Abstract
Background
There are no effective therapies for patients with coronavirus disease-2019 (COVID-19).
Methods
Forty-one patients with confirmed COVID-19 were enrolled in the study and divided into two groups: artemisinin-piperaquine (AP) (n = 23) and control (n = 18). The primary outcome were the time taken to reach undetectable levels of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the percentage of participants with undetectable SARS-CoV-2 on days 7, 10, 14, and 28. The computed tomography (CT) imaging changes within 10 days, corrected QT interval changes, adverse events, and abnormal laboratory parameters were the secondary outcomes.
Results
The mean time to reach undetectable viral RNA (mean ± standard deviation) was 10.6 ± 1.1 days (95% confidence interval [CI] 8.4–12.8) for the AP group and 19.3 ± 2.1 days (95% CI 15.1–23.5) for the control group. The percentages of patients with undetectable viral RNA on days 7, 10, 14, 21, and 28 were 26.1%, 43.5%, 78.3%, 100%, and 100%, respectively, in the AP group and 5.6%, 16.7%, 44.4%, 55.6%, and 72.2%, respectively, in the control group. The CT imaging within 10 days post-treatment showed no significant between-group differences (P > 0.05). Both groups had mild adverse events.
Conclusions
In patients with mild-to-moderate COVID-19, the time to reach undetectable SARS-CoV-2 was significantly shorter in the AP group than that in the control group. However, physicians should consider QT interval changes before using AP.
Keywords: Artemisinin, Piperaquine, COVID-19, Antimalarial, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There were 2.55 million confirmed cases and 179 000 confirmed deaths worldwide by 23 April 2020. The cumulative number of confirmed cases exceeded 12 million and deaths exceeded 540 000 By 09 July 2020 [1].
Several studies have reported the incubation periods in COVID-19 to be 3–7 days from the first contact [2,3]. According to China's Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Seventh Edition), COVID-19 patients are usually categorised into mild, moderate, severe, and critical, based on their symptoms. A large number of clinical trials for potential COVID-19 drugs are underway. Although various treatments – such as antiviral therapy, cell therapy, immunotherapy, and Chinese herbal medicine – have shown variable efficacy, no drugs or biologics had been approved by the FDA for the treatment of COVID-19 at the time of writing this article [4]. Antivirals such as lopinavir and ritonavir have not shown better antiviral efficacy than standard treatment [5]. The use of the antimalarials chloroquine and hydroxychloroquine is controversial. A few clinical trials have shown that hydroxychloroquine has failed to treat COVID-19 [6]. The National Institute of Health has advised discontinuing the clinical trials involving hydroxychloroquine because, although harmless, this drug is reported to be ineffective [7]. However, studies from China have demonstrated the efficacy and safety of chloroquine in Chinese patients with COVID-19 [8]. A study from France reported that hydroxychloroquine significantly reduced the viral load in patients with COVID-19, and demonstrated synergistic interactions with azithromycin [9]. Researchers are optimistic that a vaccine will help to lessen this epidemic; however, the development cycle of a vaccine is long and time-consuming [10,11].
Besides fast-tracking the development of COVID-19-specific treatments, other treatment strategies should also be tested. One such treatment strategy involves the use of drugs that are already in use for different indications – an approach known as drug repurposing. Artemisinin and piperaquine (AP) is a new-generation artemisinin combination therapy (ACT) based antimalarials. The first-line antimalarial drug artemisinin and its derivatives are not only potent antimalarials but also possess considerable antiviral properties [12,13,14]. Artemisinin is reported to reduce the proliferation of the hepatitis B virus [15], hepatitis C virus [16,17], and human immunodeficiency virus [18,19]. Piperaquine is a bisquinoline antimalarial drug, similar to chloroquine. Piperaquine was first synthesised in China in the 1960s and has been widely used in China and other countries ever since. Chloroquine is not only well-tolerated but also has similar potency against Plasmodium falciparum and Plasmodium vivax [20]. Chloroquine is reported to treat Middle-east respiratory syndrome and atypical respiratory syndrome (SARS) [21]. Moreover, in vitro experiments have shown that chloroquine has an inhibitory effect on SARS-CoV-2 [22]. AP is a fourth-generation ACT, which plays an essential role in the prevention and treatment of malaria. If AP also treats COVID-19, many countries will benefit, especially the developing countries affected by malaria.
