collapse of the femoral head and the development of degenerative joint disease that can incapacitate the patient with pain and reduction in hip mobility [1, 2].
While early diagnosis of osteonecrosis of the femoral head (ONFH) has been facilitated with more advanced radiological diagnostic studies such as bone scans and magnetic resonance imaging, there is little consensus on the gold standard of treatment for ONFH, especially in the early stages of the disease [1]. In terms of surgical management, several joint-preserving treatment methods have been described, including core decompression, vascularized or non-vascularized bone grafting, osteotomies and more recently tantalum rod implantation [3]. Total hip arthroplasty (THA) is often reserved for severe cases of ONFH with collapse or where joint preservation procedures have failed. In younger patients, joint preservation surgery is a priority due to the inherent risk of THA in young patient requiring revision surgery in the future [1, 4].
Tantalum rod insertion involves the technique of core decompression when drilling into the femoral head before implantation, as well as providing a porous osteoconductive and osteoinductive scaffold structure for new ingrowth of osteoblasts and progenitor cells, promoting bony ingrowth and faster return to full weight bearing [3, 5, 6]. Pedersen et al. [7] first described Tantulum rod insertion as a treatment option and hypothesized that the porous rod was a reasonable mechanical substitute for grafting and hence eliminating the morbidity associated with bone graft harvesting. The tantalum rod also has good biocompatibility and a modulus of elasticity similar to cortical bone, providing strong biomechanical support to the subchondral plate [8, 9]. Furthermore, common pathogenic bacteria such as staphylococcus aureus or epidermidis have been found to adhere less to tantalum than other commonly used implant materials [10]. Despite these advantages, its clinical efficacy remains unclear, especially in the longer term.
This meta-analysis and systematic review aims to determine the clinical and radiographic outcomes as well as complication profile of tantalum rods in treating ONFH in the adult population with a minimum follow-up of 1 year.
MATERIALS AND METHODS
Literature search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria [11]. A comprehensive search was conducted across multiple databases (PubMed, OVID Medline, EMBASE) from the date of database inception to 16 November 2019. The Medical Subject Heading and Boolean operator terms utilized for the search were: [(‘Tantalum’ OR ‘Rod’ OR ‘Screw’ OR ‘Implant’) AND (‘Femoral avascular necrosis’ OR ‘Femoral osteonecrosis’)]. Identified articles and their corresponding references were reviewed according to the selection criteria for consideration of inclusion.
Selection criteria
All articles of any study design directly studying the clinical outcomes, radiographic progression or complication profile of tantalum rod in the treatment of ONFH were considered for inclusion. Studies were then scrutinized to only include those with a minimum follow-up period of 1 year. Non-English language studies, non-peer-reviewed studies, conference proceedings and abstracts were excluded. Two independent authors reviewed records retrieved from the initial search twice and excluded irrelevant ones. Titles and abstracts of remaining articles were then screened against the inclusion criteria. Included articles were critically reviewed according to a pre-defined data extraction form. Any differences in opinions regarding the inclusion of specific articles were resolved by discussion among all authors.
Data extraction
Extracted data parameters included details on study designs, publication year, patient numbers, basic demographics, functional and radiological outcomes as well as complication profile. Functional outcomes that were extracted included the Harris Hip score (HHS) [12]. Radiological outcomes include the presence of radiographic progression of the condition in terms of the Association Research Circulation Osseous (ARCO) [13], Steinberg et al. [14] or University of Pennsylvania (UPenn) [15] classification staging system. Complications evaluated encompassed the prevalence of conversion to THA, mean time for conversion to THA and persistent hip pain. Other surgical complications analyzed included cut out, femoral fractures, infection and venous thromboembolism. Data were extracted by two authors, then compiled and consolidated via Microsoft Excel Spreadsheet (MS Excel 2008, Version 12.3.1) prior to performing statistical analysis.
Methodology assessment
Methodology quality of included studies was assessed with the Methodological Index for Non-Randomized Studies (MINORS) [16]. For non-comparative studies, MINORS [16] score by two authors. The MINORS [16] score consists of eight criteria to assess each study and each criterion was scored with a 3-points system from 0 to 2. (2: adequately reported, 1: inadequately reported or 0: not reported). An ideal observational study should score 16 points. Any differences in scores would be discussed between both authors and further disagreements will be brought up to other authors to reach a consensus.
