Abstract
Scope:
Slowly digestible starch (SDS), as a functional carbohydrate providing a slow and sustained glucose release, may be able to modulate food intake through activation of the gut-brain axis.
Methods and results:
Diet-induced obese rats were used to test the effect on feeding behavior of high fat (HF) diets containing a SDS, fabricated to digest into the ileum, as compared to rapidly digestible starch (RDS). Ingestion of the HF-SDS diet over an 11-week period reduced daily food intake, through smaller meal size, to the same level as a lean body control group, while the group consuming the HF-RDS diet remained at a high food intake. Expression levels (mRNA) of the hypothalamic orexigenic neuropeptide Y (NPY) and Agouti-related peptide (AgRP) were significantly reduced, and the anorexigenic corticotropin-releasing hormone (CRH) was increased, in the HF-SDS fed group compared to the HF-RDS group, and to the level of the lean control group.
Conclusion:
SDS with digestion into the ileum reduced daily food intake and paralleled suppressed expression of appetite-stimulating neuropeptide genes associated with the gut-brain axis. This novel finding suggests further exploration involving a clinical study and potentially development of SDS-based functional foods as an approach to obesity control.
Keywords: slowly digestible starch, gut-brain axis, obesity, food intake, meal size
Introduction
Obesity is a risk factor of hypertension, type 2 diabetes, cardiovascular disease [1], and various types of cancer [2]. Fundamentally, it is associated with positive energy balance resulting from high caloric intake and low energy expenditure. Of the two, increase in caloric intake rather than lower physical activity was noted to be the greater driver of the obesity epidemic in the United States over the last three decades [3]. Thus, there is a causative relationship between food intake and obesity. A potential way to control body weight is through activation of the gut-brain axis by functional food components to trigger hormones such as the ileal-activated glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) that affect appetitive response and food intake [4–6]. This occurs through activity of different regions of the central nervous system, particularly the hypothalamus, which plays a crucial role in regulation of food intake [7–9]. The arcuate nucleus within the hypothalamus is key in the control of appetite [10] involving orexigenic (appetite-stimulating) neuropeptides [neuropeptide Y (NPY), Agouti-related peptide (AgRP)] and anorexigenic (appetite-suppressing) neuropeptides [pro-opiomelanocortin (POMC), cocaine- and amphetamine-regulated transcript (CART)] [11]. Other neuropeptides, including the orexigenic melanin-concentrating hormone (MCH) and anorexigenic corticotropin-releasing hormone (CRH) from other regions of the hypothalamus, are additionally involved in appetite regulation [12]. In particular, NPY and AgRP have been shown to play important roles in the regulation of food intake and maintenance of energy balance [13–15]. Thus, it seems reasonable that an anti-obesity strategy could be achieved through identification and use of hypothalamus-modulating functional dietary components.
Starch, in the form of glucose, is the major dietary source of energy. For nutritional purpose, it has been classified into rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) [16]. RDS, which is high in many processed starchy foods, is digested rapidly and leads to a postprandial glucose spike; whereas SDS is digested slowly in the small intestine with measured and sustained glucose release [17]. RS is undigested in the small intestine and is largely fermented in the colon. There is a responsiveness of the small intestine to these nutritional categories of starch. In pigs, SDS caused increased glucose transporter in the ileum [18] and in humans resulted in sustained release of the incretin hormone GLP-1 [19], which has a role in appetite control and body weight regulation [20]. Considering the existence of the GLP-1-mediated ileal brake and its role in the gut-brain axis [21, 22], and evidence that glucose is a stimulator of GLP-1 secretion from L-cells in the rat ileum [23] and suppresses food intake [24], we postulated that ileal location of starch digestion is important for the activation of this feedback control of appetite [22, 25]. Although not all SDS-containing foods digest as far as the ileum, some do so and may induce the secretion of the gut hormone GLP-1 [26]. Thus, we hypothesized that a SDS material that is characterized by digestion in the ileum may have the capability to modulate food intake through the gut-brain axis.
To study properly this potential physiological effect of SDS, it was necessary to use a material in which starch digestion rate and location of digestion in the small intestine was controlled, and one in which the digestion profile would be repeatable. We had previously developed a defined SDS material by entrapping starch in a natural alginate polysaccharide matrix in the form of microspheres that contain pores for α-amylase to enter and to digest the interior starch at controlled rates [27]. The microspheres were shown to digest slowly and, in a preliminary study, we further showed that the microspheres digested to some extent in the rat ileum (~3% of ingested microspheres at 2 h after gavage). Thus, we used the SDS microspheres to assess the effect of their long-term consumption on feeding behavior and the gut-brain axis using diet-induced obese rats.