2. Methods
2.1. Study design
The study was approved by the ethical committee of Hongqi Hospital affiliated to Mudanjiang Medical University (registration number: 202014). The trial protocol and application documents were submitted to the China Clinical Trial Registration Center for online registration (registration number: ChiCTR2000033049). Initially, this trial was an open-label, randomised, parallel-group, controlled trial intended to compare the efficacy and safety of AP tablets in comparison with hydroxychloroquine to treat patients with mild-to-moderate COVID-19. Due to the rapid control of this pandemic in China, the number of patients failed to meet the trial requirements. Therefore, the trial was modified to a controlled clinical trial and conducted during the same period. Patients with confirmed SARS-CoV-2 infection were divided into two groups. Patients in the first group were treated with AP tablets and those in the second group acted as controls and received hydroxychloroquine/Arbidol as antiviral and symptomatic treatments.
2.2. Patients
Inclusion criteria were: 1) age ≥ 18 years; 2) confirmed SARS-CoV-2 infection in upper respiratory tract specimens by real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR); 3) signature on the informed consent form.
Exclusion criteria were: 1) age < 18 years; 2) pregnancy; 3) severe malignancy, heart/liver/kidney disease or poorly controlled metabolic diseases; 4) allergy to 4-aminoquinolines; 5) blood system diseases; 6) arrhythmia or chronic heart disease; 7) retinal disease or hearing loss; and 8) mental illnesses or skin diseases (including rash, dermatitis, psoriasis).
The COVID-19 patients were categorised according to China's Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Seventh Edition) as follows:
Mild: mild clinical symptoms with no pneumonia manifestation on CT imaging; Moderate: symptoms such as fever, cough, and respiratory symptoms, and pneumonia manifestation on CT imaging.
2.3. Treatment
AP group: AP (ARTEPHARM Co., Ltd.) was used as an antiviral and symptomatic treatment. AP was orally administrated with a loading dose of two tablets (artemisinin 125 mg and piperaquine 750 mg) for the first day and followed by a maintenance dose of one tablet/day (artemisinin 62.5 mg and piperaquine 375 mg) for the next six days. The total dose was eight tablets in seven days.
Control group: Hydroxychloroquine/Arbidol, according to China's Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Seventh Edition), was mainly used as an antiviral and symptomatic treatment. Hydroxychloroquine sulfate (Shanghai Zhongxi Pharmaceutical Co., Ltd.) was orally administered as a loading dose of 800 mg/day for the first three days, followed by a maintenance dose of 400 mg daily for the next five days. Arbidol hydrochloride (CSPC Ouyi Pharmaceutical Co., Ltd.) was orally administrated 600 mg/day for eight days, divided into three doses daily.
When drug doses were completed, positive patients continued to receive symptomatic treatment, and met the discharge conditions when two consecutive tests for nucleic acid became negative. All the patient were quarantined for 14-day observation after discharge. The quarantine restriction was lifted if the tests remained negative.
2.4. Assessment
Critical inspection indicators: upper respiratory tract specimens were obtained daily from each patient, and RT-PCR for SARS-CoV-2 was conducted in the local Center for Disease Control and Prevention or the hospital. The RT-PCR test kits (BioGerm) [23] could detect the ORF1ab/N gene for SARS-CoV-2 nucleic acid and the reference mean Ct value for detecting the genes was 38. For the FAM channel, the HEX/VIC channel, and the ROX channel of RT-PCR, if the Ct value of the two channels was ≤ 38, the result was positive, otherwise it was negative. The epidemiological characteristics, clinical symptoms, signs, and adverse events were recorded with case report forms. The clinical features, laboratory findings, and chest CT scan data were recorded on case report forms and the hospital case report system. The patients who were negative for SARS-CoV-2 RNA two consecutive times were placed in quarantine for 14 days.
The conclusion was drawn by the professional imaging doctors who compared the before and after images. The main analysis criteria were the number of affected lobes, presence of ground-glass nodules, patchy/punctate ground-glass opacities, patchy consolidation, fibrous stripes, and irregular solid nodules on each CT image [24].
