Abstract
Background & aims
Great interest has been raised by the possible protective role of vitamin D in coronavirus disease 2019 (COVID-19), but objective data on 25(OH)vitamin D deficiency in hospitalized COVID-19 patients are not conclusive.
The aim of this study was to determine the prevalence of 25(OH)vitamin D deficiency in COVID-19 patients admitted to an Italian referral hospital and explore its association with clinical outcomes and the markers of disease severity.
Methods
In this single-center cohort study, 129 consecutive adult COVID-19 patients hospitalized in an Italian referral center were enrolled from March to April 2020. 25(OH)Vitamin D serum levels were assessed 48 h since hospital admission and categorized into: normal (≥30 ng/mL), insufficient (<30 - ≥20 ng/mL), moderately deficient (<20 - ≥10 ng/mL), severely deficient (<10 ng/mL).
Results
The prevalence of 25(OH)vitamin D insufficiency, moderate deficiency and severe deficiency was 13.2%, 22.5% and 54.3%, respectively.
25(OH)Vitamin D deficiency (<20 ng/mL) was not associated with COVID-19 clinical features and outcomes. Unexpectedly, after adjusting for major confounders, a significant positive association between increasing 25(OH)vitamin D levels and in-hospital mortality (on a continuous logarithmic scale, odds ratio = 1.73 [95% CI, 1.11 to 2.69]; P = .016) was observed.
Conclusions
Very low 25(OH)vitamin D levels were highly prevalent and suggestive of deficiency among our hospitalized severe COVID-19 patients, but low 25(OH)vitamin D levels were not associated with outcome variables. Whether 25(OH)vitamin D adequacy may influence clinical outcomes in COVID-19 and the unexpected correlation between higher 25(OH)vitamin D levels and mortality require further investigations by large intervention trials.
Keywords: Coronavirus disease 2019, Vitamin D 25OH, Hospitalized patients, Disease severity, Mortality
1. Introduction
If effective vaccines will not be developed and deployed in the next 6 months, wintertime outbreaks of Severe Acute Respiratory Syndrome-Coronavirus–2 (SARS-CoV-2) will probably occur, with hundreds of thousands of coronavirus disease 2019 (COVID-19) patients putting again the health care systems under stress, considering that herd immunity has not been reached even in countries heavily hit by the virus. Hence, preventing strategies to mitigate the severity of future possible outbreaks should be identified without delay. In the context of COVID-19, great interest has been raised by the possible protective role of 25(OH)vitamin D, due to its known properties in modulating the endothelial function and the immune response against viral infections, and inhibiting the Renin-Angiotensin-Aldosterone System (RAAS) cascade [1,2]. 25(OH)Vitamin D plays also a protective role in acute lung injuries, down-regulating renin, Angiotensin Converting Enzyme (ACE) and angiotensin (Ang) genes, up-regulating ACE2 expression and correcting tissue sensitivity to Ang II similarly to ACE inhibitors [1]. In line with this hypothesis, epidemiological data appear to support the concept that the gradient of COVID-19 severity across different countries could be related to the inherent rate of 25(OH)vitamin D deficiency [1]. Furthermore, recent studies reported that low circulating levels of 25(OH)vitamin D increase the odds of having a critical outcome following SARS-CoV-2 infection [3], and vitamin D supplementation may mitigate the cytokine storm associated with critical COVID-19 [4].
However, caution should be used when interpreting these evidences and translating them into general recommendations, especially because detailed data on the prevalence of 25(OH)vitamin D deficiency and its association with clinical outcomes in hospitalized COVID-19 patients are still not conclusive.
2. Materials and methods
This was a single-center cohort study on consecutive COVID-19 patients (positive result on real-time reverse-transcriptase–polymerase-chain-reaction assay of nasopharyngeal swab) admitted to an Italian referral hospital in the outbreak region of Lombardy (March–April 2020). The study was approved by Ethics Committee of Pavia on (March 26th - Prot. N. 20200031199) and aimed to determine at admission: 1) the prevalence of 25(OH)vitamin D deficiency; 2) the association between 25(OH)vitamin D status and clinical outcomes (severe pneumonia, admission to intensive care units [ICU] and in-hospital mortality) and biochemical markers of disease severity (lymphocytes count, C-reactive protein, high-sensitivity troponin I, lactate dehydrogenase, international normalized ratio, D-dimer).
