Abstract
Sphingolipids are potent bioactive agents involved in the pathogenesis of various respiratory bacterial infections. To date, several sphingolipid derivatives are known, but S1P (Sphingosine-1-phosphate) and Ceramide are the best-studied sphingolipid derivatives in the context of human diseases. These are membrane-bound lipids that influence host-pathogen interactions. Based on these features, we believe that sphingolipids might control SARS-CoV-2 infection in the host. SARS-CoV-2 utilizes the ACE-II receptor (Angiotensin-converting enzyme II receptor) on epithelial cells for its entry and replication. Activation of the ACE-II receptor is indirectly associated with the activation of S1P Receptor 1 signaling which is associated with IL-6 driven fibrosis. This is expected to promote pathological responses during SARS-CoV-2 infection in COVID-19 cases. Given this, mitigating S1P signaling by application of either S1P Lyase (SPL) or S1P analog (Fingolimod / FTY720) seems to be potential approach for controlling these pathological outcomes. However, due to the immunosuppressive nature of FTY720, it can modulate hyper-inflammatory responses and only provide symptomatic relief, which may not be sufficient for controlling the novel COVID-19 infection. Since Th1 effector immune responses are essential for the clearance of infection, we believe that other sphingolipid derivatives like Cermaide-1 Phosphate with antiviral potential and adjuvant immune potential can potentially control SARS-CoV-2 infection in the host by its ability in enhancing autophagy and antigen presentation by DC to promote T cell response which can be helpful in controlling SARS-CoV-2 infection in novel COVID-19 patients.
Keywords: Sphingolipids, Immune adjuvants, M1/M2 macrophages, Th1 effector response, Covid 19
1. Introduction
Sphingolipids are bioactive agents and amphipathic molecules and are fundamentally involved in the pathogenesis of several respiratory diseases ranging from asthma, cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and pulmonary infections [1] in the host. Sphingolipids are interconvertible, and their metabolism is strictly regulated. This enables them to both integrate and regulate a plethora of cellular functions. Sphingosine-1-phosphate (S1P) and Ceramide and are two most commonly studied sphingolipid metabolites and their rheostat are important for the progression of various pathologies, which are manifested by inflammatory cascade. While Ceramide and free sphingosine induce cell death, S1P and Ceramide-1-phosphate (C1P) promote homeostasis [2]. Therefore, a fine balance in the level of Ceramide and other sphingolipid metabolites particularly S1P is critical for cellular homeostasis as well as for immunity against infections.
Sphingolipids are amphipathic bio-molecules formally derived from phosphorylation of d-sphingosine. These are produced mainly by two pathways: de novo sphingolipid biosynthesis or by breakdown of ceramide. In de novo biosynthesis, the first step is the condensation of l-serine and palmitoyl-CoA through the action of serine palmitoyltransferase to form 3-ketodihydrosphingosine, which is then reduced to dihydro-sphingosine [3]. Acylation of dihydro-sphingosine produces ceramide, which can form sphingomyelin after conjugating with phosphocholine. However, sphingosine cannot be synthesized by the de novo pathway and is generated instead via the deacylation of ceramide catalyzed by ceramidase by salvage pathway [4]. Phosphorylation of sphingosine and ceramide by sphingosine kinases and ceramide kinase respectively generates S1P and ceramide-1-phosphate (C1P). This gets further acylated to di-hydro-ceramide which gets de saturated ceramide by the action of desaturase. Sphingomyelin is formed by the action sphingomyelin synthase. In the salvage pathway, sphingomyelin is hydrolyzed to ceramide, and then ceramide is further hydrolyzed to sphingosine, which is phosphorylated to S1P. This gets further acylated to form Ceramide which after coupling with phosphocholine leads to the formation of sphingomyelin [5]. The production of Ceramide from Sphingomyelin by sphingomyelinase is of particular pathophysiological relevance [6].