Statistical analysis
Meta-analysis was performed based on weighted PPs and weighted mean differences. Random-effects models were used, with the assumption that there were variations amongst studies. Chi squared tests were used to study heterogeneity between trials. I2 statistic was employed to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance. An I2 value greater than 50% was deemed to be of substantial heterogeneity. I2 is calculated as: I2 = 100% × (Q − df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. Specific analyses considering confounding factors were not possible because raw data were not always available. Subgroup analysis was performed on certain parameters where needed. All P-values were two-sided, with P values <0.05 indicating statistical significance. R studio 3.4 and Review Manager (version 5.3, Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) were used for statistical analysis.
RESULTS
Literature search
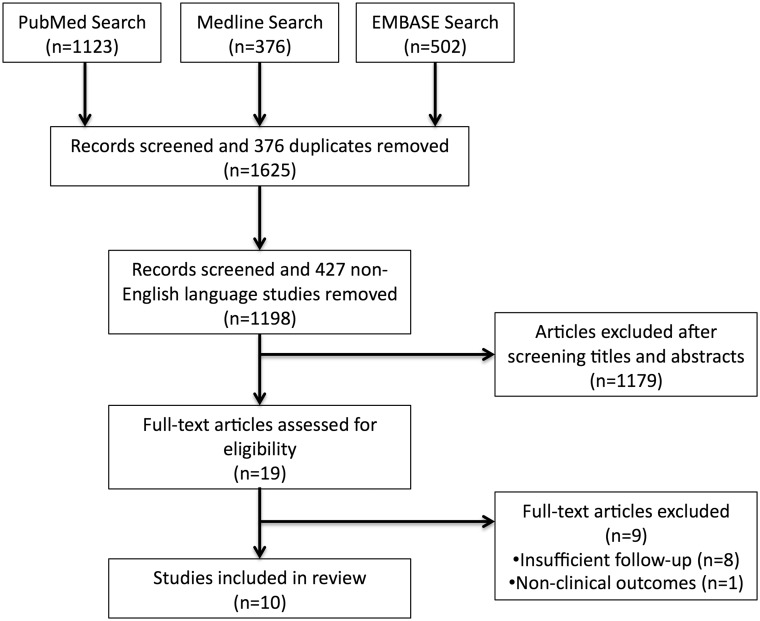
A selection process flowchart to identify included studies is illustrated in Fig. 1. No additional studies were identified from citation search and 19 full-text articles were assessed for eligibility. A total 10 studies [17–26] were included, consisting of nine prospective [17–22, 24–26] and one retrospective [23] study.
Fig. 1.
PRISMA search flowchart.
Demographics
A total of 550 hips were included in this study. Six studies (n = 315) reported pre-operative staging with the Steinberg classification, consisting of 86 Stage I, 172 Stage II, 47 Stage III and 10 Stage IV hips [18–20, 22, 23, 25]. Varitimidis et al. [25] had one death for reasons unrelated to the procedure. The hip was included in basic demographics but was not included in the Steinberg classification. Three studies (n = 210) used the ARCO classification consisting of 4 Stage I, 100 Stage II, 94 Stage III and 12 Stage IV hips [17, 21, 26]. Shuler et al. [24] used the UPenn classification criteria and had 2 Stage I and 22 Stage II hips. Steroid-related avascular necrosis is the most common etiology amongst the patient cohort. Zimmer (NJ, USA) tantalum rod was used in 493 hips, while Runze (Chongqing, China) tantalum rod was used in 21 hips. One study did not specify the tantalum rod that was used in their patient cohort [22]. Four studies included bone grafting during the implantation of the tantalum rod [18, 21, 23, 26]. There was a weighted mean follow-up period of 3.1 years, with minimum follow-up of 1 year [19, 21] and maximum of 6.5 years [26]. Further details are found in Table I.
Table I.