Serum 25(OH)vitamin D serum was assessed within 48 h since hospital admission (chemiluminescence immunoassay [Abbott Diagnostics, Lake Forest, IL, USA]) and categorized into: normality (≥30 ng/mL), insufficiency (<30 - ≥20 ng/mL), moderately deficiency (<20 - ≥10 ng/mL), severe deficiency (<10 ng/mL).
Between-group comparisons (25(OH)vitamin D ≥ 20 vs. <20 ng/mL) related to continuous variables were performed using parametric or non-parametric tests, while categorical variables were analyzed by the Fisher's exact test. Then, adjusted logistic regression was used to investigate the association between 25(OH)vitamin D (<20 ng/mL and log-transformed) and death. Analyses were performed using Stata 16 (StataCorp, College Station, TX, USA).
3. Results
One hundred twenty-nine patients (54.3% males, mean age 73.6 ± 13.9 years) were enrolled and 34 (26.4%) died during the hospital stay. The prevalence of 25(OH)vitamin D insufficiency, deficiency and severe deficiency was 13.2%, 22.5% and 54.3%, respectively.
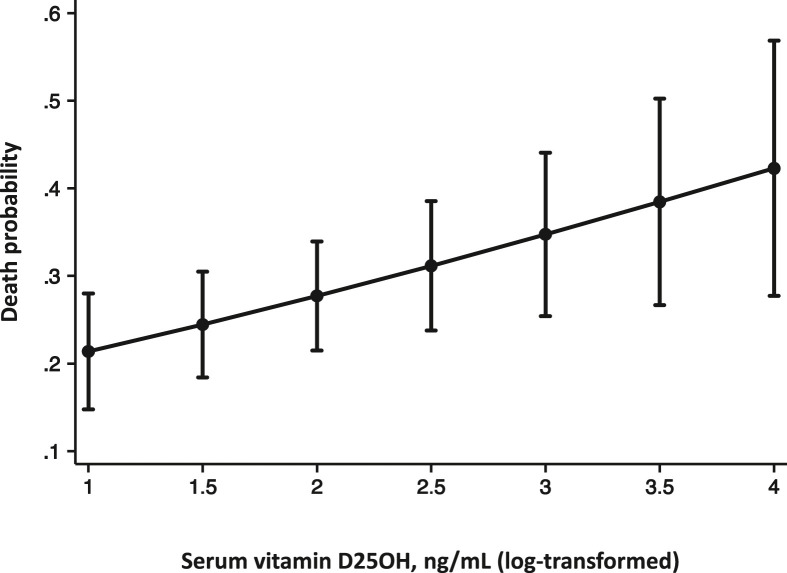
25(OH)Vitamin D deficiency (<20 ng/mL) was not associated with COVID-19 clinical features and outcomes (Table 1 ). Unexpectedly, after adjusting for major confounders (age, sex, C-reactive protein, ischemic heart disease and severe pneumonia), a significant positive association between increasing 25(OH)vitamin D levels and in-hospital mortality was observed (Table 2 and Fig. 1 ).
Table 1.
Features of the study population at admission by serum 25(OH)vitamin D.