2. Sphingosine -1-phosphate (S1P) / Sphingosine kinase (SK) and viral infections
A plethora of evidence suggested that S1P promotes the pathogenesis in several inflammatory and tumor diseases. Although hundreds of sphingolipid species have been identified in the past, S1P / Ceramide [[7], [8], [9]] are the two best-studied sphingolipid derivatives studied in the context of respiratory diseases [[10], [11], [12]]. In view of this, we here discussed various approaches of how modulating sphingolipids derivatives may help in controlling SARS-CoV-2 infection in COVID-19 patients. S1P is known to influence mast cells' allergic response and other alveolar cells like macrophages and epithelial cells, which serve as a significant barrier for various pathogens and are expected to be relevant for controlling pathogens. In this context, studies have demonstrated the association of sphingolipids with viral tropism, viral-attachment, viral-replication, and viral-pathogenicity of several viral infections [13,14]; thus, sphingolipids indeed represent one of the potential targets for controlling the viral disease. On account of this we here discussed the significance of sphingolipid-based interventions for controlling SARS-CoV-2 infection, developing effective therapeutics for controlling the novel COVID-19 disease. Furthermore, several studies have suggested that sphingolipid metabolites are involved in the replication of Influenza A Virus (IAV). Increased SK1 activity in the IAV infected promotes the synthesis and stability of viral ribo-nucleoprotein complexes [15] in the infected epithelial cells.
Several reports have demonstrated a correlation between increased turnover of S1P in virus-infected cells supporting viral replication in the host. Additionally, increased levels of SK1/S1P lead to activation of ERK-1/2 (extracellular signal-regulated kinases), MAPK (mitogen-activated protein kinases), and AKT signaling pathways, which further promote viral replication in the host. Most intriguingly, impeded expression of SK1 leads to a decrease in glycoproteins' activity in the IAV infected cells [16]. Therefore, the perturbation in the biosynthesis or depletion of host sphingomyelin impairs viral maturation, budding, and release of the infected cells' viral nuclear particle [16]. Therefore, the perturbation in the biosynthesis or depletion of host sphingomyelin impairs viral maturation, budding, and release [16] of the infected cells' viral nuclear particle. Similarly, it was demonstrated that SK1 is critical for the nuclear export of viral proteins (NP, NS2, and M1) involved in transporting vRNPs from the nucleus to the cytoplasm. Later, Tafesse et al. projected that perturbation of host sphingomyelin biosynthesis inhibited the transport of influenza virus HA and NA to the cell surface, which in turn impaired viral maturation, budding, and release. Influenza virus activates multiple signal transduction pathways (ERK pathways) to make the intracellular environment extremely affordable for viral propagation [17]. Interestingly SK1/S1P is associated with the inhibition of ERK and NF-κB pathway (nuclear factor kappa-light-chain-enhancer of activated B cells) which are essential for viral replication [18] and nuclear transport of viral ribo-nucleoprotein complex respectively in the infected cell [19] and indicates that increased levels of S1P / SK1 enzymatic activity support virus infection in the host. On account of this, either pharmacological activation of S1P Lyase or inhibition of SK1 is likely to control replication of SARS-Cov-2 virus as well in COVID-19 patients. Like IAV, human cytomegalovirus (HCMV) increases SK1 activity which contributes to the efficient virus replication. Blockade of SK1 expression decreased the expression of immediate-early IE1 protein, over expression of same elevated the expression of IE1 proteins and virus particles [20]. Similarly, respiratory syncytial virus (RSV) increased the activity of SK1 and the mRNA expression of SK1 as well. Elevated activation of SK1 has shown to enhance RSV-induced activation of ERK MAPK and AKT signaling pathways which regulate the cell survival pathway upon infection.SARS-CoV2 interacts with angiotensin-converting enzyme 2 (ACE2) receptors on alveolar epithelial cells for its attachment and entry [21,22]. Since ACE2 / Angiotensin-II receptors and S1P receptor 1 (S1PR1) signaling is known to cooperate to promote IL-6 induced myopathy and fibrosis [23] in a mouse model cardiac hypertrophy. On the basis of this report, it is possible that a crosstalk of these receptors may contribute to fibrosis in COVID-19 patients as well. Interestingly, S1PR1 signaling is associated with Th1/ Th2 / 17 responses [24,25] in context dependent manner. Apart from this, S1PR1 signaling promotes hypoxia, asthmatic reactions [26] and anti-inflammatory response [27,28] in cancer patients. On account of this and increased expression of SK1 and S1P turnover in the virus-infected host, it is logical to presume that increased S1P signaling is likely to promote SARS–CoV-2 infection / replication in COVID-19 patients and warrant investigation.
