Abstract
Since December 2019, an outbreak of coronavirus disease 2019 (COVID-19) which initially occurred in the city of Wuhan, located in China's Hubei province, spread around the world and on March 11, 2020, the World Health Organization declared the new Coronavirus disease 2019 (COVID-19) as a pandemic. The presence of comorbidities (eg, cardiovascular disease, obesity), Sepsis Induced Coagulopathy score >4, elevation of D-dimer (>6 times the normal value), C-reactive protein, troponins and other disseminated intravascular coagulation markers; is associated to a worse prognosis in hospitalized patients with severe COVD-19, reaching a hospital mortality of 42%. Initial anticoagulant treatment with low molecular weight heparin has been shown to reduce mortality by 48% at 7 days and 37% at 28 days and achieve a significant improvement in the arterial oxygen pressure/inspired fraction of O2 (PaO2/FiO2) by mitigating the formation of microthrombi and associated pulmonary coagulopathy.
Epidemiology
Since December 2019, an outbreak of coronavirus disease 2019 (COVID-19) which initially occurred in the city of Wuhan, located in China's Hubei province, spread around the world. On February 11, 2020, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses officially named the new coronavirus that causes COVID-19 as "severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)," and on March 11, 2020, the World Health Organization declared the new Coronavirus disease 2019 (COVID-19) as a pandemic.1, 2, 3, 4, 5, 6, 7, 8
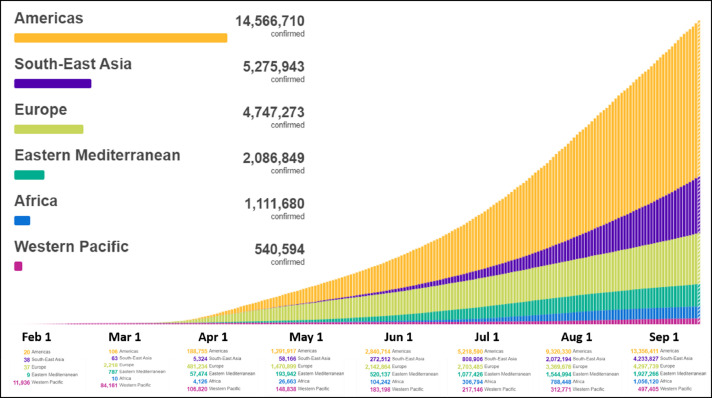
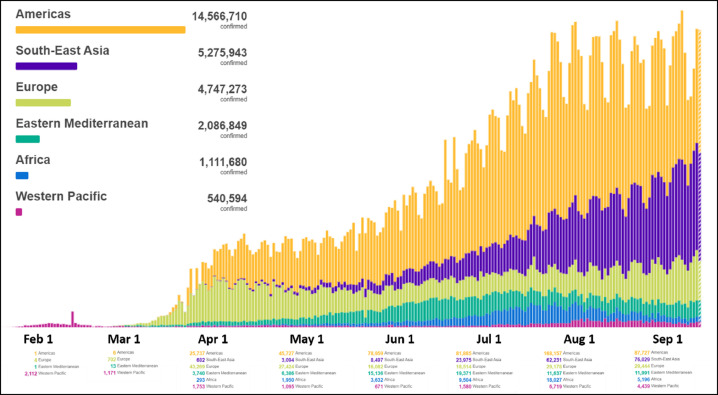
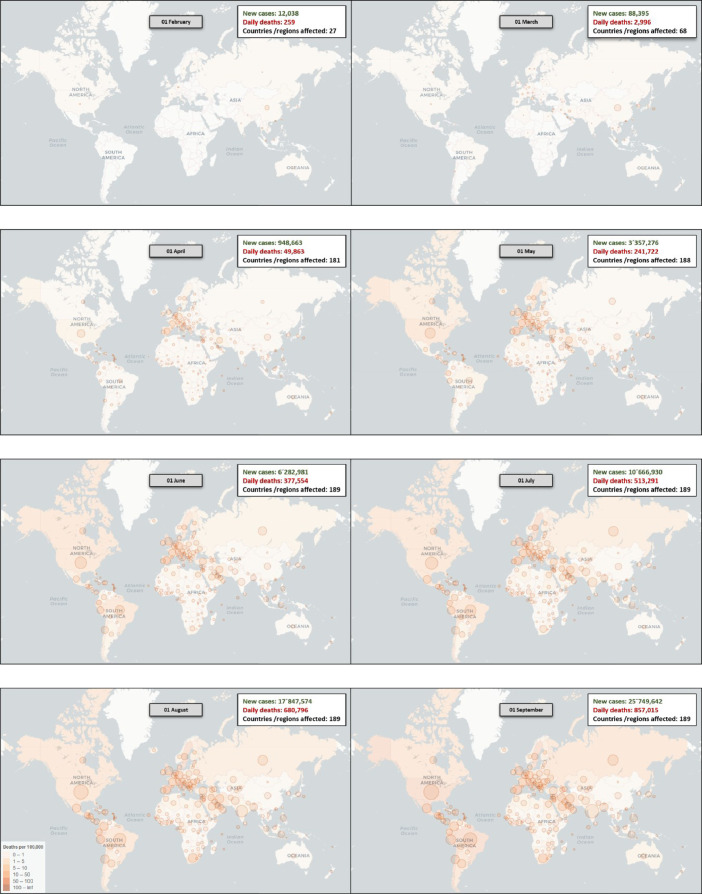
This pandemic had its epicenter in the Asian continent (China), which later moved to the European continent (mainly Italy and Spain), and currently to the American continent, initially in the United States and now in United States and Latin America (mainly Mexico and Brazil) (Graphs 1 -3 ).
Graph 2.
Cumulative cases until September/2020. Comparison by regions. Image adapted from: https://covid19.who.int/.