Basic characteristics and demographics
| Articles | Year | Study design | No of hips |
No of patients |
Prosthesis type | Mean age (year) | Steinberg staging (hips) |
ARCO staging (hips) |
Etiology |
Minimum follow-up (years) | Mean follow-up (range) (years) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | I | II | III | IV | I | II | III | IV | Idiopathic | Steroid | alcohol | Others | |||||||
| Huang et al. [17] | 2019 | Randomized prospective | — | — | 41 | 29 | 10 | 39 | Runze, Zimmer | 42.5 (26–63) | — | — | — | — | 4 | 37 | 0 | 0 | 5 | 14 | 20 | 0 | 3 | 3.23 (3–3.58) |
| Liu et al. [18] | 2013 | Prospective cohort | — | — | 94 | 55 | 14 | 69 | Zimmer | 37.3 (19–57) | 27 | 49 | 18 | 0 | — | — | — | — | 18 | 39 | 37 | 0 | 1.5 | 2.95 (1.5–5) |
| Liu et al. [19] | 2010 | Prospective cohort | — | — | 49 | 23 | 12 | 35 | Zimmer | 38.2 (22–50) | 21 | 26 | 2 | 0 | — | — | — | — | 25 | 14 | 7 | 3 | 1 | 1.27 (1–3) |
| Liu et al. [20] | 2016 | Prospective cohort | — | — | 52 | 37 | 5 | 42 | Zimmer | 40.7 (20–63) | 22 | 30 | 0 | 0 | — | — | — | — | 10 | 19 | 23 | 14 | 3.16 | 4 (3.16–5.16) |
| Liu et al. [21] | 2014 | Prospective cohort | 71 | 67 | 138 | — | — | 130 | Zimmer | 33.4 (20–48) | — | — | — | — | 0 | 63 | 75 | 0 | 24 | 75 | 39 | 0 | 2.42 | 3.21 (2.42–4.17) |
| Miao et al. [22] | 2015 | Randomized prospective | — | — | 36 | 12 | 18 | 30 | — | 32.6 (20–45) | 16 | 20 | 0 | 0 | — | — | — | — | 7 | 22 | 4 | 0 | 1 | 2.13 (1–2.33) |
| Pakos et al. [23] | 2015 | Retrospective cohort | — | — | 58 | 28 | 21 | 49 | Zimmer | 38 a (22–55) | 0 | 38 | 20 | 0 | — | — | — | — | 22 | 19 | 2 | 6 | 5 | — |
| Shuler et al. [24] | 2007 | Prospective cohort | 20 | 4 | 24 | 20 | 4 | 24 | Zimmer | 43.2 (28–60) | — | — | — | — | — | — | — | — | 8 | 6 | 10 | 0 | 2.25 | 3.25 (2.25–4.92) |
| Varitimidis et al. [25] | 2009 | Prospective cohort | 23 | 4 | 27a | 23 | 4 | 27 | Zimmer | 36 a (15–55) | 0 | 9 | 7 | 10 | — | — | — | — | 8 | 15 | 2 | 1 | 1.25 | 3.13 (1.25–5.92) |
| Zhao et al. [26] | 2015 | Randomized prospective | — | — | 31 | 13 | 11 | 24 | Zimmer | 33.2 (23–45) | — | — | — | — | 0 | 0 | 19 | 12 | 4 | 14 | 4 | 2 | 2.17 | 5.36 (2.17–6.5) |
—, not specified.
One patient died and was not further analyzed.
Methodology assessment
The MINORS [16] score assessment for every included study is shown in Table II. The mean MINORS16] score among studies was 12.9, with a lowest of 10 and highest of 16. There was an inter-rater reliability of 80%.
Table II.
Methodological index for non-randomized studies (MINORS) score
| Articles | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate study aims | Unbiased assessment of endpoints | Follow-up period appropriate | Loss to follow-up <5% | Adequate statistical analyses | Total |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al. [17] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Liu et al. [18] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 12 |
| Liu et al. [19] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 13 |
| Liu et al. [20] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 15 |
| Liu et al. [21] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 12 |
| Miao et al. [22] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 13 |
| Pakos et al. [23] | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 10 |
| Shuler et al. [24] | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Varitimidis et al. [25] | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 14 |
| Zhao et al. [26] | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 1 | 11 |
2, adequately reported; 1, inadequately reported; 0, not reported.