S1P is a potent "find me" signal inducing a sterile inflammatory response, and may polarize M1 effector macrophages and CD4 + T cells toward M2 (foamy macrophages), and regulatory T cell in the infected organs (Ref). Moreover, S1PR1 signaling is known to activate Ras, MAPK, PI3K/AKT, and mTOR pathways, which drive substantial Th2 / 17 responses [23] hypoxia, allergic manifestations [26] and aberrant pathology which are anticipated to promote replication of SARS-CoV-2 infection and would account for novel COVID-19 related death. Given S1P related pathogenic inflammation and association of S1PR1 and ACE2 linked signaling, it is likely that S1P, despite its antibacterial potential [29], might not be effective in controlling SARS-CoV-2 infection. Hence blocking S1P response either by enhancing S1P lyase activity [30] or inhibiting its binding to its receptor by use of analogue known as FTY720 (Fingolimod) [31,32] may modulate the pathogenesis of novel COVID-19 cases. Currently, FTY720 is being explored in the Phase-2 clinical trial against COVID-19 patients (NCT04280588, MRCTA, ECFAH of FMU), and results are still awaited. Due to immunosuppressive nature FTY720 is only expected to lower down the hyper-inflammatory response and afford symptomatic/temporary relief and unlikely to afford clearance of infection [33] in novel COVID-19 cases.
3. Ceramide/Ceramide-1 Phosphate as proposed anti-Covid 19 agents
In view of proviral attributes of S1P, exploring other sphingolipid derivatives like Ceramide-1 Phosphate (C1P) with immune adjuvant and antiviral potential [34,35] may help the host in controlling the novel COVID-19 disease. Since free ceramide is potentially pro-apoptotic in nature, phosphorylated ceramide or C1P can be used for the management of disease pathology in COVID -19 patients. Unlike S1P, not much is known how C1P could influence the viral replication in host. In this context, one compelling study has demonstrated its potent anti-retroviral and immune augmenting potential [46] against HIV infection and on account of this, C1P is expected to qualify pharmacological criteria of being introduced/used as adjunct therapy against COVID-19 disease. In view of this, mobilizing C1P in COVID--19 patients seems to be appropriate strategies for interventions. Although there are various ways by which C1P could be enhanced in the COVID-19 patients for affording therapy. However due to feasibility and toxicity issues, we here described two potential modalities that could be used as therapy components. One of those is to supplement infected host or patients with l-serine essential amino acid in conjunction with Palmitoyl CoA or its analogs (due to kidney clearance issue) for increased synthesis of ceramide [[36], [37], [38]] in their plasma. Another strategy is to use ceramide kinase enzymes or mimetic compounds [39] for enhancing C1P levels in the infected cells. Both of these strategies are in their infancy and deserve further attention. Since C1P is a potent activator of resting macrophages [[40], [41], [42], [43]], phagocytosis [44] and antigen presentation by DC for promoting CTL responses [45] as depicted in Fig. 1 a thus certainly have potential of augmenting required adaptive immunity for controlling SARS-CoV-2 virus in the host.