Graph 1.
Daily cases until September/2020. Comparison by regions. Image adapted from: https://covid19.who.int/.
Graph 3.
Worldwide change of the Epicenter of the pandemic: Starting in Asia, then Europe and, currently, America (North America and Latin America). Image adapted from: https://vac-lshtm.shinyapps.io/ncov_tracker/.
Coronavirus-19 Infection
The “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” is a single stranded RNA coronavirus that enters the human cell mainly through binding to angiotensin converting enzyme 2 (ACE 2), expressed in increased amounts in the alveolar cell of the lung, cardiac myocytes, vascular endothelium, and other cells. SARS-CoV-2 is transmitted mainly after the viral particles are inhaled and enters the respiratory tract. This virus can survive for up to 24-72 hours on surfaces that allow its transmission.1 , 6
This respiratory viral infection produces the COVID-19, which is generally asymptomatic or with mild symptoms including fever, cough, fatigue, dyspnea, diarrhea, headache, and myalgia (up to 81.4% of patients). Severe cases are characterized by respiratory rate >30 bpm, arterial oxygen saturation <93% at rest, PaO2/FiO2 <300 mm Hg and/or infiltrates in >50% of lung fields in 24-48 hours (up to 13.9% of patients) and can progress to critically ill patients (up to 4.7% of patients), presenting rapid deterioration and development of acute respiratory distress syndrome, septic shock, metabolic acidosis and coagulopathy, including disseminated intravascular coagulation (DIC) and cytokine storm.2 , 4 , 7, 8, 9, 10, 11, 12, 13
These clinical manifestations, as well as the imaging and paraclinical alterations, vary as the pandemic evolves worldwide, and they also depend on the severity of the infection. A registry of 1099 laboratory-confirmed COVID-19 patients in 552 institutions in 30 provinces of China described some of these most frequent and relevant findings, observed in the first two months of this pandemic (Table 1 ).8
TABLE 1.