| Feature | Overall population (N = 129) | 25(OH)Vitamin D ≥ 20 ng/mL (N = 30) |
25(OH)Vitamin D < 20 ng/mL (N = 99) |
P value |
|---|---|---|---|---|
| Female, N (%) | 59 (45.7) | 17 (56.7) | 42 (42.4) | .12 |
| Age (years), Median (IQR) | 77 (65.0–85.0) | 77.5 (65.0–86.0) | 77.0 (64.0–85.0) | .66 |
| Body mass index (kg/m2), Median (IQR) | 24.7 (22.5–27.6) | 24.4 (20.8–26.2) | 24.7 (22.9–27.9) | .22 |
| COPD, N (%) | 16 (12.6) | 5 (16.7) | 11 (11.3) | .31 |
| Diabetes, N (%) | 39 (30.7) | 11 (36.7) | 28 (28.9) | .28 |
| Hypertension, N (%) | 89 (70.1) | 24 (80.0) | 65 (67.0) | .13 |
| Ischemic heart disease, N (%) | 52 (40.9) | 10 (33.3) | 42 (43.3) | .23 |
| Cancer, N (%) | 27 (21.3) | 10 (33.3) | 17 (17.5) | .059 |
| Chronic kidney disease, N (%) | 24 (18.9) | 8 (26.7) | 16 (16.5) | .16 |
| Number of comorbidities, Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | .11 |
| >2, N (%) | 44 (34.1) | 14 (46.7) | 30 (30.3) | .077 |
| Lymphocytes (x10ˆ3/ul), Median (IQR) | 0.80 (0.50–1.11) | 0.69 (0.37–1.05) | 0.81 (0.56–1.18) | .12 |
| Platelets (x10ˆ3/ul), Median (IQR) | 204 (154–266) | 184 (135–253) | 216 (162–278) | .22 |
| Creatinine (mg/dL), Median (IQR) | 0.97 (0.72–1.38) | 0.95 (0.67–1.12) | 0.98 (0.74–1.42) | .37 |
| Aspartate aminotransferase (U/L), Median (IQR) | 31 (22–49) | 34 (29–46) | 29 (22–57) | .097 |
| Alanine aminotransferase (U/L), Median (IQR) | 24 (17–39) | 26 (18–43) | 23 (16–38) | .32 |
| Creatine kinase (U/L), Median (IQR) | 87 (46–211) | 108 (60–193) | 82 (42–228) | .24 |
| Lactate dehydrogenase (U/L), Median (IQR) | 315 (245–396) | 333 (273–495) | 313 (244–394) | .20 |
| C-reactive protein (mg/dL), Median (IQR) | 10.38 (5.19–16.46) | 6.81 (4.00–14.39) | 11.15 (5.56–17.14) | .11 |
| Albumin (g/dL), Median (IQR) | 2.85 (2.50–3.10) | 2.72 (2.50–3.10) | 2.90 (2.50–3.10) | .74 |
| INR, Median (IQR) | 1.08 (1.02–1.19) | 1.12 (1.03–1.23) | 1.08 (1.01–1.19) | .35 |
| D-Dimer (ug/L), Median (IQR) | 1271 (679–2467) | 2125 (748–4085) | 1162 (679–2275) | .15 |
| Hs-Troponine I (ng/L), Median (IQR) | 23 (8–56) | 28 (15–56) | 22 (7–56) | .23 |
| Severe pneumoniaa, N (%) | 70 (54.3) | 15 (50.0) | 55 (55.6) | .37 |
| ICU admission, N (%) | 5 (3.9) | 0 (0.0) | 5 (5.1) | .26 |
| Death, N (%) | 34 (26.4) | 10 (33.3) | 24 (24.2) | .22 |
Abbreviations:COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
According to the American Thoracic Society guidelines.
Table 2.
Association between 25(OH)vitamin D and in-hospital mortality (logistic regression model).
| Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|
| Predictors | OR (95% CI) | P-value | Predictors | OR (95% CI) | P value |
| 25(OH)Vitamin D < 20 ng/mL | 0.28 (0.09–0.99) | .038 | Ln-25(OH)vitamin D | 1.73 (1.11–2.69) | .016 |
| Age >77 years | 13.27 (4.01–43.91) | <.001 | Age >77 years | 15.53 (4.51–53.54) | <.001 |
| Ln CRP | 3.17 (0.87–11.50) | .080 | Ln CRP | 3.17 (0.89–11.34) | .076 |
| Ischemic heart disease | 3.74 (1.34–10.44) | .012 | Ischemic heart disease | 3.90 (1.31–11.55) | .018 |
| Severe pneumoniaa | 3.87 (1.27–11.80) | .017 | Severe pneumoniaa | 4.21 (1.34–13.19) | .014 |
Abbreviations:OR (95%CI), odds ratio and 95% confidence intervals; Ln, natural logarithm; CRP, C-reactive protein.
According to the American Thoracic Society guidelines.