Materials and Methods
Preparation of SDS microspheres
SDS microspheres (in the range of 200–600 μm) were made according to the method of Venkatachalam et al [27] by dropping a mixture of sodium alginate (0.5% w/v) and waxy cornstarch (10% w/v) through a 21 gauge dispensing needle (EFD Inc., East Providence, RI, USA) into a 2% w/v calcium chloride bath. The in vitro Englyst starch digestibility test [16] was conducted on the cooked microspheres and values were 62.8, 33.3, and 4.2% for RDS, SDS, and RS. As mentioned above, in a preliminary experiment, rats were gavaged with the 0.5% alginate-prepared microspheres and some starch was recovered in the ileum, which showed that they were digested throughout the length of the rat small intestine. For purpose of this study, the microspheres are referred to as “SDS”. SDS microspheres were cooked in boiling water for 10 min to gelatinize the starch and were freeze-dried prior to incorporation into a pelleted chow-type diet, as explained below. The RDS material for the study was fast digesting pregelatinized waxy cornstarch (Ultra-Sperse A, National Starch, Bridgewater, NJ, USA).
Animals and diets
Forty-five 8-wk old Sprague Dawley male rats (Harlan Laboratories, Indianapolis, IN, USA) were individually housed in stainless steel hanging wire cages maintained at a constant temperature (21°C) on a 12:12 h light/dark cycle (lights on at 6:00 a.m.) with ad libitum access to rodent chow (5001 Rodent Diet, Purina LabDiet, St. Louis, MO, USA) and water. After 1 wk of acclimation to the laboratory, rats were randomly assigned to a control low-fat (LF) diet group (14% fat calories purified control diet, Teklad TD.10026, Harlan Laboratories, Madison, WI, USA) (n=6) or a high fat (HF) diet group (45% fat calories diet, Harlan Laboratories) (n=39) for 14 wks to induce obesity. At the end of the diet-induced obesity (DIO) phase, the body fat percentage (%) was determined using a rat/mice body composition analyzer (Echo-MRI, Echo MRI LLC, Houston, TX, USA). The animals were screened using percent (%) body fat as the criteria to exclude the rats in the LF group that were more prone to being obese (higher % body fat), and the rats in the HF group that were less prone to being obese (lower % body fat), and also the most obese (highest % body fat) rats. The remaining HF-fed obese rats were randomly assigned to three experimental diet groups: HF (n=6), HF-containing RDS (HF-RDS; n=6), HF-containing SDS microspheres (HF-SDS; n=5), and then transferred to the food intake monitoring cages for the study phase. Lean control rats continued to be fed on the LF diet (n=4). Diet compositions are found in Table 1. The experimental diets were formulated at Harlan Laboratories (Harlan Teklad, Madison, WI, USA). All husbandry procedures followed the guidelines of The NIH Guide for the Care and Use of Laboratory Animals (8th ed., The National Academy Press, Washington, D.C.), and were approved by the Purdue University Animal Care and Use Committee (approval no. 1111000321). Every effort was made to minimize suffering and the number of animals used.
Table 1.
Composition of the experimental diets in g/kg and energy kcal (%).
| Nutrients (g/kg) | LF1 | HF2 | HF-RDS | HF-SDS |
|---|---|---|---|---|
| Protein | ||||
| Casein | 210 | 245 | 245 | 245 |
| Kcal (%) | 20.1 | 19.4 | 19.3 | 19.3 |
| Carbohydrate | ||||
| Corn starch | 449 | 85 | ||
| Pregelatinized starch3 | 283 | 83 | ||
| Maltodextrin | 100 | 115 | 115 | 115 |
| Starch-microspheres4 | 200 | |||
| Sucrose | 90 | 200 | ||
| Kcal (%) | 65.9 | 34.6 | 35.0 | 35.0 |
| Fat | ||||
| Lard | 28 | 195 | 195 | 195 |
| Soybean Oil | 28 | 30 | 30 | 30 |
| Kcal (%) | 14.1 | 45.9 | 45.7 | 45.7 |
LF: low-fat
HF: high-fat
Source of rapidly digestible starch (RDS), waxy cornstarch
Waxy cornstarch. DS: rapidly digestible starch
SDS: slowly digestible starch.
Automated food intake monitoring
The BioDAQ Episodic Food Intake Monitor (Research Diets, Inc., New Brunswick, NJ, USA) was used for continuous data collection of meal patterns in undisturbed rats. The BioDAQ system weighs the food hopper every second and detects “not eating” as weight stable and “eating” as weight unstable. Meals that consisted of one or more bouts were separated by an inter-meal interval (IMI) set as 900 sec (15 min) and the minimal meal size was set as 0.2 g, which means that food intake was considered to be one meal when a feeding bout occurred within 15 min from the previous response and the total sum of the intake was equal to or greater than 0.2 g. Meal parameters including meal size (g/meal), number of meals, and amount of food consumed were extracted and assessed from the software (BioDAQ 2.3.07).