Most frequent clinical, imaging and paraclinical findings
| All (1.099) | Nonsevere (926) | Severe (173) | |
|---|---|---|---|
| Most frequent symptoms | |||
| Cough | 745 (67.8%) | 623 (67.3%) | 122 (70.5%) |
| Fever on admission (>37.5°C) | 473/1081 (43.8%) | 391/910 (43%) | 82/171 (48%) |
| Fatigue or tiredness | 419 (38.1%) | 350 (37.8%) | 69 (39.9%) |
| Sputum production | 370 (33.7%) | 309 (33.4%) | 61 (35.3%) |
| Shortness of breath | 205 (18.7%) | 140 (15.1%) | 65 (37.6%) |
| Myalgia or arthralgia | 164 (14.9%) | 134 (14.5%) | 30 (17.3%) |
| Odynophagia | 153 (13.9%) | 130 (14.0%) | 23 (13.3%) |
| Headache | 150 (13.6%) | 124 (13.4%) | 26 (15.0%) |
| Chill | 126 (11.5%) | 100 (10.8%) | 26 (15.0%) |
| Imaging findings | |||
| Chest X-ray changes | 162/274 (59.1%) | 116/214 (54.2%) | 46/60 (76.7%) |
| Chest CT alterations | 840/975 (86.2%) | 682/808 (84.4%) | 158/167 (94.6%) |
| Laboratory findings | |||
| White blood cell count <4,000 mmᶾ | 330/978 (33.7%) | 228/811 (28.1%) | 102/167 (61.1%) |
| Lymphocyte count <1,500 mmᶾ | 731/879 (83.2%) | 584/726 (80.4%) | 147/153 (96.1%) |
| Platelet count <150,000 mmᶾ | 315/869 (36.2%) | 225/713 (31.6%) | 90/156 (57.7%) |
| C-reactive protein ≥10 mg / L | 481/793 (60.7%) | 371/658 (56.4%) | 110/135 (81.5%) |
| D-dimer ≥0.5 mg/L | 260/560 (46.4%) | 195/451 (43.2%) | 65/109 (59.6%) |
The most consistent hemostatic alterations with COVID-19 are thrombocytopenia and elevation of D-dimer, which are associated with a higher requirement for mechanical ventilation, admission to intensive care, and death. It has been described that older patients and those with comorbidities have a higher risk of in-hospital mortality, and in these 2 groups of patients there are also higher levels of D-dimer. Taking into account the clinical implications of the elevated D-dimer value or the marked elevations during follow-up (3-4 times), hospital management can be considered in this setting in the absence of other severe symptoms since this indicates an increase in thrombin generation and a greater risk of complications (Tables 2 and 3 ).14, 15, 16
TABLE 2.
Conditions associated with hospital mortality
| Total (n = 191) | Death (n = 54) | Alive (n = 137) | P value | OR (95% CI) |
||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Demographic conditions | ||||||
| Age,years. Median (IQR) | 56 (46 - 67) | 69 (63 – 76) | 52 (45 – 58) | <0.0001 | 1.14 (1.09-1.18) P < 0.0001 | 1.10 (1.03-1.17) P = 0.0043 |
| Arterial hypertension. n (%) | 58 (30%) | 26 (48%) | 32 (23%) | 0.0008 | 3.05 (1.57-5.92) P = 0.001 | |
| Diabetes mellitus. n (%) | 36 (19%) | 17 (31%) | 19 (14%) | 0.0051 | 2.85 (1.35-6.05) P = 0.0062 | |
| Coronary heart disease. n (%) | 15 (8%) | 13 (24%) | 2 (1%) | <0.0001 | 21.40 (4.64-98.76) P < 0.0001 | 2.14 (0.26-17.79) P =0.48 |
| COPD. n (%) | 6 (3%) | 4 (7%) | 2 (1%) | 0.047 | ||
| Respiratory rate> 24 bpm. n (%) | 56 (29%) | 34 (63%) | 22 (16%) | <0.0001 | 8.89 (4.34-18.19) P < 0·0001 | |
| SOFA score. Median (IQR) | 2 (1 - 4) | 4.5 (4 – 6) | 1 (1 – 2) | <0.0001 | 6.14 (3.48-10.85) P < 0·0001 | |
| Laboratory findings | ||||||
| Leukocytes, >10,000 mm3. n (%) | 40 (21%) | 25 (46%) | 15 (11%) | <0·0001 | 6.60 (3.02-14.41) P < 0·0001 | |
| Lymphocytes, <800 mm3. n (%) | 77 (40%) | 41 (76%) | 36 (26%) | <0.0001 | 0.02 (0.01-0.08) P < 0·0001 | 0.19 (0.02-1.62) p = 0·13 |