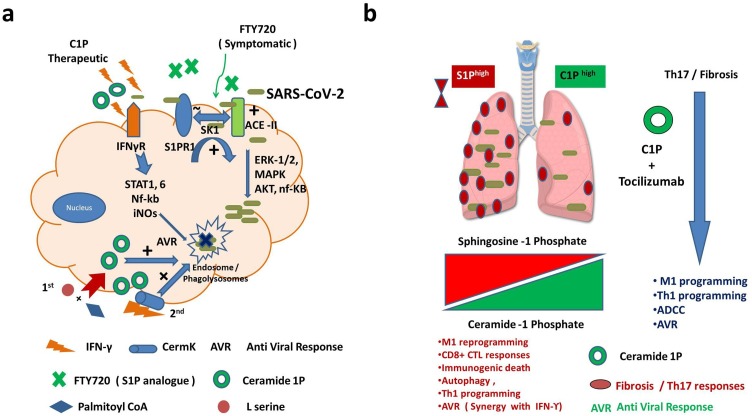
Fig. 1.
Impact of Ceramide-1 phosphate rheostat (Cer-1Phigh/S1Plow) for controlling SARS-Cov-2 in Covid 19 disease.
a. SARS-CoV-2 utilizes ACE-II receptors on epithelial cells for its entry and replication. Activation of ACE-II receptors is associated with the activation of S1PR1 signaling which is known to promote Th2 effector immune responses and subsequent fibrosis. Activation of Sphingosine kinase 1 (SK1) is believed to promote viral replication in ERK-1/2, MAPK and AKT dependent manner in lipid rafts and endosomal compartment. Blocking SK1 activity or use of S1P analogue (FTY720) can lower down infection induced cytokine storm / fibrosis. This is anticipated to protect severely infected patients from harmful inflammatory response even after virus is cleared. b. Mobilizing Ceramide-1 phosphate in the host by Ceramide Kinase (discussed in the text) represent another potential strategy for controlling SARS-CoV-2 infection by its potential of enhancing autophagy, M1 retuning of Th2/17 programmed macrophages and MHC-I restricted viral antigen presentation by M1 macrophages to CTL for augmenting M1 / Th1 programming in the host. Combining Ceramide-1 Phosphate can repurpose Remedsvir / Tocilizumab and polarize Th17 response towards Th1 and mitigate fibrosis for effective eradication of viral burden in lungs. High C1P and low S1P rheostat is anticipated to promote M1/ Th1 programming inhibit pulmonary fibrosis and Th17 programming of lung which is pathogenic in nature and contribute to the infected related death. This will afford help immune system to inhibit replication of virus effectively and contribute to anti-Covid-19 responses.
Taken together, we believe that enhancing the C1P gradient or its synthesis by either L serine / Palmitoyl Co A ceramide analog [46] or Ceramide kinase [47,48] is believed to augment required adaptive immunity [41,49,50] for controlling infection effectively as shown in Fig. 1b.
4. Conclusion and perspectives
We here propose that Sphingolipid derivatives are promising drug candidates for the management of novel COVID-19 disease. Furthermore, C1P based tailoring of Th1 effector immunity for the eradication of infection is a translationally viable approach and deserves immediate attention. We strongly anticipate that C1P would promote the killing of infected cells and resolve infection in moderate to severely infected cases. On account of this, ceramide derivatives can be exploited as drug candidates for controlling SARS-CoV-2 against novel COVID-19 disease.
Author contributions
HP conceived the idea and supervised the entire study. DJU, ORB, AKJ, contributed to the research. HP and BK: Critical analysis. HP and AJ wrote the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
This work was supported by the Extramural funding from the Department of Biotechnology BT/PR8282/MED/29/722/2013 to HP. The funder had no role to play in the decision-making process.