Satiety ratio was calculated as IMI (min) between the first and second meal/the first meal size (kcal) and expressed in min/kcal, thus a higher number indicates that the meals were more satiating. First and last meals consumed during the dark cycle were excluded from the calculation of satiety ratios.
Analysis of total starch retained in microspheres in feces
Another treatment group, HF-SDS+RS, was run, but feeding behavior results are not reported due to poor digestibility of the starch. Microspheres were visible in the feces of HF SDS+RS group, and were recovered and analyzed for residual starch content. Three fecal droppings from each of two rats were used for the analysis. They were mixed 1:10 with 1x PBS buffer, and microspheres were manually separated from the suspension. Recovered microspheres were washed in deionized water three times and dried at 40°C overnight. Microspheres were then pulverized and total starch content determined as described above.
Gene expression of hypothalamic neuropeptides
At week 12 of the study phase, animals were euthanized with ether and decapitated following a 12 h overnight fasting. Brains of all animals were harvested and collected on iced isopropanol before being stored at −80°C. The hypothalamus from each brain was excised and homogenized in Trizol reagent (Invitrogen, Carsbad, CA, USA) to extract total mRNA according to the manufacturer’s protocol. RT-qPCR was performed in duplicate using a StepOne Real-Time PCR Thermal Cycler (Applied Biosystems, Carlsbad, CA, USA) with SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) for 40 cycles in which GAPDH was served as a housekeeping gene. Gene expression changes were calculated using a protocol of 2-ΔΔCt after normalization based on the housekeeping gene. The primers for PCR are listed in Supplemental Table 1.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test for post hoc analyses or one-way repeated measures ANOVA followed by the paired t-test. A power of 0.83 was achieved for food intake difference assuming a mean difference of 5 kcal with a statistical significance of p = 0.05 and an animal size of 5. Hypothalamic neuropeptide data were analyzed using the t-test. Differences between groups were considered significant at P < 0.05. Statistical analyses were performed using SAS 9.3 program (SAS, Inc; Cary, NC, USA).
Results
Daily intake (dark cycle feeding time, kcal)
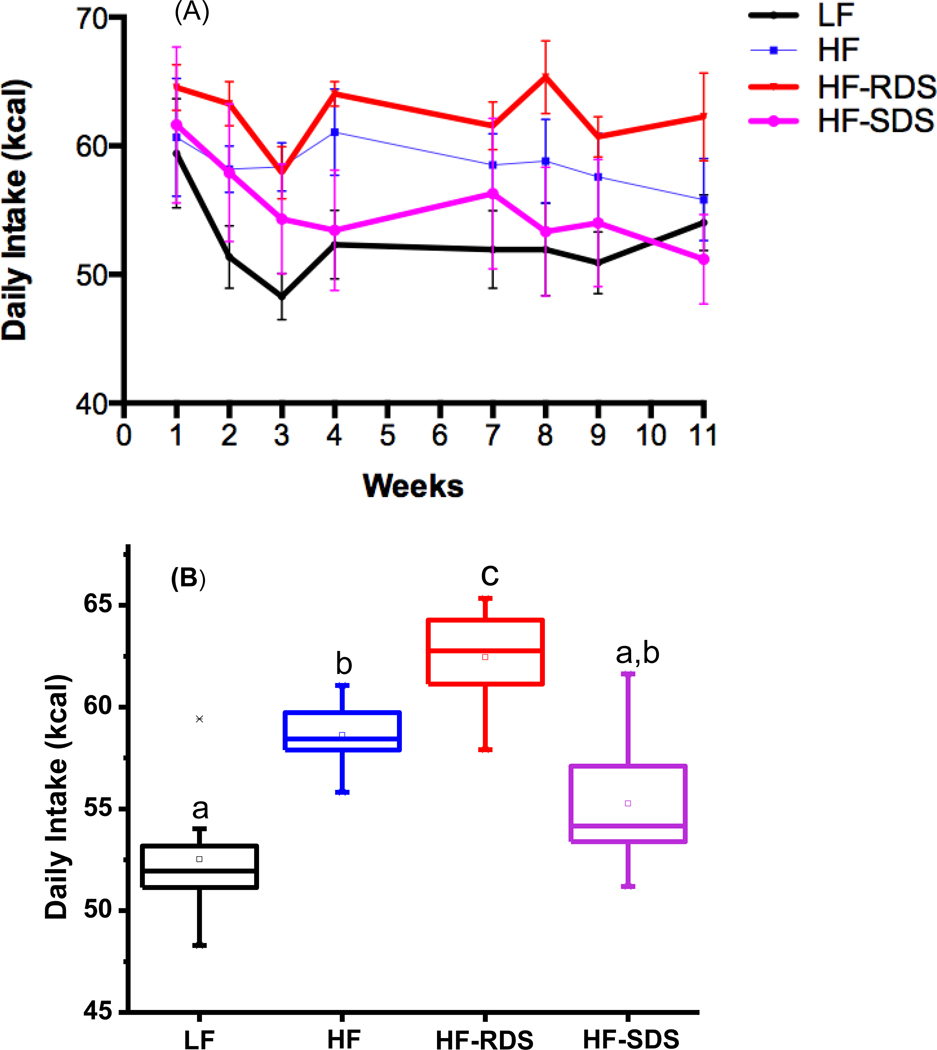