| Anemia. n (%) | 29 (15%) | 14 (26%) | 15 (11%) | 0.0094 | ||
| Platelets, <100,000 mm3. n (%) | 13 (7%) | 11 (20%) | 2 (1%) | <0.0001 | ||
| Albumin, mg/dL. n (%) | 3.2 (2.9-3.5) | 2.91 (2.65-3.13) | 3.36 (3.06-3.64) | <0.0001 | ||
| ALT >40, U/L. n/N (%) | 59/189 (31%) | 26 (48%) | 33/135 (24%) | 0.0015 | 2.87 (1.48-5.57) P = 0·0018 | |
| LDH >245, U/L. n/N (%) | 123/184 (67%) | 53 (98%) | 70/130 (54%) | <0.0001 | 45.43 (6.10-338.44) P = 0·0002 | |
| Troponin I HS >28, pg/mL. n/N (%) | 24/145 (17%) | 23/50 (46%) | 1/95 (1%) | <0.0001 | 80.07 (10.34-620.36) P =< 0·0001 | |
| D-dimer >1, μg/mL. n/N (%) | 72/172 (42%) | 44 (81%) | 28/118 (24%) | <0·0001 | 20.04 (6.52-61.56) P =< 0·0001 | 18.42 (2.64-128.55) P = 0.0033 |
| Prothrombin time, ≥16. n/N (%) | 11/182 (6%) | 7 (13%) | 4/128 (3%) | 0·0004 | 4.62 (1·29-16.50) P = 0.019 | |
| Ferritin, ug/L (>300). n/N (%) | 102/128 (80%) | 44/46 (96%) | 58/82 (71%) | 0·0008 | 9.10 (2.04-40.58) P = 0.0038 | |
| Interventions | ||||||
| Steroid use. n (%) | 57 (30%) | 26 (48%) | 31 (23%) | 0.0005 | ||
| Immunoglobulin IV. n (%) | 46 (24%) | 36 (67%) | 10 (7%) | <0.0001 | ||
| Oxygen by high flow nasal cannula. n (%) | 41 (21%) | 33 (61%) | 8 (6%) | <0.0001 | ||
| Noninvasive MV. n (%) | 26 (14%) | 24 (44%) | 2 (1%) | <0.0001 | ||
| Invasive VM. n (%) | 31 (17%) | 31 (57%) | 1 (1%) | <0.0001 | ||
| ECMO. n (%) | 3 (2%) | 3 (6%) | 0 | 0.0054 | ||
| Renal replacement therapy. n (%) | 10 (5%) | 10 (19%) | 0 | <0.0001 | ||
IV, intravenous; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation system; SOFA, sequential organ failure assessment.
TABLE 3.
Poor prognosis indicators
| Parameter | Value |
|---|---|
| Age | 52 years (alive) vs 69 years (dead) |
| SOFA score | >2.0 |
| D-dimer | >0.5 mg/L |
| Thrombocytopenia | <100,000 |
| Prothrombin time | Increase >3 seconds |
| Activated partial thromboplastin time | Increase >5 seconds |
| Fibrinogen | <1.5 gm/l |
| Sepsis-Induced Coagulopathy (SIC) score | ≥4 |
| Respiratory frequency | >24 bpm |
| Heart rate | >125 bpm |
Coagulopathy
In hospitalized patients for suspected or confirmed COVID-19, a coagulation profile should be performed, including D-dimer, partial thromboplastin, partial thromboplastintime, platelet count, and fibrinogen. Alterations in these parameters can occur 7-11 days after the onset of symptoms or 4-10 days after hospitalization. Repeating these coagulopathy parameters (D-dimer, prothrombin time, and platelet count) are recommended in patients with severe COVID-19, at least every 2-3 days.6 , 15
The combination of thrombocytopenia, prolonged PT, and elevated D-dimer suggests DIC, however, its presentation is different from the presentation seen in sepsis, where thrombocytopenia is much more profound and the elevation of D-dimer does not reach the values observed in COVID-19 cases. Current evidence suggests COVID-19 associated coagulopathy is a combination of low-grade DIC and pulmonary thrombotic microangiopathy, which could have a significant impact on organ dysfunction in most patients with severe disease.14