Clinical and radiological outcomes
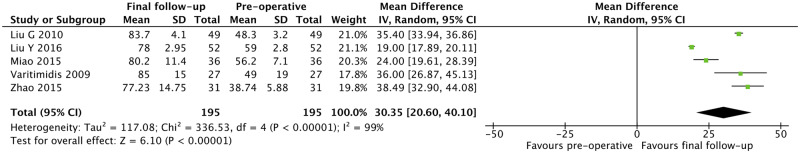
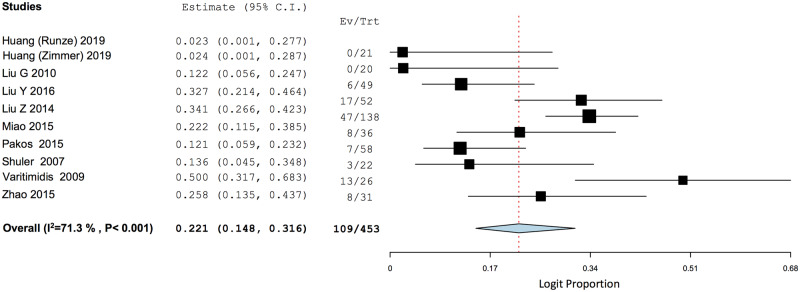
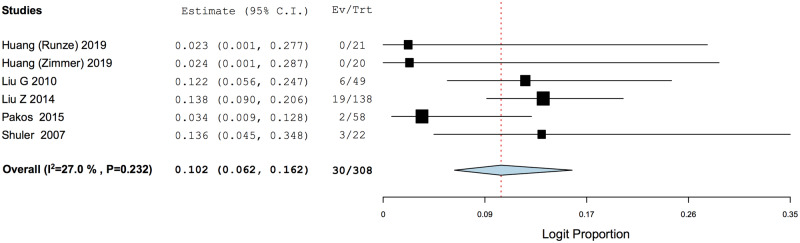
There was a statistically significant increase in HHS when comparing between final follow-up and pre-operative scores (MD = 30.35, 95% CI: 20.60–40.10, P < 0.001) (Fig. 2). The PP of radiographic progression of ONFH was 0.221 (95% CI: 0.148–0.316), while that of progression into femoral head collapse was 0.102 (95% CI: 0.062–0.162) (Figs 3 and 4).
Fig. 2.
Meta-analysis of Harris Hip Score at final follow-up.
Fig. 3.
Weighted pooled proportion of radiographic progression of disease.
Fig. 4.
Weighted pooled proportion of progression into femoral head collapse.
Complications
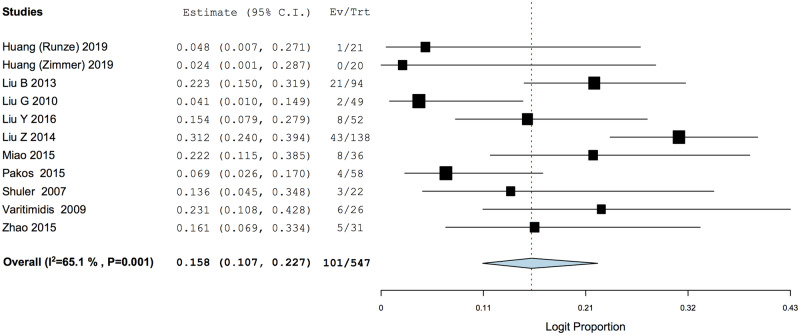
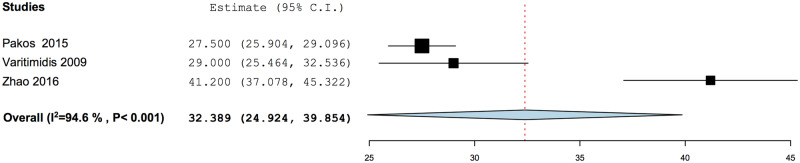
Conversion to THA had a PP of 0.158 (95% CI: 0.107–0.227), with a mean weighted time period of 32.4 months (95% CI: 24.9–39.9 months) after tantalum rod implantation (Figs 5 and 6). Subgroup analysis of conversion to THA when tantalum rod was used in conjunction with bone grafting was marginally lower with a PP of 0.150 (95% CI: 0.092–0.235), while that without concomitant bone grafting had a PP of 0.154 (95% CI: 0.078–0.282) (Fig. 5a and b).
Fig. 5.
Weighted pooled proportion analysis of conversion to THA. (a) Subgroup analysis of conversion to THA with concomitant bone grafting. (b) Subgroup analysis of conversion to THA without bone grafting.
Fig. 6.
Mean time from index procedure to conversion to total hip arthroplasty.
Other complications analyzed include cut out (PP = 0.033, 95% CI: 0.017–0.061), femoral fractures (PP = 0.015, 95% CI: 0.006–0.035), infections (PP = 0.016, 95% CI: 0.007–0.036), persistent hip pain post tantalum rod implantation (PP = 0.132, 95% CI: 0.056–0.278) and venous thromboembolism (PP = 0.014, 95% CI: 0.006–0.035) (Supplementary Appendix 1).
DISCUSSION
The literature on the use of tantalum rods in ONFH is limited and to our knowledge, this is the first review of the efficacy and safety of this technique in the English language literature with a minimum of 1-year follow-up. Our most prominent finding was a significant improvement in clinical function despite a moderate risk of radiographic progression and persistent hip pain and eventual conversion into THA in less than a mean time of 3 years.