Rats fed on the different diets had notable differences in food intake over the 11-week study period (Fig. 1A). The HF-SDS group showed lower daily food intake than the HF-RDS group at wk 8 (P= 0.013), and the HF-RDS group had higher food intake compared to the lean group with significant differences at wks 2, 3, and 8 (P = 0.018, 0.025, and 0.010) (Fig. 1A, Table S1). Rats fed the HF-SDS diet had a significant decrease in food intake over the 11 wk study period (between wk 1 and 11, P = 0.042), and food intake of the HF-SDS group trended similar to the LF lean control group. The Average daily food intake over the entire treatment phase of the HF-SDS group was lower than the HF-RDS group (P < 0.05), and trended close to the LF lean control group (Fig. 1B). In addition, average daily food intake of the HF-RDS group was higher than the HF control group.
Figure 1.
Daily intake (kcal) measured during the study phase feeding period (dark cycle). (A) and the box-plot of the overall average of the 11 wk treatment (B). Different letters represent significance at P < 0.05.
A second SDS-containing diet was tested in the study design with microspheres made using a higher alginate concentration for slower starch digestion than the HF-SDS diet (termed HF-SDS+RS). Rats consumed significantly more of the HF-SDS+RS diet than HF-SDS diet (Fig. 1S) and it was later found that about 25% of consumed starch was recovered in SDS+RS microspheres from the feces. Rats in this treatment group apparently consumed more of the diet because of there was a lower caloric load due to lost starch than in the HF-SDS diet. This result was instructive, however, as it showed that the physical property of the microspheres was not a negative factor for the rats to consume the microsphere-containing diets.
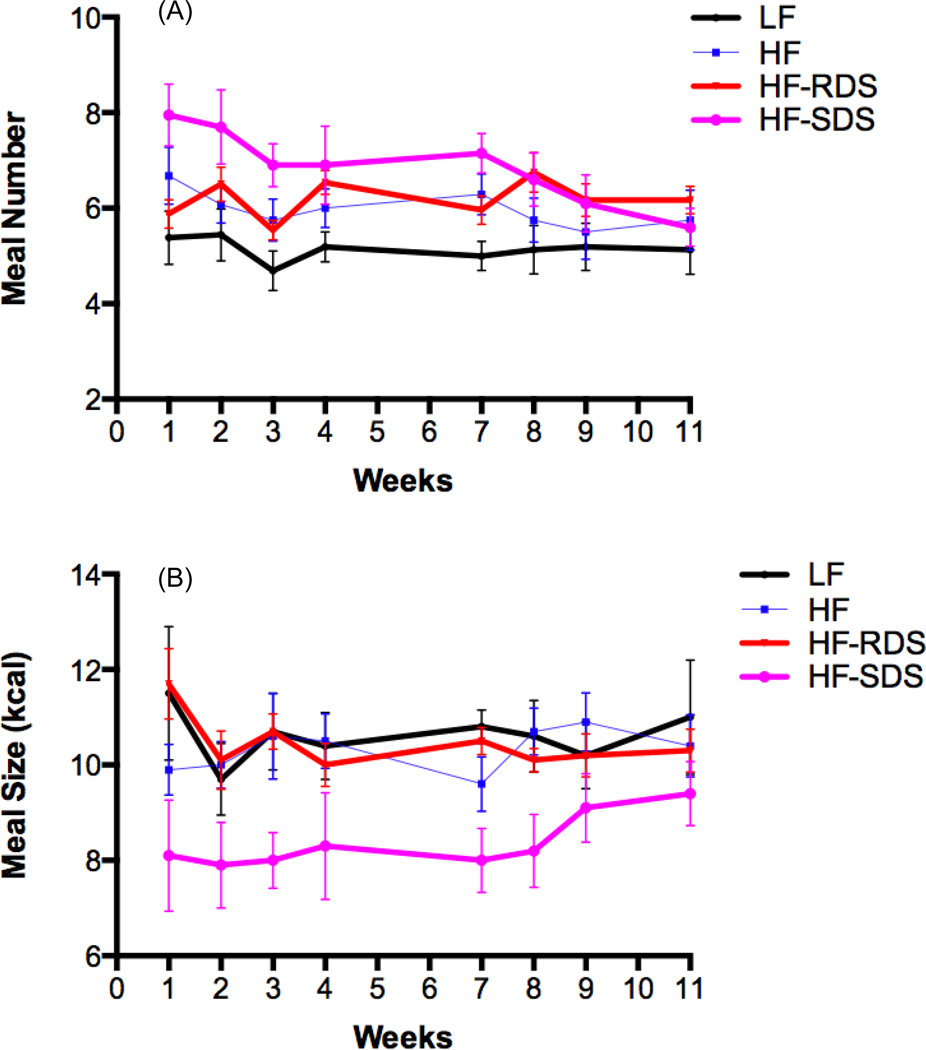
Rats on the HF-SDS diet had a substantially decreased meal size throughout the study compared to other treatment groups (Fig. 2A, Table S1). Compared to the HF-RDS group, the decrease in meal size of the HF-SDS group was significant at wks 1 (P = 0.014), 2 (P=0.020), 3 (P=0.007), 7 (P=0.003), and 8 (P=0.028). Compared to the LF control group, decrease in meal size of the HF-SDS group was significant at wks 1 (P = 0.035), 3 (P = 0.015), 7 (P = 0.003), and 8 (P = 0.012). The range of meal size reduction of the HF-SDS group compared to the HF-RDS group and the LF control group was 11.1 – 30.5% and 11.2 – 29.1%, respectively. Meal size was not different between the HF-RDS and the LF lean control groups at all time points.
Figure 2.
The daily meal size (kcal) (A) and meal number (B) during the study phase feeding period (dark cycle).