‘The time to undetectable viral RNA’ was defined as the time between the first dose of drug administration and the time when the RT-PCR for SARS-CoV-2 was negative for the first time. The rate of patients to undetected SARS-CoV-2 by RT-PCR at days 7, 10, 14, 21, and 28 during drug administration, CT images results within 10 days, abnormal laboratory index and adverse events were compared between the two treatments. To assess the safety of AP, ECG (Japanese photoelectric electrocardiograph ECG-1250C six-channel automatic analysis of 12 leads) was used to monitor the QT change before and 3–8 days after treatment in the AP group. Bazett's formula corrected the QTc algorithm:
2.5. Statistical analysis
Qualitative indicators were described by percentage or composition ratio for descriptive statistical analysis; the mean and standard deviation described the quantitative indicators. For qualitative data, the χ2, Fisher's exact probability method and the Wilcoxon rank-sum test were used. The quantitative data conformed to the normal distribution with the t-test and did not conform to the normal distribution with the Wilcoxon rank-sum test for the two groups. The hypothesis test uses a two-sided test uniformly. A P-value < 0.05 is considered statistically significant. The overall negative conversion probability was estimated by analysing the time taken by the patient to reach undetectable viral RNA levels. The Kaplan-Meier method was used for this and the findings were compared with a log-rank test. SPSS19.0 was used to perform statistical analyses.
3. Results
3.1. Baseline information of participants
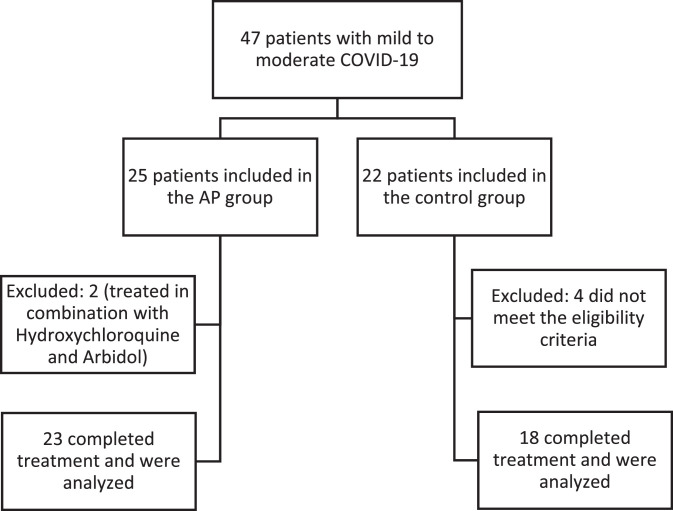
Of 25 patients accepted in the AP group, 23 (92%) completed the treatment and were included in the final analysis. Similarly, of 22 control group patients, 18 (82%) completed the treatment and were included in the final analysis (Figure 1 ). The demographic baseline data and clinical characteristics of the patients were summarised in Table 1 . The average age of the AP group and control group patients was 42.7 years and 45.8 years, respectively. A total of 82.6% of the patients in the AP group and 88.9% of the patients in the control group were diagnosed with moderate COVID-19, and the rest were mild COVID-19 patients. Patients in the two groups were relatively balanced in terms of clinical features, and the laboratory indicators also showed no significant difference (P > 0.05).
Figure 1.
Study flowchart.
Table 1.
Baseline demographic and clinical characteristics of the patients.
| Characteristicsa | AP (n = 23) | Control (n = 18) | P-value |
|---|---|---|---|
| Age (years) | 42.7 ± 11.8 | 45.8 ± 16.3 | 0.472 |
| Males (%) | 9 (39.1) | 6 (33.3) | 0.754 |
| Disease severity (%) | |||
| Mild | 4 (22.2) | 2 (1.1) | 0.679 |
| Moderate | 19 (82.6) | 16 (88.9) | 0.679 |
| Coexisting conditions (%) | |||
| Hypertension | 5 | 6 | 0.489 |
| Diabetes | 3 | 4 | 0.679 |
| others | 5 | 5 | 1.000 |
| Vital signals | |||
| Body temperature (°C) | 36.6 ± 0.5 | 36.8 ± 0.7 | 0.321 |
| Respiratory rate (breaths/min) | 19.3 ± 2.0 | 18.8 ± 1.7 | 0.345 |
| Pulse (beats/min) | 89.7 ± 17.0 | 87.5 ± 14.5 | 0.658 |