The improvement in HHS of 30.4 from pre-operative to final follow-up (mean 3.1 years) is a great improvement. While the minimum clinically important difference (MCID) of HHS is not specifically defined in ONFH cases, Cao et al. [27] adopted an arbitrary value of 10 in his study, while Chahal et al. [28] reported an MCID value of 20 at 12 months for patients with femoroacetabular impingement. Compared with the quoted MCID, the improvement in HHS reported by our study is not only statistically, but also clinically significant. Nadeau et al. [29] compared patients who did not need conversion into THA and those who required conversion into THA after tantalum rod insertion, and found a higher mean age in the conversion group (50.1 versus 36.8 years old, P = 0.04) and a lower mean pre-operative HHS in the conversion group (59.5 versus 44.3 points, P = 0.039). Three patients who had conversion to THA in <12 months had an average pre-operative HHS of 27.6 points. While these results may suggest that a younger age and higher pre-operative HSS are associated with better outcomes, it should be noted that there might be selection bias for older patients to more likely receive a THA conversion than younger patients. Nadeau et al. [29] also did not report if there were a difference in the pre-operative staging between those who required and did not require THA conversion. Furthermore, Nadeau et al. [29] acknowledged that a small sample size of 18 hips may have insufficient statistical power to firmly conclude any associations. Hence, this raises questions regarding patient selection for performing hip preservation surgery in patients with advanced disease.
In 2007, Shuler et al. [24] published the results of an RCT that demonstrated overall better outcome of tantalum rod insertion when compared with vascularized bone grafting. Miao et al. [22], however, more recently concluded that tantalum rod was comparable to core decompression with a minimum follow-up period of 1 year. Further studies that directly compare joint preservation procedures are required to determine the optimal technique.
In comparison to our results of 15.8% conversion to THA, a recent meta-analysis on the efficacy and safety of core decompression found a conversion rate of 34% while that of core decompression with concomitant autologous bone grafting was 18% [30]. Of note, the proportion of patients with advanced disease (ARCO III or Steinberg/UPenn IV and above) was similar between our study and Hua et al. [30] (21.1%, n = 116/550 and 23.6%, n = 575/2441, respectively). Aurégan et al. [31] conducted a systematic review of seven studies to reveal a 24.6% THA conversion after a combination of core decompression and/or tantalum rod insertion, with a mean delay of 14.8 months from time of index surgery and a mean follow-up time of 2.2 years. This rate is higher than that of our results despite a shorter follow-up and we are unsure if core decompression may have a negative influence when used in combination with tantalum rod. We note that ONFH is a rapidly progressive disease and the difference in our conversion rate to THA is likely due to the discrepancy in mean follow-up time (3.1 years in our study, 4.5 years in Hua et al. [30] and 2.2 years in Aurégan et al. [31]). Therefore we cannot say with certainty if tantalum rod insertion is superior to core decompression and bone grafting in delaying conversion to THA, since the follow-up end points are of a different duration.
Liu et al. [18] compared advanced and early stage (ARCO I/II or Steinberg/UPenn I/II/III) ONFH in tantalum rod insertion and concluded that advanced disease patients fared worse than early disease patients. Liu et al. [18] reported a statistically significant lower improvements in HHS (P < 0.05) and survival time to THA conversion (P < 0.05) for the advanced than early stage ONFH patient. This is consistent with Hua et al. [30] who reported a similar trend of results for core decompression treatment of ONFH. Ma et al. [32] also conducted a retrospective cohort study on 104 hips and reported that patients older than 35 years old and pre-operative ARCO Stage III are more likely to require conversion to THA with a 5-year conversion rate of 61.9 and 64%, respectively. This suggests that advanced age and pre-operative stage may be poor prognosis for ONFH treatment with tantalum rod. Since we were unable to stratify our analysis according to the different stages of disease or age groups of patients, the inclusion of advanced disease patients (21.1%, n = 116/550) in our cohort may suggest that the overall PP for conversion to THA and radiographic progression that we are presenting may in fact be higher than if hip joint sparing procedure was performed purely for pre-femoral head collapse patients.