In the HF-SDS group, rats initially had a higher meal number (about 8 versus 6 meals per day), presumably to maintain same caloric intake in light of the reduced meal size they consumed. However, over the course of the 11-week treatment phase, daily meal number of the HF-SDS group decreased to 5.6 meals per day, a level similar to the other treatment groups, and significantly less than at the beginning of the study (P = 0.01) (Fig. 2B, Table S1). Compared to the HF-RDS group, meal number was significantly higher for the HF-SDS group at wks 1, 3, and 7 (P = 0.006, 0.019, and 0.030, respectively), and to the LF lean control group at wks 1, 2, 3, and 7 (P = 0.003, 0.005, 0.001, and 0.001, respectively); but no differences were observed compared to either group after wk 7 and until the end of the study.
Starting at wk 3, the HF SDS group showed a trending of higher satiety ratio compared to the other groups, though values were not statistically significant from the other treatment groups (Fig. S2).
Hypothalamic neuropeptides
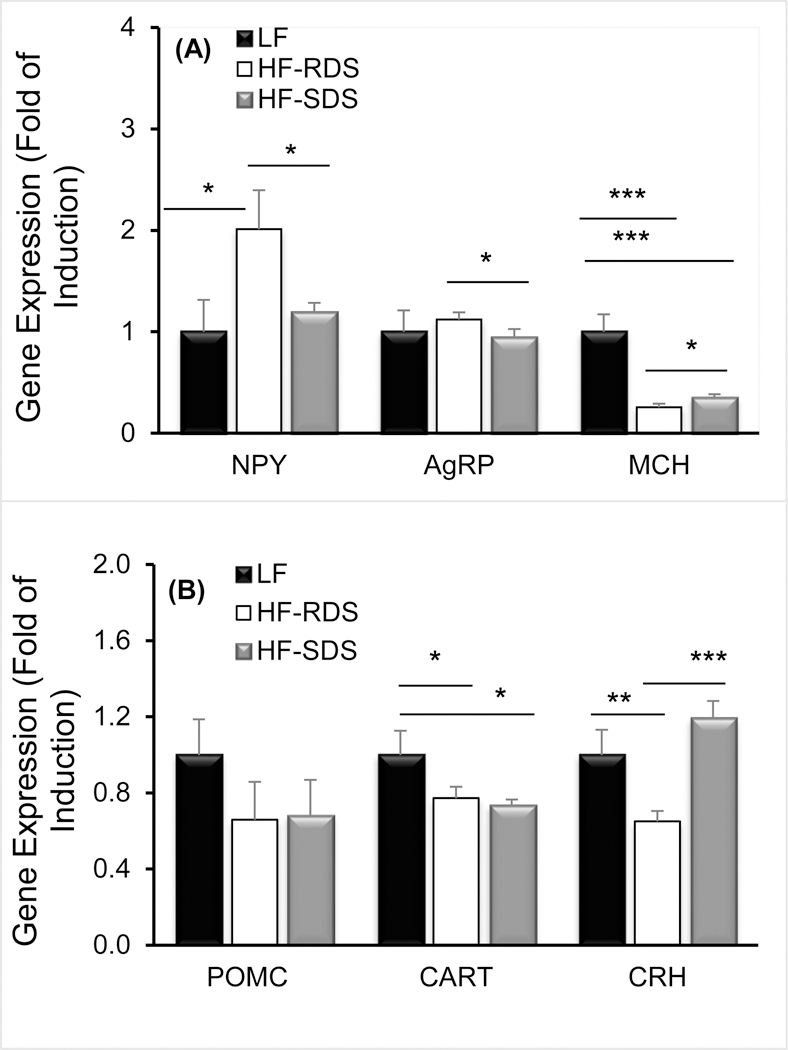
For the appetite-stimulating orexigenic neuropeptides, rats fed the HF-SDS diet showed a substantial decrease in gene expression of NPY compared to the HF-RDS group (P = 0.019; Fig 3A) that was similar and not significantly different from the LF control group. Gene expression of AgRP was also lower for the HF-SDS group compared to the HF-RDS group (P = 0.020). Gene expression of MCH was reduced in the obese rats and there was a slight, though significant increase, in MCH expression in the HF-SDS compared to the HF-RDS group (P < 0.05). For the appetite-suppressing anorexigenic neuropeptides, there were no differences in POMC among the three groups, though the obese rats had lower gene expression of CART than the LF control (P = 0.011 and P = 0.029 for HF-RDS and HF-SDS groups). For CRH, there was a significant increase in expression in the HF-SDS and LF groups compared to the HF-RDS group (P < 0.001 and P < 0.01, respectively) (Fig. 3B).
Figure 3.
Gene expression of the orexigenic neuropeptides Y (NPY), agouti-related peptide (AgRP), and melanin-concentrating hormone (MCH) (A), and anorexigenic neuropeptides proopiomelanocortin (POMC), cocaine-and amphetamine-regulated transcript (CART), and corticotropin-releasing hormone (CRH) (B)in the hypothalamus of rats fed the LF control, HF-RDS, and HF-SDS diets at the week of euthanasia (wk 12) following the study phase, * p<0.05, ** p<0.01, and *** p<0.001.
Discussion