Fig. 1.
Adjusted probability of death according to 25(OH)vitamin D levels.
4. Discussion
This was one of the few prospective studies which assessed the prevalence of 25(OH)vitamin D deficiency in COVID-19 patients hospitalized in a referral hospital during the first 2 months of the pandemic in Western countries and evaluated its association with clinical features and outcomes.
We are aware of the study limitations, which consist mainly in being monocentric, like the vast majority of those already published, and in the limited sample size. Another potential limitation could be the inclusion bias associated with the setting of care. Our population consisted of particularly 25(OH)vitamin D -deficient severe patients, being our Institution a tertiary referral hospital. However, we think that our results may be relevant to deepen the analysis on the role of 25(OH)vitamin D in COVID-19.
Our findings are consistent with an English prospective study including 134 COVID-19 inpatients, which showed a correlation between 25(OH)vitamin D levels and ICU admission rates, but not with mortality or the biochemical markers of disease severity [5].
On the other hand, our results are in contrast with those reported by a prospective German study of 185 COVID-19 cases, of whom 93 hospitalized, which showed a correlation between 25(OH)vitamin D levels and disease severity [3]. Interestingly, our population consisted of severe and old patients with a prevalence of 25(OH)vitamin D deficiency more than triple that reported by the German colleagues (76.8% vs 22%, respectively) but similar to that detected by an Iranian registry of 235 patients (67.2%), which also showed a positive association of 25(OH)vitamin D with clinical outcomes [6]. Our findings are also inconsistent with another prospective Iranian study, which included 63 confirmed COVID-19 patients and showed a correlation between 25(OH)vitamin D levels and prognosis [7].
Our observed absence of a correlation between clinical outcomes and 25(OH)vitamin D levels may not be surprising, considering the heavy clinical impact of COVID-19 in old comorbid patients, particularly in the first months of the pandemic outbreak, when adequate treatment was almost a challenging bet. Our population indeed consisted of fragile high-risk patients, which may explain the high mortality registered. In this context, it appears very unlikely that a single nutrient deficiency would have been able to affect clinical outcomes.
On the other hand, promising results from a randomized pilot study on 76 patients, showed the potential efficacy of 25(OH)vitamin D supplementation in reducing the disease severity [8].
The unexpected correlation between higher vitamin D levels and mortality observed doesn't have an immediate and unambiguous explanation and it has certainly to be considered that, although our sample size was not really small, it was not large enough to be conclusive about mortality.
Current models of COVID-19 pathogenesis propose distinct crucial immune stages, from the early activation of the innate immune system to a deleterious hyperinflammatory response characterized by excessive macrophages activation [9].
In this perspective, the role of 25(OH)vitamin D in the initial innate immune response and the ineffective control by infected and exhausted T lymphocytes in the adaptive response could play a role in the up-regulation of the monocyte-macrophagic system, with important pro-inflammatory manifestations observed in the critically ill patients [9].
Furthermore, the recent hypothesis that macrophages expressing ACE2 may serve as Trojan horses for SARS-CoV, causing a continuous loop [9] enhanced by the 25(OH)vitamin D -mediated up-regulated expression of ACE2 [1], may partially explain our unexpected observation.
Nonetheless, another hypothesis consists in the fact that, based on cross-sectional studies, the association between low 25(OH)vitamin D levels and the disease could be also an example of reversed causality. Severe illnesses characterized by robust inflammatory responses, like COVID-19, may be responsible for a reduction in vitamin binding proteins (due to shorter half-life) and an increase in total body water and volume distribution volume, which, in turn, results in the dilution of solutes, thus low serum concentrations [2]. Interestingly, in our population median C-reactive protein (CRP) levels were higher in patients with 25(OH)vitamin D < 20 ng/mL, but the difference was not statistically significant (Table 1). Although this observation could be biased by the limited sample size, this may make our findings more intriguing, as it has long been known that as soon as CRP increases >8 mg/dl, nearly all blood determinations return 25(OH)vitamin D values < 20 ng/ml, without any real evidence of deficiency [10].