| Systolic blood pressure (mmHg) | 136.5 ± 20.6 | 143.7 ± 25.0 | 0.319 |
| Diastolic blood pressure (mmHg) | 88.1 ± 17.1 | 91.5 ± 12.2 | 0.460 |
| Symptoms | |||
| Dyspnoea | 0 | 1 | 0.439 |
| Dysgeusia | 0 | 1 | 0.439 |
| Fever | 3 | 7 | 0.075 |
| Cough | 6 | 8 | 0.322 |
| Sputum production | 3 | 4 | 0.679 |
| Fatigue | 0 | 1 | 0.439 |
| Pharyngalgia | 1 | 3 | 0.303 |
| Pharyngoxerosis | 3 | 1 | 0.618 |
| Chest tightness | 0 | 3 | 0.077 |
| Dizziness | 1 | 0 | 1.000 |
| Muscular soreness | 2 | 2 | 1.000 |
| Insomnia | 3 | 1 | 0.618 |
| Nasal congestion | 0 | 2 | 0.187 |
| Diarrhoea | 0 | 2 | 0.187 |
| Laboratory parameters | |||
| Alanine aminotransferase (U/L) | 24.48 ± 14.00 | 28.83 ± 21.31 | 0.436 |
| Aspartate aminotransferase (U/L) | 19.39 ± 5.17 | 20.78 ± 7.38 | 0.484 |
| Lactate dehydrogenase (U/L) | 171.43 ± 41.71 | 179.94 ± 40.66b | 0.523 |
| White cell count (× 10^9/L) | 6.41 ± 1.71 | 5.99 ± 1.84 | 0.457 |
| Neutrophil count (× 10^9/L) | 3.87 ± 1.39 | 3.97 ± 1.38 | 0.825 |
| Lymphocyte count (× 10^9/L) | 1.97 ± 0.60 | 1.84 ± 0.81 | 0.555 |
| Haematocrit | 0.42 ± 0.04 | 0.39 ± 0.05 | 0.014 |
| Platelet count (× 10^9/L) | 262.00 ± 81.31 | 264.89 ± 83.67 | 0.912 |
| Total bilirubin (μmol/L) | 16.91 ± 6.69 | 13.12 ± 4.95 | 0.052 |
| Urea (mmol/L) | 3.96 ± 0.73 | 3.77 ± 1.37 | 0.601 |
| Creatinine (μmol/L) | 58.75 ± 10.09 | 54.27 ± 12.76b | 0.612 |
| Uric acid (μmol/L) | 331.17 ± 87.98 | 278.06 ± 95.72 | 0.072 |
| Creatine kinase (μmol/L) | 72.22 ± 42.71 | 54.50 ± 33.29 | 0.241 |
| Myoglobin (ng/mL) | 31.29 ± 10.85 | 36.58 ± 11.95 | 0.146 |
| Troponin 1 (μg/L) | 0.63 ± 0.18 | 0.53 ± 0.22 | 0.111 |
Abbreviations: AP, artemisinin-piperaquine; control, hydroxychloroquine/Arbidol.
± values are means ± SD.
Lactate dehydrogenase and creatinine with 5.6% (1/18) missing data.
As there was no treatment to prove that the virus has a specific effect, based on ethical requirements, other drugs may interfere with the antiviral effect that has been clinically used, as shown in the list shown in Table 2 . The enumeration of other antiviral drugs was based on facts and might affect the inevitable factors in evaluating the efficacy. Interferon α-1b, ribavirin, lopinavir, oseltamivir, and carrimycin might have certain effects on COVID-19 patients; however, there was no significant statistical difference between the two groups (P > 0.05). Herbal medicine was used for symptomatic treatment in this study and the results showed that there was a significant between-group difference (P = 0.030 ).
Table 2.
Other antiviral treatments for the two groups.
| Treatments N (%) | AP (n = 23) | Control (n = 18) | P-value |
|---|---|---|---|
| Interferon α-1b | 12 (52.2) | 6(33.3) | 0.343 |
| Hydroxychloroquine | 0 (0) | 5 (27.8) | 0.011 |
| Arbidol | 0 (0) | 15 (83.3) | < 0.001 |
| Carrimycin | 0 (0) | 3 (16.7) | 0.077 |
| Oseltamivir | 2 (8.7) | 0 (0) | 0.495 |
| Ribavirin | 7 (30.4) | 2 (11.1) | 0.254 |
| Lopinavir | 2 (8.7) | 0 (0) | 0.495 |
| Lianhua Qingwen capsules | 18 (78.3) | 12 (66.7) | 0.489 |
| Herbal | 23 (100) | 14 (77.8) | 0.030 |
Abbreviations: AP, artemisinin-piperaquine; control, hydroxychloroquine/Arbidol.