The presence of coagulopathy as part of the systemic inflammatory response syndrome is a common feature of severe COVID-19. Approximately 20%-50% of hospitalized patients with COVID-19 have hematologic changes in coagulation tests (elevated D-dimer, prolonged PT, thrombocytopenia, and/or low fibrinogen levels). This condition is characterized by more thrombotic than hemorrhagic events that are associated with coagulopathy (specifically venous thromboembolism [VTE]). On the other hand, endothelial dysfunction results in high levels of D-dimer, thrombin and fibrin degradation products, thrombocytopenia and prolonged clotting times, which leads to hypoxia and pulmonary congestion mediated by thrombosis and microvascular occlusion, in addition to thrombosis of central lines and catheters and vascular occlusive events (cerebrovascular events, limb ischemia, etc) that generally occur in the intensive care units.6 , 10 , 15 , 17, 18, 19
Fibrin and thrombin deposition occurs mainly in the pulmonary microvasculature, being a factor that contributes to acute respiratory distress syndrome and coagulopathy in patients who die from COVID-19. Furthermore, the hypoxia that occurs in severe COVID-19 can aggravate thrombosis not only by increasing the viscosity of the blood, but also through the hypoxia-inducible transcription factor-dependent signaling pathway.10 , 17 , 20
Similar to the endothelial dysfunction of sepsis induced coagulopathy (SIC), in which there is excessive thrombin generation and impaired fibrinolysis, there is a type of endotheliopathy that appears to contribute to the pathophysiology of microcirculatory changes in SARS-CoV-2 infection. The receptor for viral adhesion is an angiotensin-converting enzyme-2 receptor on endothelial cells, and viral replication causes inflammatory cell infiltration, endothelial apoptosis, and microvascular prothrombotic events. Viral inclusions within endothelial cells and mononuclear and polymorphonuclear cell infiltration have been observed, with evidence of endothelial apoptosis in postmortem analysis of SARS-Cov-2 infection. As a result of this, microcirculatory dysfunction contributes to the clinical sequelae of COVID-19 patients.6 , 10 Other abnormalities that may be relevant in the context of coagulopathy are decreased fibrinogen, elevated Lactate dehydrogenase (LDH), and, in some patients, markedly elevated serum ferritin values.20
Another important characteristic of COVID-19 infection is the procoagulant response in its acute phase, where acute phase reactants (such as Factor VIII, Von Willebrand Factor, and fibrinogen) are associated with an increased risk of thrombosis directly related to elevated levels of fibrinogen. In severe stages of the disease, there is an increase in inflammatory cytokines (tumor necrosis factor and interleukins, including interleukin 1 and interleukin 6). IL-6 induces expression of tissue factor in macrophages, which initiates the activation of coagulation and generation of thrombin. Tumor necrosis factor and IL-1 are the main mediators of the suppression of the endogenous coagulation cascade. In a group of severely compromised COVID-10 patients, a cytokine storm characterized by high concentrations of proinflammatory cytokines and chemokines may be found.12 , 14
The International Society of Thrombosis and Haemostasis proposed a new category to identify an early stage of DIC associated with sepsis, which is called SIC. This score can be applied to COVID-19 patients, and those who meet these criteria benefit from anticoagulant management (Table 4 ).7 , 10
TABLE 4.