While apparently not confirming a correlation between the degree of 25(OH)vitamin D deficiency and clinical outcomes in high-risk COVID-19 patients, the high prevalence of hypovitaminosis D observed doesn't exclude the recent hypothesized protective role of vitamin D in COVID-19 primary prevention [1], which has been shown for other acute respiratory tract infections [2].
5. Conclusions
Very low 25(OH)vitamin D levels were highly prevalent and suggestive of deficiency among our hospitalized severe COVID-19 patients, but low 25(OH)Vitamin D levels were not associated with outcome variables. Whether 25(OH)vitamin D adequacy may prevent COVID-19 infection or influence clinical outcomes needs to be assessed by adequately sized and designed population-based studies and intervention trials, respectively, which could be very relevant in the unfortunate occurrence of new outbreaks.
Funding/support
None.
Author contributions
Caccialanza, Cereda and Bogliolo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
Caccialanza is chief investigators and act as guarantors for this work.
Concept and design: Caccialanza, Cereda, Bogliolo, Klersy.
Acquisition, analysis, or interpretation of data: Caccialanza, Cereda, Bogliolo, Klersy, Lobascio, Masi, Crotti, De Stefano, Mariani, Ludovisi, Muggia, Croce, Barteselli, Mambella, Di Terlizzi, Belliato.
Drafting of the manuscript: Caccialanza, Cereda, Bogliolo, Klersy.
Critical revision of the manuscript for important intellectual content: Montecucco, Di Sabatino, Corsico, Perlini, Bruno.
Statistical analysis: Klersy.
Administrative, technical, or material support: Ferrari.
Supervision: Caccialanza.
Other - Research facilitator responsible for data collection from participants: Mariani, Ludovisi, Muggia, Croce, Barteselli, Mambella, Di Terlizzi.
Other - Trial management: Cereda, Bogliolo, Masi, Crotti, De Stefano, Lobascio.
Additional contributions
We are grateful to all the employees of the Fondazione IRCCS Policlinico San Matteo for their courageous efforts in struggling against the clinical and social COVID-19 emergency.
Conflict of interest
None of the authors have conflicts of interest to disclose.
Contributor Information
NUTRI-COVID19 IRCCS San Matteo Pavia Collaborative Group:
Mirko Belliato, Serena Ludovisi, Francesca Mariani, Alessandra Ferrari, Valeria Musella, Chiara Muggia, Gabriele Croce, Chiara Barteselli, Jacopo Mambella, and Francesco Di Terlizzi
Appendix A.
NUTRI-COVID19 IRCCS San Matteo Pavia Collaborative Group information: NUTRI-COVID19 IRCCS San Matteo Pavia Collaborative Group investigators included the article authors and the following individuals: Mirko Belliato, Serena Ludovisi, Francesca Mariani, Alessandra Ferrari, Valeria Musella, Chiara Muggia, Gabriele Croce, Chiara Barteselli, Jacopo Mambella, Francesco Di Terlizzi.
References
- 1.Rhodes J.M., Subramanian S., Laird E., Griffin G., Kenny R.A. Perspective: vitamin D deficiency and COVID-19 severity - plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2020 Jul 2 doi: 10.1111/joim.13149. Epub ahead of print. PMID: 32613681; PMCID: PMC7361294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reijven P.L.M., Soeters P.B. Vitamin D: a magic bullet or a myth? Clin Nutr. 2020;39(9):2663–2674. doi: 10.1016/j.clnu.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):E2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020;32(10):2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panagiotou G., Tee S.A., Ihsan Y., Athar W., Marchitelli G., Kelly D., et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf) 2020 Jul 3 doi: 10.1111/cen.14276. Epub ahead of print. PMID: 32621392; PMCID: PMC7361912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montazeri M., Nasiri M., Shirvani A., Holick M.F. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PloS One. 2020;15(9) doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Mardani R., Alamdary A., Mousavi Nasab S.D., Gholami R., Ahmadi N., Gholami A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289:198148. doi: 10.1016/j.virusres.2020.198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan A., Talwar D., McMillan D.C., Stefanowicz F., O'Reilly D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95:64–71. doi: 10.3945/ajcn.111.023812. [DOI] [PubMed] [Google Scholar]