3.2. Overall time to achieve undetectable levels of SARS-CoV-2 RNA
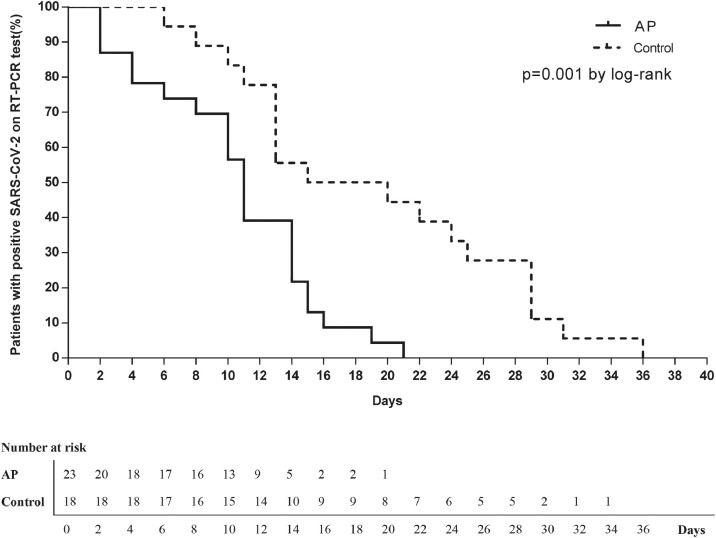
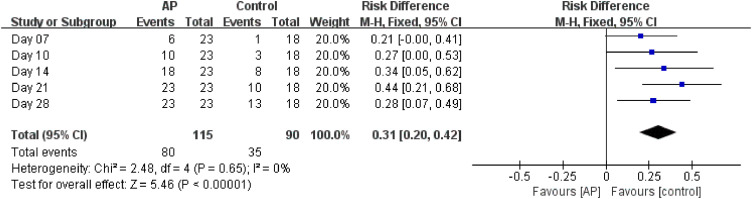
The average time to achieve undetectable SARS-CoV-2 RNA in the AP group was significantly less than that in the control group (AP: 10.6 ± 1.1 days (95% CI 8.4–12.8), control: 19.3 ± 2.1 days (95% CI 15.1-23.5)) (P = 0.001 ) (Figure 2 ). The percentages of the patients to achieve undetectable SARS-CoV-2 at days 7, 10, 14, 21, and 28 during drug administration in the AP group were 26.1%, 43.5%, 78.3%, 100%, and 100%, respectively, while that in the control group were 5.6%, 16.7%, 44.4%, 55.6%, and 72.2%, respectively (Table 3 ). Analysis of these data indicated that the elimination rate of SARS-CoV-2 RNA in the AP group was significantly higher than that in the control group (RD = 0.28; 95% CI 0.07–0.49) (Figure 3 ). The length of hospital stay for the AP group was 13.3 ± 4.8 days and 21.3 ± 9.1 days for the control group (Table 3). Non of the patients progressed to the severe form of COVID-19.
Figure 2.
Kaplan-Meier curves for the time taken to achieve undetectable viral RNA levels in different treatment groups.
Table 3.
The time to undetectable viral RNA in patients.
| AP (n = 23) | Control (n = 18) | P-value | |
|---|---|---|---|
| Time to undetectable viral RNA in days (Mean ± SD) | 10.6 ± 1.1 | 19.3 ± 2.1 | < 0.005 |
| Patients with undetectable viral RNA, N (%) | |||
| Day 7 | 6 (26.1) | 1 (5.6) | 0.112 |
| Day 10 | 10 (43.5) | 3 (16.7) | 0.095 |
| Day 14 | 18 (78.3) | 8 (44.4) | 0.049 |
| Day 21 | 23 (100.0) | 10 (55.6) | < 0.005 |
| Day 28 | 23 (100.0) | 13 (72.2) | 0.011 |
| Duration of hospitalisation (days, mean ± SD) | 13.3 ± 4.8 | 21.3 ± 9.1 | < 0.005 |
Abbreviations: AP, artemisinin-piperaquine; control, hydroxychloroquine/Arbidol.
Figure 3.
Overall time taken to achieve undetectable viral RNA levels in different treatment groups.