Food carbohydrates have gained a negative connotation in the view of many consumers and researchers. There is the concept of “carbohydrate quality” that divides foods into high and low glycemic index or glycemic response foods, however there is rather little known about the real nature of these materials and how they interact with the body. Here, we show for the first time that when starch is digested slowly and into the ileum that the gut-brain axis is activated and, at least in obese rats, reduce their food intake.
The lower food intake of the HF-SDS treatment group occurred through a marked reduction in meal size. Although rats on the SDS diet initially consumed more meals, they adjusted to a normal meal frequency over the course of the 11 wk feeding period. Concurrently, by wk 11 there was a significant decrease in orexigenic NPY and AgRP in the HF-SDS versus HF-RDS groups, and for NPY the effect was substantial and to the same level as the LF lean control. Thus, foods containing slow digesting glycemic carbohydrates that reach the ileum appear to activate the gut-brain axis through endoendocrine L-cells and their effect on hypothalamic neurons to modulate food intake.
Notably, meal size was reduced in the HF-SDS group compared to the HF-RDS group. Meal size has been related to satiation and meal number to satiety [28, 29]. Satiation, which refers to processes leading to meal termination, is a within-meal effect governed by neural and humoral signals generated in response to ingested food through nutrient sensing [30]. That the ileal digestion property of the SDS used in this study is linked to changes in hypothalamic neuropeptides implies activation of the gut-brain axis, possibly through GLP-1 [31, 32] and its related positive effect on satiation [33]. The reduction in NPY gene expression in DIO rats fed on the HF-SDS diet appears to have reduced appetite by signaling the animal to stop eating earlier in the same meal (higher satiation) than when consuming the HF-RDS diet. NPY is a potent appetite-stimulating peptide [7], and it is known to be overproduced during obesity [34]. Additionally, gene expression of the anorexigenic neuropeptide CRH, which is present in the paraventricular nucleus of hypothalamus, was significantly increased by HF-SDS diet. CRH is a potent neuropeptide to suppress food intake and reduce body weight [35, 36], and its increased expression in the HF-SDS group is consistent with HF-SDS reduction of food intake. Furthermore, the NPY neuron directly provides input into CRH cell bodies [37] to exert its anorexigenic function, and it is therefore logical that down regulation of NPY may lead to an increase in expression of CRH which suppresses food intake [38]. Both the decrease of NPY and AgRP and increase of CRH could contribute to the increased satiation response of rats consuming the HF-SDS diet.