The main aim of joint preserving surgery such as tantalum rod insertions is to delay disease progression and the need for THA [1]. Therefore, radiographic progression and progression to femoral head collapse are important clinical indicators of the efficacy of tantalum rod insertions. Our results report that over a mean of 3.1 years, tantalum rods had led to stable lesions in 77.9% of patients. This result suggests that tantalum rods could be effective in delaying the progression of ONFH. However, it is noted that of the nine studies [17, 19–26] reporting radiographic progression, three studies [17, 21, 26] used the ARCO system, with two [21, 26] employing plain radiography and one [17] using MRI for post-operative imaging. One study [24] used the UPenn classification system with plain radiography for post-operative imaging. Of the five remaining studies [19, 20, 22, 23, 25] employing the Steinberg system, two studies [20, 23] used MRI and three [19, 22, 25] used plain radiography for post-operative imaging. Hence, the majority of patients only received plain radiography (305 versus 151 patients) without subsequent MRI scans for follow-up imaging and determination of radiographic progression. This introduces significant subjectivity and inter-rater reliability issues especially for early stages and subtle progressions of ONFH. While severe cases such as femoral head collapse and subchondral fractures have specific pathognomonic signs on plain radiography, such as flattening of femoral head and crescent sign, respectively, plain radiography only has 41% sensitivity and subtle non-specific signs for early stage disease [33, 34]. On the other hand, the sensitivity and specificity of MRI in diagnosing early ONFH has been reported as 93% (95% CI: 92.0–94.0%) and 91% (95% CI: 89.0–93.0%), respectively [35]. Furthermore, accurate measurement of the size of lesion in early disease may not be possible on plain radiograph alone. Overall, MRI should ideally be the gold standard pre- and post-operative imaging for accurate comparison and monitoring of progression.
Another concern in management is the lack of a standardized classification system for ONFH in the literature, while most classification systems are based on important prognostic factors, including extent of osteonecrotic lesion [36, 37], presence of subchondral fracture [34] and location of lesion [37], the classification systems used in these studies have relatively low intra- and inter-observer reliability. The MRI evaluation using Steinberg and ARCO classification system was reported to have a mean inter-observer reliability of 0.56 (range: 0.24–0.84) and 0.35 (range: 0.06–0.56), respectively [38, 39]. Intra-observer reliability for MRI evaluation using the ARCO system is only 0.44 (0.26–0.56) [38]. This can create difficulties in accurate diagnosis and monitoring of disease progression.
The difference in PP analysis of tantalum with and without bone grafting with THA as outcome measure was only 0.004. This is unlikely to be clinically significant. The authors are unable to explain why bone grafting did not synergistically reduce the risk of conversion to THA in the autologous bone grafting group, as Hua et al. [30] did with core decompression (0.43 versus 0.19). However, Liu et al. [21] performed a prospective cohort study and reported promising results of a statistically significant lower rate of conversion to THA in tantalum rod patients with concomitant bone grafting when compared with those without (13.6% versus 44.3%, P < 0.001, Chi-square test). A previous meta-analysis by Zhang et al. [40] comparing tantalum rod implantation versus vascularized or non-vascularized bone grafting concluded that tantalum rod was less invasive with better HHS improvement and lower radiographic progression than bone grafting. However, Vaishya et al. [41] conducted a prospective study on 53 Ficat II or III hips treated with Sartorius muscle pedicle iliac bone graft and showed excellent results. Vaishya et al. [41] showed a 3.77% rate of progression to osteoarthritis (Ficat IV) with 96.3% of patients having a HHS of more than 70 at a mean follow-up of 4.2 years. With these contradicting results, it will be challenging to draw any concrete conclusion. Hence, the question remains if autologous bone grafting may provide osteostimulation effects as well as augment the osteoinductive and osteoconductive properties of the tantalum rod. It is important to note that these are early results being presented and longer term studies would be required to further investigate if a synergistic benefit truly exist for this combined technique.
Given that 15.8% of tantalum patients had conversion to THA in our analysis, it is important to appreciate the effect of this index operation on the THA procedure. Liu et al. [20] noted that despite meticulous debridement and removal of the tantalum rod during conversion to THA, post-operative radiographs often demonstrated accumulated metallic debris in the periarticular soft tissues. This metallic debris, like those from metal-on-metal bearings, could potentially induce local inflammatory processes and leading to third-body wear [42]. Liu et al. [20] thus recommended the use of ceramic-on-ceramic bearings in order to minimize the risk of osteolysis and improvement survival rates of THA after previous tantalum rod insertion. Long-term studies after THA following initial tantalum rod insertion will be required to make further recommendations on THA technique and prosthesis choice for these patients.
There is also emerging evidence for the role of hip arthroscopy and biologics as treatment adjuncts in ONFH. A systematic review by Papavasiliou et al. [43] reported the adjunct use of hip arthroscopy to improve accuracy of drill tip placement into the osteonecrotic area in fluoroscopic-assisted retrograde drilling. Direct visualization of the femoral head also reduces the possibility of over-drilling, penetration and damage of articular cartilage, which is imperative in young patients to prevent early osteoarthritis. A later update by Papavasiliou et al. [44] explored the added option of supplementing arthroscopically assisted procedures with biologic therapies including bone morphogenetic proteins, platelet-rich plasma, peripheral blood stem cells and bone marrow mononuclear cells, which all showed promising results. Furthermore, hip arthroscopy allows for direct visualization to ensure accurate placement of biologics and a much more minimally invasive approach compared with the trapdoor technique proposed by Mont et al. [45].