3.3. CT imaging
Before treatment, the lungs of 82.6% (19/23) of the patients in the AP group and 83.3% (15/18) of the patients in the control group had visible inflammation. The CT imaging changes of the patients were analysed within the next 10 days. Improvements in CT imaging were detected in 36.8% (7/19) of the AP group patients and 46.7% (7/15) of the control group patients. A total of 21.1% (4/19) and 6.7% (1/15) of the patients in the AP and control groups, respectively, had no significant change. Exacerbations occurred in 10.5% (2/19) of the AP group patients and 13.3% (2/15) of the control group patients. A total of 31.6% (6/19) of the AP group patients and 33.3% (5/15) of the control group patients were not re-examined within 10 days (Table 4 ).
Table 4.
Computed tomography imaging changes in patients.
| Group/cases | Before | After (≤ 10 days) | |||
|---|---|---|---|---|---|
| Improved | No significant change | Exacerbation | No review | ||
| AP | 19 | 7 | 4 | 2 | 6 |
| Control | 15 | 7 | 1 | 2 | 5 |
Abbreviations: AP, artemisinin-piperaquine; control, hydroxychloroquine/Arbidol.
aAP group: four cases had no significant inflammatory reaction; control group: two cases had no significant inflammatory reaction, and one case had unrecorded.
bχ2 test by Fisher's exact probability method showed that P = 0.669, no significant between-group difference.
3.4. Safety analysis
The adverse reactions in both the groups were mild and disappeared quickly after treatment. Three (13%) patients in the AP group and three (17%) patients in the control group encountered adverse reactions. The adverse reactions in the AP group patients included nausea, muscle aches and fatigue, while that in the control group patients included nausea, rash and itching . In the AP group, three patients had alanine aminotransferase (ALT) levels > 80 U/L and one patient had aspartate aminotransferase (AST) levels > 60 U/L post-treatment. In the control group, two patients had ALT levels > 80 U/L and one patient had AST levels > 60 U/L post-treatment.
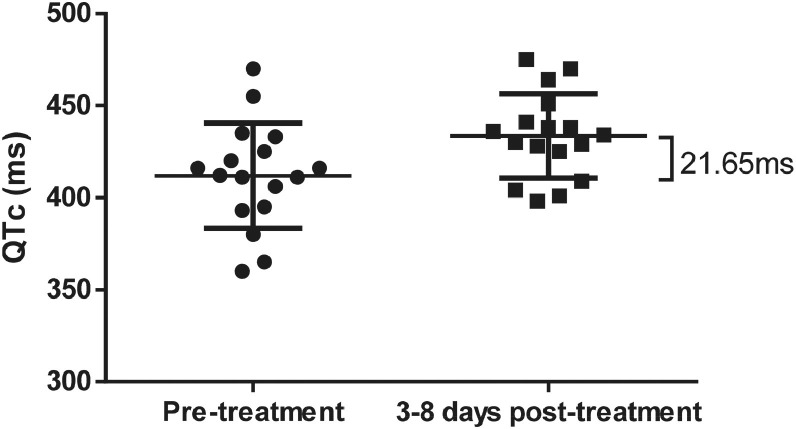
In 17 patients in the AP group, the ECG results indicated that the average QTc interval value was 411.94 ms before treatment and 433.59 ms 3–8 days post-treatment. The average prolongation was 21.65 ms (95% CI 3.58–39.71 ms) after treatment. Twelve patients (70.59%) with varying degrees of prolongation: six (35.29%) with mild prolongation (< 30 ms), four (23.53%) with moderate prolongation (30–60 ms), and two (11.76%) with severe prolongation (> 60 ms). Furthermore, the paired sample t-test showed significant between-group differences (P < 0.05) (Figure 4 ).
Figure 4.
Electrocardiogram monitoring of the corrected QT interval change in the artemisinin-piperaquine group.
*Paired sample t-test showed significant differences in the artemisinin-piperaquine (AP) group between before treatment and 3–8 days after treatment (P = 0.011 ).
AP treatment did not cause TdP or other arrhythmias in the patients. The patients with prolonged QT intervals returned to normal after the treatment was discontinued. ECG changes for the control group patients were not collected and recorded.
4. Discussion
Although this study has various limitations, including that of insufficient sample size and trial design, it was decided to publicly share the research data to help in meeting the urgent treatment needs.