ISTH score - Sepsis Induced Coagulopathy (SIC)
| ITEM | SCORE | VALUE |
|---|---|---|
| Platelet count (× mmᶾ) | 1 | 100.000-150.000 |
| 2 | <100.000 | |
| PT - INR | 1 | 1.2-1.4 |
| 2 | >1.4 | |
| SOFA score | 1 | 1 |
| 2 | ≥2 | |
| ≥ 4 |
Up to 71.4% of patients who die from COVID-19 have DIC, while it occurs in only 0.6% in those who survive. The main alteration of this coagulopathy is the marked elevation of D-dimer without a drop in platelets or a prolongation of clotting times, which suggests a process of generation of thrombin and local rather than systemic fibrinolysis. D-dimer value >2.0 ug/mL at admission or its increase during hospitalization (up to 3-4 times) have been associated with higher hospital mortality.18 , 20, 21, 22
The worsening of laboratory parameters related to coagulation indicates progression in the severity of COVID-19 infection and predicts the need for greater and more aggressive intensive care, while the improvement of these parameters, together with the improvement or clinical stability suggests an adequate evolution.18
VTE
COVID-19 infection can predispose to VTE or arterial due to the presence of increased inflammatory response, hypoxia, immobilization, and DIC.7 , 10 , 21
The risk of developing VTE in critically ill patients is higher in the presence of COVID-19. In addition to hemostatic alterations; immobility, systemic inflammatory status, mechanical ventilation, and central catheters increase the risk of thromboembolic events, while nutritional and hepatic alterations vary the production of coagulation factors.1 There are several studies that support the increased incidence of VTE in COVID-19 patients and their risk factors,13 , 21 , 23, 24, 25 (Table 5 ). Other study found that the proportion of patients with VTE was higher in the intensive care unit (ICU) patients (47%; 95% confidence interval, 36-58) than in the general ward patients (3.3%; 95% confidence interval, 1.3-8.1) (Table 6 ); the risk factors for VTE that were identified include ICU hospitalization, higher leukocyte count, higher neutrophil/lymphocyte ratio, and higher D-dimer value.25
TABLE 5.
Venous thromboembolism incidence studies for COVID-19 patients
| Author, year | n | Outcome | Tests | Treatment | Findings |
|---|---|---|---|---|---|
| Cui S et al, 202023 | 81 | Incidence of VTE in ICU |
-rTR-PCR for SARS-COV-2 -CT -LL venous Doppler ultrasound -Clinical examination -Laboratory tests |
-Antiviral -Supportive -None -Thromboprophylaxis |
-20/81 (25%) VTE -8/81 (10%) died -D-dimer cut-off point of 1.5 ug/L for VTE with a S: 85%, E:88.6%, PPV: 70.8% and NPV: 94.7% |
| Zhang L et al, 202024 | 143 | Incidence of DVT in hospitalized |
-LL venous Doppler ultrasound -Ecocardiography -Laboratory test -CT -Prediction scores for risk of VTE |
-Antiviral -Antibiotic -Glucocorticoid -Antihypertensive -Immunoglobulin -Thromboprophylaxis |
-66/143 (46%) DVT. -23/66 (34.8%)proximal DVT -43/66 (65.2%) distal DVT -CURB-65 score 3 to 5, Padua score ≥4 and D-dimer >1.0 ug/mL for DVT screening with S: 88.52% and E: 61.43%. |
| Middeldrop S et al, 202025 | 198 | Incidence of VTE in hospitalized patients |
-rTR-PCR for SARS-COV-2 -CT -LL venous Doppler ultrasound -Laboratory test |
-Thromboprophylaxis -Anticoagulation |
-39/198 (20%) VTE -14/198 (7.1%) proximal DVT -11/198 (5.6%) distal DVT -13/198 (6.6%) PTE with/without DVT |
| Klok FA et al, 202021 |
184 | Incidence of the composite outcome of symptomatic acute PTE, DVT, ischemic stroke, myocardial infarction or systemic arterial embolism in ICU |
-Laboratory test -LL venous Doppler ultrasound -CT angiogram |
Thromboprophylaxis (nadroparin) |
-31% cumulative incidence of composite outcome -27% cumulative incidence for VTE -3.7% cumulative incidence for arterial thrombotic events |
| Lodigiani C et al, 202013 | 388 | Rate of venous and arterial thrombo embolic complication in hospitalized patients |
-Laboratory test -LL venous Doppler ultrasound -CT angiogram -ISTH score |
Thromboprophylaxis |
-28/362 (7.7%) at least one thromboembolic complication -16/362 (4.4%) VTE -9/362 (2.5%) ischemic stroke -4/362 (1.1%) acute coronary syndrome |
TABLE 6.