Our analysis was limited by the heterogeneity of study protocols including patient selection, radiographic classification and assessment of disease, surgical technique and clinical outcome measures. Variations in the follow-up period of patients could have also influenced the accuracy and reliability of evidence presented. Furthermore, the lack of a standardized classification system and follow-up radiographic imaging modality for ONFH could have introduced bias and perhaps over classification of lesions, thus leading to discrepancies in the results of individual studies. Peri-operative parameters such as operative time and estimated blood loss could not be quantitatively analyzed due to the lack of raw data.
CONCLUSION
Tantalum rod is a safe alternative option to the current joint-preserving procedures available in the treatment of ONFH. However, more studies are needed to investigate and identify the most appropriate patients who would benefit most as well as the synergistic effect brought on by the use of complementary biological augmentation of bone grafting or stem cells with tantalum rods.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Hip Preservation Surgery online.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
Contributor Information
James Randolph Onggo, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
Mithun Nambiar, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
Jason Derry Onggo, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
Guan Tay, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
Parminder J Singh, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
Sina Babazadeh, Department of Orthopaedic Surgery, Maroondah Hospital, Ringwood East, Melbourne City, VIC 3135, Australia.
REFERENCES
- 1. Baig SA, Baig MN. Osteonecrosis of the femoral head: etiology, investigations, and management. Cureus 2018; 10: e3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995; 77: 459–74. [DOI] [PubMed] [Google Scholar]
- 3. Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: current concepts. Ind J Orthop 2015; 49: 28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee PT, Lakstein DL, Lozano B et al. Mid-to long-term results of revision total hip replacement in patients aged 50 years or younger. Bone Joint J 2014; 96-b: 1047–51. [DOI] [PubMed] [Google Scholar]
- 5. Bobyn JD, Stackpool GJ, Hacking SA et al. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br 1999; 81-B: 907–14. [DOI] [PubMed] [Google Scholar]
- 6. Lu MM, Wu PS, Guo XJ et al. Osteoinductive effects of tantalum and titanium on bone mesenchymal stromal cells and bone formation in ovariectomized rats. Eur Rev Med Pharmacol Sci 2018; 22: 7087–104. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen DR, Brown TD, Poggie RA. Finite element characterization of a porous tantalum material for treatment of avascular necrosis. Trans Orthop Res Soc 1997; 22: 598. [Google Scholar]
- 8. Kato H, Nakamura T, Nishiguchi S et al. Bonding of alkali- and heat-treated tantalum implants to bone. J Biomed Mater Res 2000; 53: 28–35. [DOI] [PubMed] [Google Scholar]
- 9. Balla VK, Bodhak S, Bose S et al. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 2010; 6: 3349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schildhauer TA, Robie B, Muhr G et al. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma 2006; 20: 476–84. [DOI] [PubMed] [Google Scholar]
- 11. Moher D The PRISMA GroupLiberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 1969; 51: 737–55. [PubMed] [Google Scholar]
- 13. Gardeniers J. A new international classification of osteonecrosis of the ARCO committee on terminology and classification. ARCO Newsl 1992; 4: 41–6. [Google Scholar]
- 14. Steinberg ME, Hayken GD, Steinberg DR. A new method for evaluation and staging of avascular necrosis of the femoral head In: Arlet J, Ficat RP, Hungerford DS (eds). Bone Circulation. Baltimore, MD, USA: Williams & Wilkins, 1984, 398–403. [Google Scholar]
- 15. Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br 1995; 77-B: 34–41. [PubMed] [Google Scholar]
- 16. Slim K, Nini E, Forestier D et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–6. [DOI] [PubMed] [Google Scholar]
- 17. Huang W, Gong X, Sandiford S et al. Outcome after a new porous tantalum rod implantation for treatment of early-stage femoral head osteonecrosis. Ann Transl Med 2019; 7: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu B, Sun W, Yue D et al. Combined tantalum implant with bone grafting for the treatment of osteonecrosis of the femoral head. J Invest Surg 2013; 26: 158–62. [DOI] [PubMed] [Google Scholar]
- 19. Liu G, Wang J, Yang S et al. Effect of a porous tantalum rod on early and intermediate stages of necrosis of the femoral head. Biomed Mater 2010; 5: 065003. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Yan L, Zhou S et al. Tantalum rod implantation for femoral head osteonecrosis: survivorship analysis and determination of prognostic factors for total hip arthroplasty. Int Orthop 2016; 40: 1397–407. [DOI] [PubMed] [Google Scholar]