In AP treatment, artemisinin is reported to have a fast and complete oral absorption, widespread distribution, rapid excretion, and a short half-life (1.93 hours) [25]. However, piperaquine has a long half-life (11.7 days) and a slow elimination rate [26]. Continuous administration of AP might result in toxicity and cause QT prolongation [27]. Therefore, after the first dose of two tablets was administered, one tablet a day was chosen to minimise the side effects and maximise the efficacy of artemisinin.
Treatment of COVID-19 patients with herbal medicine such as Lianhua Qingwen and Huoxiang Zhengqi significantly alleviates the patient's symptoms and improves prognosis without having serious adverse reactions, but has not been found to significantly contribute to viral assay findings [28,29,30]. Based on this, the focus of this study was that AP could significantly shorten the time to reach undetectable SARS-CoV-2. Due to the use of herbal medicine and other symptomatic treatment measures, no further analysis and inferences were made on the improvement of clinical symptoms.
COVID-19 spreads from human to human through droplets, contaminated hands or surfaces. SARS-CoV-2 has an incubation time of 2–14 days. The spread index of this virus, as estimated by most studies, is between 2.24 and 3.58, which is slightly higher than SARS [31]. COVID-19 can quickly progress from mild to severe [32]. Traditional public health measures – including isolation, quarantine, social distancing, and community containment – are effective in curbing this pandemic [33].
This study found that AP shortens the time the virus remains in the body. CT imaging results within 10 days of taking AP showed a similar effect on lung improvement as the control group. As there are no effective antiviral drugs, it is recommended that AP be used as eight tablets in seven days for patients with mild-to-moderate COVID-19. For regions lacking medical facilities, AP is also recommended during isolation and quarantine periods, if the condition of the patient worsens. For the patients with suspected close contacts of COVID-19, AP is recommended (eight tablets within seven days) as a precautionary treatment.
SARS-CoV-2 directly infects endothelium and causes immune cell recruitment that results in extensive endothelial dysfunction and apoptosis. These manifestations make the blood more viscous and result in thrombus formation [34]. As per studies, the adult dose of AP for malaria consists of four tablets (artemisinin 250 mg and piperaquine 1500 mg), which has shown prolonged QT-intervals in some people [35]. The total recommended AP adult dose for the treatment of COVID-19 consists of eight tablets (artemisinin 500 mg and piperaquine 3000 mg). The current study found severe prolongation in two patients (11.76%) and significant differences between the two groups. Although AP treatment can cause QT interval prolongation in some patients, it did not cause TdP or other arrhythmias. Moreover, patients with prolonged QT intervals returned to normal after the drug was discontinued. However, considering the effect of SARS-CoV-2 on blood vessels and the side effects of AP, close monitoring of QT segment changes during AP treatment is still recommended.
The mechanisms of how AP is improving the health of patients with COVID-19 is still being investigated.
Acknowledgments
We would like to thank all the patients who participated in this study and their families. We would like to thank all the healthcare workers at the field sites who are fighting this pandemic. The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Declarations
Funding: This work was supported by the Natural Science Foundation of China [Grant Number 81873218], China Postdoctoral Science Foundation [Grant Number 2019M662875], Scientific Research Projects for Novel Coronavirus-infected Pneumonia Prevention and Control of the Education Department of Guangdong province, China (2020KZDZX1055), the Emergent Scientific and Technological Special Project for Novel Coronavirus-infected Pneumonia Prevention and Control of Guangdong Province, China (2020A111128013), National Major Science and Technology Special Project (2018ZX09303008) and Bureau of Traditional Chinese Medicine Project of Guangdong Province, China (No. [2019]43).
Competing Interests: The funding agencies had no role in study design, data collection, and analyses, decision to publish, or preparation of the manuscript
Ethical Approval: Hongqi Hospital affiliated with Mudanjiang Medical University (Registration Number: 202014) and China Clinical Trial Registration Center (Registration Number: ChiCTR2000033049).
Author contributions: G. Li and M. Yuan contributed equally to this study. X. Guan, F. Xie, C. Deng, and J. Song designed, organised and directed the trial. X. Guan, F. Xie, M. Yuan, H. Li, and C. Jin carried out the fieldwork. G. Li, H. Li, M. Yuan, Q. Wang, X. Tang, H. Zhang Y. Zou, Y. Yuan, J. Guo, and W. Yu collected and analysed the data. G. Li, Q. Xu, C. Deng, and J. Song wrote and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.WHO. Data from who.int/emergencies/diseases/novel-coronavirus-2019. n.d.