Despite the observed lower daily food intake of the HF-SDS diet group, no significant reduction of the body weight of the rats was found (Fig. S3). We think this could have been due to a combination of factors, but mainly that reduction in daily food intake of the HF-SDS group only occurred in the latter part of the treatment phase, and that the DIO rats stayed on a high-fat diet instead of being placed on a normal lower fat diet. Change in treatment design DIO rats are placed on a low-fat diet during the treatment phase is needed.
It is noteworthy that only a small amount of the starch from the microspheres was measured in the ileum (~3% of ingested, as mentioned above), suggesting that it is sensitive to the stimulatory effect of starch digestion. All cooked starches digest more extensively in the proximal than in the distal small intestine, and even for SDS relatively little or no starch typically reaches the ileum. One might speculate that ileal-located starch digestion fits diets of early man, which presumably included starchy foods eaten in relatively dense food matrices; where a small portion of starch would digest into the ileum to trigger the gut-brain axis, thus naturally controlling appetite.
In conclusion, our study is the first to demonstrate that SDS that digests into the ileum of the small intestine alters feeding behavior of diet-induced obese rats by reducing meal size and over time modulating meal frequency through triggering of the gut-brain axis. Gene expression of appetite-stimulating orexigenic neuropeptides was reduced and a hypothalamic appetite-suppressing neuropeptide was increased. Weight reduction was not seen in the treatment phase and a future study on weight reduction of obese animals needs to be done using a longer treatment period and with changing obese rats from a high-fat to normal diet. Overall, a dietary approach using slow digesting carbohydrates to control food intake may have practical preventive or treatment implications for the obesity problem.
Supplementary Material
Acknowledgments
We are thankful for the support of the U.S. Department of Agriculture (USDA) Agriculture and Food Research Initiative competitive grant program, no. 08–555-03–18793; U.S. Department of Health and Human Services, National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, no. DK027627; and the Whistler Center for Carbohydrate Research at Purdue University, West Lafayette, IN, USA.
Footnotes
Conflict of Interest
There is no commercial conflict of interest in this work.
References
- [1].Must A, S J, Coakley EH, Field AE, Colditz G, Dietz WH, The disease burden associated with overweight and obesity. JAMA. 1999, 282, 1523–1529. [DOI] [PubMed] [Google Scholar]
- [2].Louie SM, Roberts LS, and Nomura DK, Mechanisms linking obesity and cancer. Biochim. Biophys. Acta 2013, 1831, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Swinburn B, Sacks G, and Ravussin E, Increased food energy supply is more than sufficient to explain the us epidemic of obesity. Am. J. Clin. Nutr 2009, 90,1453–6. [DOI] [PubMed] [Google Scholar]
- [4].Delgado-Aros S, Kim D-Y, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M, Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am. J. Physio 2002, 282, G424–G31. [DOI] [PubMed] [Google Scholar]
- [5].Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE, Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physio 2005, 288, R384–R388. [DOI] [PubMed] [Google Scholar]
- [6].Scott KA, Moran TH, The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am. J. Physio 2007, 293, R983–R987. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz MW, Woods SC, Porte D, Seeley RJ, and Baskin DG, Central nervous system control of food intake. Nature, 2000, 404, 661–671. [DOI] [PubMed] [Google Scholar]
- [8].Cummings DE and Overduin J, Gastrointestinal regulation of food intake. J. Clin. Invest 2007, 117, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hussain SS and Bloom SR, The regulation of food intake by the gut-brain axis: Implications for obesity. Int. J. Obes 2013, 37, 625–633. [DOI] [PubMed] [Google Scholar]
- [10].Könner AC, Klöckener T, and Brüning JC, Control of energy homeostasis by insulin and leptin: Targeting the arcuate nucleus and beyond. Physio. Behav 2009, 97, 632–638. [DOI] [PubMed] [Google Scholar]
- [11].Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, and Low MJ, The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes 2001, 25 Suppl 5, S63–7. [DOI] [PubMed] [Google Scholar]
- [12].Parker JA and Bloom SR, Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [DOI] [PubMed] [Google Scholar]
- [13].Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van der Ploeg LHT, Woods SC, and Seeley RJ, Long-term orexigenic effects of agrp-(83—132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp 2000, 279, R47–R52. [DOI] [PubMed] [Google Scholar]
- [14].Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, and Matsukura S, A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [DOI] [PubMed] [Google Scholar]