- 21. Liu ZH, Guo WS, Li ZR et al. Porous tantalum rods for treating osteonecrosis of the femoral head. Genet Mol Res 2014; 13: 8342–52. [DOI] [PubMed] [Google Scholar]
- 22. Miao H, Ye D, Liang W et al. Effect of osteonecrosis intervention rod versus core decompression using multiple small drill holes on early stages of necrosis of the femoral head: a prospective study on a series of 60 patients with a minimum 1-year-follow-up. Open Orthop J 2015; 9: 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pakos EE, Megas P, Paschos NK et al. Modified porous tantalum rod technique for the treatment of femoral head osteonecrosis. World J Orthop 2015; 6: 829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty 2007; 22: 26–31. [DOI] [PubMed] [Google Scholar]
- 25. Varitimidis SE, Dimitroulias AP, Karachalios TS et al. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop 2009; 80: 20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao D, Liu B, Wang B et al. Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end-stage osteonecrosis of the femoral head. Biomed Res Int 2015; 2015: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao L, Guo C, Chen J et al. Free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. Clin Orthop Rel Res 2017; 475: 2230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chahal J, Thiel GSV, Mather RC et al. The minimal clinical important difference (MCID) and patient acceptable symptomatic state (PASS) for the modified harris hip score and hip outcome score among patients undergoing surgical treatment for femoroacetabular impingement. Orthop J Sports Med 2014; 2(7_Suppl 2): 2325967114S0010. [DOI] [PubMed] [Google Scholar]
- 29. Nadeau M, Séguin C, Theodoropoulos JS et al. Term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J Med 2007; 10: 4–10. [PMC free article] [PubMed] [Google Scholar]
- 30. Hua KC, Yang XG, Feng JT et al. The efficacy and safety of core decompression for the treatment of femoral head necrosis: a systematic review and meta-analysis. J Orthop Surg Res 2019; 14: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aurégan J-C, Villain B, Bégué T. What is the rate of patients undergoing a total hip arthroplasty after core decompression and insertion of a tantalum rod in osteonecrosis of the femoral head: a systematic review. Int Orthop 2018; 42: 1631–8. [DOI] [PubMed] [Google Scholar]
- 32. Ma J, Sun W, Gao F et al. Porous tantalum implant in treating osteonecrosis of the femoral head: still a viable option? Sci Rep 2016; 6: 28227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoica Z, Dumitrescu D, Popescu M et al. Imaging of avascular necrosis of femoral head: familiar methods and newer trends. Curr Health Sci J 2009; 35: 23–8. [PMC free article] [PubMed] [Google Scholar]
- 34. Choi H-R, Steinberg ME, Y Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med 2015; 8: 210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y-Z, Cao X-Y, Li X-C et al. Accuracy of MRI diagnosis of early osteonecrosis of the femoral head: a meta-analysis and systematic review. J Orthop Surg Res 2018; 13: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nam KW, Kim YL, Yoo JJ et al. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am 2008; 90: 477–84. [DOI] [PubMed] [Google Scholar]
- 37. Mont MA, Zywiel MG, Marker DR et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 2010; 92: 2165–70. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt-Sody M, Kirchhoff C, Mayer W et al. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop 2008; 32: 283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takashima K, Sakai T, Hamada H et al. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin Orthop Rel Res 2018; 476: 1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Li L, Shi ZJ et al. Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol 2013; 23: 211–7. [DOI] [PubMed] [Google Scholar]
- 41. Vaishya R, Agarwal AK, Gupta N et al. Sartorius muscle pedicle iliac bone graft for the treatment of avascular necrosis of femur head. J Hip Preserv Surg 2016; 3: 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lohmann CH, Singh G, Willert H-G et al. Metallic debris from metal-on-metal total hip arthroplasty regulates periprosthetic tissues. World J Orthop 2014; 5: 660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papavasiliou A, Yercan HS, Koukoulias N. The role of hip arthroscopy in the management of osteonecrosis. J Hip Preserv Surg 2014; 1: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papavasiliou AV, Triantafyllopoulos I, Paxinos O et al. The role of cell therapies and hip arthroscopy in the management of osteonecrosis: an update. J Hip Preserv Surg 2018; 5: 202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Rel Res 2003; 417: 84–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.