References
- 1.Sharma L., Prakash H. Sphingolipids are dual specific drug targets for the management of pulmonary infections: perspective. Front. Immunol. 2017;8:378. doi: 10.3389/fimmu.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawai H., Hannun Y.A. Ceramide and sphingomyelinases in the regulation of stress responses. Chem. Phys. Lipids. 1999;102(1–2):141–147. doi: 10.1016/s0009-3084(99)00082-1. Nov. [DOI] [PubMed] [Google Scholar]
- 3.Merrill A.H., Jr., Lingrell S., Wang E., Nikolova-Karakashian M., Vales T.R., Vance D.E. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J. Biol. Chem. 1995;270(23):13834–13841. doi: 10.1074/jbc.270.23.13834. Jun 9. [DOI] [PubMed] [Google Scholar]
- 4.Mullen T.D., Hannun Y.A., Obeid L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012;441(3):789–802. doi: 10.1042/BJ20111626. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng C., Dong H., Shi L., Deng Y., Mu J., Zhang J., et al. Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis. Plant Physiol. 2008;146(3):1322–1332. doi: 10.1104/pp.107.113506. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worgall T.S. Sphingolipid synthetic pathways are major regulators of lipid homeostasis. Adv. Exp. Med. Biol. 2011;721:139–148. doi: 10.1007/978-1-4614-0650-1_9. [DOI] [PubMed] [Google Scholar]
- 7.Hait N.C., Oskeritzian C.A., Paugh S.W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758(12):2016–2026. doi: 10.1016/j.bbamem.2006.08.007. Dec. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S., Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011;11(6):403–415. doi: 10.1038/nri2974. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arana L., Gangoiti P., Ouro A., Trueba M., Gomez-Munoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. doi: 10.1186/1476-511X-9-15. Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker K.A., Riethmuller J., Luth A., Doring G., Kleuser B., Gulbins E. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;42(6):716–724. doi: 10.1165/rcmb.2009-0174OC. Jun. [DOI] [PubMed] [Google Scholar]
- 11.Oskeritzian C.A., Milstien S., Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol. Ther. 2007;115(3):390–399. doi: 10.1016/j.pharmthera.2007.05.011. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teichgraber V., Ulrich M., Endlich N., Riethmuller J., Wilker B., De Oliveira-Munding C.C., et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008;14(4):382–391. doi: 10.1038/nm1748. Apr. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi M., Tasaki T., Ninomiya H., Ueda Y., Kuremoto K.I., Mitsutake S., et al. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci. Rep. 2016;28(6):37829. doi: 10.1038/srep37829. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yager E.J., Konan K.V. Sphingolipids as potential therapeutic targets against enveloped human RNA viruses. Viruses. 2019;11(10) doi: 10.3390/v11100912. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo Y.J., Blake C., Alexander S., Hahm B. Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity. J. Virol. 2010;84(16):8124–8131. doi: 10.1128/JVI.00510-10. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tafesse F.G., Sanyal S., Ashour J., Guimaraes C.P., Hermansson M., Somerharju P., et al. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc. Natl. Acad. Sci. U. S. A. 2013;110(16):6406–6411. doi: 10.1073/pnas.1219909110. Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael P., Brabant D., Bleiblo F., Ramana C.V., Rutherford M., Khurana S., et al. Influenza A induced cellular signal transduction pathways. J. Thorac. Dis. 2013;5(Suppl 2):S132–S141. doi: 10.3978/j.issn.2072-1439.2013.07.42. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michael P., Brabant D., Bleiblo F., Ramana C.V., Rutherford M., Khurana S., et al. Influenza A induced cellular signal transduction pathways. J. Thorac. Dis. 2013;(5 Suppl 2):S132–S141. doi: 10.3978/j.issn.2072-1439.2013.07.42. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo Y.J., Pritzl C.J., Vijayan M., Bomb K., McClain M.E., Alexander S., et al. Sphingosine kinase 1 serves as a pro-viral factor by regulating viral RNA synthesis and nuclear export of viral ribonucleoprotein complex upon influenza virus infection. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo Y.J., Hahm B. Sphingosine analog AAL-R promotes activation of LCMV-infected dendritic cells. Viral Immunol. 2014;27(2):82–86. doi: 10.1089/vim.2013.0096. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkura S.I., Usui S., Takashima S.I., Takuwa N., Yoshioka K., Okamoto Y., et al. Augmented sphingosine 1 phosphate receptor-1 signaling in cardiac fibroblasts induces cardiac hypertrophy and fibrosis through angiotensin II and interleukin-6. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder M., Richter C., Juan M.H., Maltusch K., Giegold O., Quintini G., et al. The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen. Mol. Immunol. 2011;48(9–10):1139–1148. doi: 10.1016/j.molimm.2011.02.007. May. [DOI] [PubMed] [Google Scholar]
- 25.Schulze T., Golfier S., Tabeling C., Rabel K., Graler M.H., Witzenrath M., et al. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25(11):4024–4036. doi: 10.1096/fj.10-179028. Nov. [DOI] [PubMed] [Google Scholar]
- 26.Saluja R., Kumar A., Jain M., Goel S.K., Jain A. Role of Sphingosine-1-phosphate in mast cell functions and asthma and its regulation by non-coding RNA. Front. Immunol. 2017;8:587. doi: 10.3389/fimmu.2017.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez Y.I., Campos L.E., Castro M.G., Aladhami A., Oskeritzian C.A., Alvarez S.E. Sphingosine-1 phosphate: a new modulator of immune plasticity in the tumor microenvironment. Front. Oncol. 2016;6:218. doi: 10.3389/fonc.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigert A., Weichand B., Brune B. S1P regulation of macrophage functions in the context of cancer. Anticancer Agents Med. Chem. 2011;11(9):818–829. doi: 10.2174/187152011797655096. Nov. [DOI] [PubMed] [Google Scholar]
- 29.Nadella V., Sharma L., Kumar P., Gupta P., Gupta U.D., Tripathi S., et al. Sphingosine-1-phosphate (S-1P) promotes differentiation of naive macrophages and enhances protective immunity against Mycobacterium tuberculosis. Front. Immunol. 2019;10:3085. doi: 10.3389/fimmu.2019.03085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijayan M., Xia C., Song Y.E., Ngo H., Studstill C.J., Drews K., et al. Sphingosine 1-phosphate lyase enhances the activation of IKKepsilon to promote type I IFN-Mediated innate immune responses to influenza a virus infection. J. Immunol. 2017;199(2):677–687. doi: 10.4049/jimmunol.1601959. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadopoulos D., Rundle J., Patel R., Marshall I., Stretton J., Eaton R., et al. FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J. Neurosci. Res. 2010;88(2):346–359. doi: 10.1002/jnr.22196. Feb 1. [DOI] [PubMed] [Google Scholar]
- 32.Penuelas-Rivas G., Dominguez-Perles R., Brinkmann V., Del Rio M.L., Munoz-Luna A., Ramirez-Romero P., et al. FTY720 inhibits TH1-mediated allogeneic humoral immune response. Transplant Proc. 2005;37(9):4124–4126. doi: 10.1016/j.transproceed.2005.09.184. Nov. [DOI] [PubMed] [Google Scholar]
- 33.Walsh K.B., Marsolais D., Welch M.J., Rosen H., Oldstone M.B. Treatment with a sphingosine analog does not alter the outcome of a persistent virus infection. Virology. 2010;397(2):260–269. doi: 10.1016/j.virol.2009.08.043. Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finnegan C.M., Rawat S.S., Puri A., Wang J.M., Ruscetti F.W., Blumenthal R. Ceramide, a target for antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 2004;101(43):15452–15457. doi: 10.1073/pnas.0402874101. Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritzl C.J., Seo Y.J., Xia C., Vijayan M., Stokes Z.D., Hahm B. A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J. Immunol. 