- 2.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jean S-S, Lee P-I, Hsueh P-R. Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infec. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020:369. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine. n.d.
- 8.Huang M, Li M, Xiao F, Pang P, Liang J, Tang T. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci Rev. 2020 doi: 10.1093/nsr/nwaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautret P, Lagier J-C, Parola P, Meddeb L, Mailhe M, Doudier B. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Singh P, Srivastav SK, Mittal A, Singh M. COVID-19, the novel coronavirus 2019: current updates and the future. Int J Res Med Sci. 2020;8:1939. [Google Scholar]
- 11.Triggle CR, Bansal D, Abd Farag EAB, Ding H, Sultan AA. COVID-19: learning from lessons to guide treatment and prevention interventions. Msphere. 2020:5. doi: 10.1128/mSphere.00317-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L, Huang H. Immune suppressive properties of artemisinin family drugs. Pharmacol Ther. 2016;166:123–127. doi: 10.1016/j.pharmthera.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakir L, Hussain M, Javeed A, Ashraf M, Riaz A. Artemisinins and immune system. Eur J Pharmacol. 2011;668:6–14. doi: 10.1016/j.ejphar.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Yao W, Wang F, Wang H. Immunomodulation of artemisinin and its derivatives. Sci Bull. 2016;61:1399–1406. doi: 10.1007/s11434-016-1105-z. [DOI] [Google Scholar]
- 15.Romero MR, Efferth T, Serrano MA, Castaño B, Macias RIR, Briz O. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Paeshuyse J, Coelmont L, Vliegen I, Hemel J Van, Vandenkerckhove J, Peys E. Hemin potentiates the anti-hepatitis C virus activity of the antimalarial drug artemisinin. Biochem Biophys Res Commun. 2006;348:139–144. doi: 10.1016/j.bbrc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Obeid S, Alen J, Pham VC, Meuleman P, Pannecouque C, Le TN. Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS One. 2013;8:e81783. doi: 10.1371/journal.pone.0081783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung M, Schinazi RF. Synthesis and in vitro anti-human immunodeficiency virus activity of artemisinin (qinghaosu)-related trioxanes. Bioorg Med Chem Lett. 1994;4:931–934. doi: 10.1016/S0960-894X(01)80266-3. [DOI] [Google Scholar]
- 19.Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJG, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47:804–811. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 20.Davis TME, Hung T-Y, Sim I-K, Karunajeewa HA, Ilett KF. Piperaquine. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Dyall J, Gross R, Kindrachuk J, Johnson RF, Olinger GG, Hensley LE. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77:1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Liu J, Li S, Peng Z, Xiao Z, Wang X. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020 doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birgersson S, Van Toi P, Truong NT, Dung NT, Ashton M, Hien TT. Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers. Malar J. 2016;15:90. doi: 10.1186/s12936-016-1134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Röshammar D, Hai TN, Friberg Hietala S, Van Huong N, Ashton M. Pharmacokinetics of piperaquine after repeated oral administration of the antimalarial combination CV8 in 12 healthy male subjects. Eur J Clin Pharmacol. 2006;62:335–341. doi: 10.1007/s00228-005-0084-9. [DOI] [PubMed] [Google Scholar]
- 27.Wu W, Liang Y, Wu G, Su Y, Zhang H, Zhang Z. Effect of artemisinin-piperaquine treatment on the electrocardiogram of malaria patients. Rev Soc Bras Med Trop. 2019;52:1–6. doi: 10.1590/0037-8682-0453-2018. [DOI] [PubMed] [Google Scholar]
- 28.Hu K, Guan W-J, Bi Y, Zhang W, Li L, Zhang B. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of coronavirus disease 2019 (Covid-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9:1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Ide S, Saito S, Kinoshita N, Kutsuna S, Moriyama Y. COVID-19 can suddenly become severe: a case series from Tokyo, Japan. Global Health Med. 2020 doi: 10.35772/ghm.2020.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilder-Smith A, Isolation Freedman DO. quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa020. taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Liang Y, Wu G, Su Y, Zhang H, Zhang Z. Effect of artemisinin-piperaquine treatment on the electrocardiogram of malaria patients. Rev Da Soc Bras Med Trop. 2019;52 doi: 10.1590/0037-8682-0453-2018. [DOI] [PubMed] [Google Scholar]