- [15].Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, and Bi S, Role of dorsomedial hypothalamic neuropeptide y in modulating food intake and energy balance. J. Neurosci 2009, 29, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Englyst HN, Kingman SM, and Cummings JH, Classification and measurement of nutritionally important starch fractions. Euro. J. Clin. Nutr 1992, 46 Suppl 2, S33–50. [PubMed] [Google Scholar]
- [17].Seal CJ, Daly ME, Thomas LC, Bal W, Birkett AM, Jeffcoat R, and Mathers JC, Postprandial carbohydrate metabolism in healthy subjects and those with type 2 diabetes fed starches with slow and rapid hydrolysis rates determined in vitro. Br. J. Nutr 2003, 90, 853–864. [DOI] [PubMed] [Google Scholar]
- [18].Woodward AD, Regmi PR, Ganzle MG, van Kempen TA, and Zijlstra RT, Slowly digestible starch influences mrna abundance of glucose and short-chain fatty acid transporters in the porcine distal intestinal tract. J. Anim. Sci 2012, 90 Suppl 4, 80–2. [DOI] [PubMed] [Google Scholar]
- [19].Wachters-Hagedoorn RE, Priebe MG, Heimweg JAJ, Heiner AM, Englyst KN, Holst JJ, Stellaard F, and Vonk RJ, The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr 2006, 136, 1511–1516. [DOI] [PubMed] [Google Scholar]
- [20].Larsen PJ, Mechanisms behind glp-1 induced weight loss. Br. J. Diabetes Vasc. Dis 2008, 8, S34–S41. [Google Scholar]
- [21].Romijn JA, Corssmit EP, Havekes LM, and Pijl H, Gut-brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–21. [DOI] [PubMed] [Google Scholar]
- [22].Zhang G, Hasek LY, Lee BH, and Hamaker BR, Gut feedback mechanisms and food intake: A physiological approach to slow carbohydrate bioavailability. Food Func. 2015, 6, 1072–89. [DOI] [PubMed] [Google Scholar]
- [23].Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, and Goke B, Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–26. [DOI] [PubMed] [Google Scholar]
- [24].Williams DL, Baskin DG, and Schwartz MW, Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology, 2009, 150,1680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee B-H, Bello-Pérez LA, Lin AH-M, Kim CY, and Hamaker BR, Importance of location of digestion and colonic fermentation of starch related to its quality. J. Cereal Chem 2013, 90, 335–343. [Google Scholar]
- [26].Little TJ, Doran S, Meyer JH, Smout AJPM, O’Donovan DG, Wu K-L, Jones KL, Wishart J, Rayner CK, Horowitz M, and Feinle-Bisset C, The release of glp-1 and ghrelin, but not gip and cck, by glucose is dependent upon the length of small intestine exposed. Am. J. Physiol. Endocrinol.Metab 2006, 291, E647–E655. [DOI] [PubMed] [Google Scholar]
- [27].Venkatachalam M, Kushnick MR, Zhang G, and Hamaker BR, Starch-entrapped biopolymer microspheres as a novel approach to vary blood glucose profiles. J. Am. Coll. Nutr 2009, 28, 583–90. [DOI] [PubMed] [Google Scholar]
- [28].Meguid MM, Yang ZJ, and Koseki M, Eating induced rise in lha-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res. Bull 1995, 36, 487–90. [DOI] [PubMed] [Google Scholar]
- [29].Meguid MM, Yang ZJ, and Laviano A, Meal size and number: Relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am. J. Physio 1997, 272, R1925–30. [DOI] [PubMed] [Google Scholar]
- [30].Posovszky C. and Wabitsch M, Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: Characteristics of enteroendocrine cells and their capability of weight regulation. Horm Res. Paediatr 2015, 83, 1–10. [DOI] [PubMed] [Google Scholar]
- [31].Cummings DE and Overduin J, Gastrointestinal regulation of food intake. J. Clin. Invest 2007, 117, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wachters-Hagedoorn RE, Priebe MG, Heimweg JA, Heiner AM, Englyst KN, Holst JJ, Stellaard F, and Vonk RJ, The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr 2006, 136, 1511–6. [DOI] [PubMed] [Google Scholar]
- [33].Stanley S, Wynne K, McGowan B, and Bloom S, Hormonal regulation of food intake. Physio. Rev 2005, 85, 1131–58. [DOI] [PubMed] [Google Scholar]
- [34].Bi S, Ladenheim EE, Schwartz GJ, and Moran TH, A role for npy overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of oletf rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 2001, 281, R254–R260. [DOI] [PubMed] [Google Scholar]
- [35].Glowa JR and Gold PW, Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides 1991, 18, 55–61. [DOI] [PubMed] [Google Scholar]
- [36].Glowa JR, Barrett JE, Russell J, and Gold PW, Effects of corticotropin releasing hormone on appetitive behaviors. Peptides 1992, 13, 609–21. [DOI] [PubMed] [Google Scholar]
- [37].Li C, Chen P, and Smith MS, Corticotropin releasing hormone neurons in the paraventricular nucleus are direct targets for neuropeptide y neurons in the arcuate nucleus: An anterograde tracing study. Brain Res. 2000, 854, 122–9. [DOI] [PubMed] [Google Scholar]
- [38].Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, and Koob GF, Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide y. Brain res. 1993, 611,18–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.