2015;194(9):4339–4349. doi: 10.4049/jimmunol.1402672. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wigger D., Gulbins E., Kleuser B., Schumacher F. Monitoring the sphingolipid de novo synthesis by stable-isotope labeling and liquid chromatography-mass spectrometry. Front. Cell Dev. Biol. 2019;7:210. doi: 10.3389/fcell.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirabayashi Y., Furuya S. Roles of l-serine and sphingolipid synthesis in brain development and neuronal survival. Prog. Lipid Res. 2008;47(3):188–203. doi: 10.1016/j.plipres.2008.01.003. May. [DOI] [PubMed] [Google Scholar]
- 38.Weiss B., Stoffel W. Human and murine serine-palmitoyl-CoA transferase--cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 1997;249(1):239–247. doi: 10.1111/j.1432-1033.1997.00239.x. Oct 1. [DOI] [PubMed] [Google Scholar]
- 39.Rovina P., Graf C., Bornancin F. Modulation of ceramide metabolism in mouse primary macrophages. Biochem. Biophys. Res. Commun. 2010;399(2):150–154. doi: 10.1016/j.bbrc.2010.07.034. Aug 20. [DOI] [PubMed] [Google Scholar]
- 40.Arana L., Gangoiti P., Ouro A., Rivera I.G., Ordonez M., Trueba M., et al. Generation of reactive oxygen species (ROS) is a key factor for stimulation of macrophage proliferation by ceramide 1-phosphate. Exp. Cell. Res. 2012;318(4):350–360. doi: 10.1016/j.yexcr.2011.11.013. Feb 15. [DOI] [PubMed] [Google Scholar]
- 41.Ouro A., Arana L., Gangoiti P., Rivera I.G., Ordonez M., Trueba M., et al. Ceramide 1-phosphate stimulates glucose uptake in macrophages. Cell. Signal. 2013;25(4):786–795. doi: 10.1016/j.cellsig.2013.01.009. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangoiti P., Granado M.H., Wang S.W., Kong J.Y., Steinbrecher U.P., Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 2008;20(4):726–736. doi: 10.1016/j.cellsig.2007.12.008. Apr. [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Munoz A. Ceramide-1-phosphate: a novel regulator of cell activation. FEBS Lett. 2004;562(1–3):5–10. doi: 10.1016/s0014-5793(04)00211-x. Mar 26. [DOI] [PubMed] [Google Scholar]
- 44.Hinkovska-Galcheva V., Boxer L.A., Kindzelskii A., Hiraoka M., Abe A., Goparju S., et al. Ceramide 1-phosphate, a mediator of phagocytosis. J. Biol. Chem. 2005;280(28):26612–26621. doi: 10.1074/jbc.M501359200. Jul 15. [DOI] [PubMed] [Google Scholar]
- 45.Pritzl C.J., Seo Y.J., Xia C., Vijayan M., Stokes Z.D., Hahm B. A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J. Immunol. 2015;194(9):4339–4349. doi: 10.4049/jimmunol.1402672. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finnegan C.M., Rawat S.S., Puri A., Wang J.M., Ruscetti F.W., Blumenthal R. Ceramide, a target for antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 2004;101(43):15452–15457. doi: 10.1073/pnas.0402874101. Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ordonez M., Rivera I.G., Presa N., Gomez-Munoz A. Implication of matrix metalloproteinases 2 and 9 in ceramide 1-phosphate-stimulated macrophage migration. Cell. Signal. 2016;28(8):1066–1074. doi: 10.1016/j.cellsig.2016.05.005. Aug. [DOI] [PubMed] [Google Scholar]
- 48.Baumruker T., Bornancin F., Billich A. The role of sphingosine and ceramide kinases in inflammatory responses. Immunol. Lett. 2005;96(2):175–185. doi: 10.1016/j.imlet.2004.09.001. Jan 31. [DOI] [PubMed] [Google Scholar]
- 49.Avni D., Philosoph A., Meijler M.M., Zor T. The ceramide-1-phosphate analogue PCERA-1 modulates tumour necrosis factor-alpha and interleukin-10 production in macrophages via the cAMP-PKA-CREB pathway in a GTP-dependent manner. Immunology. 2010;129(3):375–385. doi: 10.1111/j.1365-2567.2009.03188.x. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Munoz A., Presa N., Gomez-Larrauri A., Rivera I.G., Trueba M., Ordonez M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 2016;61:51–62. doi: 10.1016/j.plipres.2015.09.002. Jan. [DOI] [PubMed] [Google Scholar]