Cumulative incidence of VTE24
| Total VTE | VTE in ICU | VTE in general ward | |||
|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | Asymptomatic | Symptomatic | Asymptomatic and symptomatic | |
| 7 days | 16% (95% CI, 10-22) | 10% (95% CI, 5.8-16) | 26% (95% CI, 17-37) | 15% (95% CI, 8.0-24) | (95% CI, 1.4-15) |
| 14 days | 33% (95% CI, 23-43) | 21% (95% CI, 14-30) | 47% (95% CI, 34-58) | 28% (95% CI, 18-39) | 9.2% (95% CI, 2.6-21) |
| 21 days | 42% (95% CI, 30-54) | 25% (95% CI, 16-36) | 59% (95% CI, 42-72) | 34% (95% CI, 21-46) | 9.2% (2.6-21) |
In patients with sudden deterioration in oxygen saturation, respiratory distress, low blood pressure, or right ventricular (RV) dysfunction, the possibility of pulmonary embolism (PE) should be considered. Diagnosis can be difficult as COVID-19 patients may have an elevated D-dimer value even in the absence of VTE. Imaging studies cannot be done routinely due to the risk of transmission of the infection, the limitations to transfer and the clinical instability that the patient could present at any given time. In these cases, and taking into account the value of D-dimer, the use of anticoagulants at therapeutic, intermediate doses or as prophylaxis could be considered. The use of tests at the patient side, such as compression ultrasonography for the diagnosis of deep vein thrombosis and echocardiography to evaluate RV strain associated with PE, can be difficult in unstable, prone, or critically ill patients; also, without having sufficient specificity and sensitivity to diagnose VTE, in certain clinical scenarios they can increase the index of clinical suspicion, and its use may be considered.1 , 25
Thromboprophylaxis
Hospitalized patients with COVID-19 present similar intrinsic and extrinsic risk factors for VTE to the rest of the hospitalized population, such as advanced age, obesity, immobilization, neurological events, cancer, ICU management, previous thromboembolic events, or thrombophilia, however, prophylactic management in this population is currently a challenge (Table 7 ).25
TABLE 7.
Recommendations for thromboprophylaxis and/or anticoagulation
| COVID-19 positive | Coagulation tests | Conventional thromboprophylaxis | Thromboprophylaxis in scalating doses | Anticoagulation |
|---|---|---|---|---|
| Ambulatory | Consider | |||
| Hospitalized | X | |||
| General ward | X | X | ||
| ICU | X | X | ||
| VTE confirmed | X | X | ||
| Confirmed PE | X | X | ||
| ARDS | X | X |
Pharmacological thromboprophylaxis should then be considered in all hospitalized COVID-19 patients who are immobilized or severely ill, unless there are contraindications (such as active bleeding or severe thrombocytopenia). Different scales can be used to assess this hospital risk (Padua, Caprini, IMPROVE). The dose should be adjusted according to renal function. Although drug selection should be guided by available institutional protocols, the World Health Organization recommends the use of unfractionated or low molecular weight heparins (LMWHs) and, if contraindicated, mechanical thromboprophylaxis should be considered. Pharmacological thromboprophylaxis is recommended once a day, since it reduces the risk of missing additional doses and is also associated with less exposure of health personnel for its administration. If LMWH is not available, unfractionated heparin can be considered, keeping in mind that this requires more frequent injections and, therefore, greater exposure of health personnel. Fondaparinux can also be considered, but there is no evidence that this molecule has the same anti-inflammatory properties as heparins. Patients with more severe infections may require higher doses of thromboprophylaxis due to their hypercoagulable state. The use of direct anticoagulants in thromboprophylaxis is not recommended in this context due to the possible drug interactions that may occur with the different drugs and therapies available and under investigation for the treatment of COVID-19.4 , 14 , 15 , 25
Some of the nonanticoagulant properties of LMWH include the potential for binding to inflammatory cytokines, inhibition of neutrophil chemotaxis and leukocyte migration, neutralization of positively charged complement factor C5a, and sequestration of acute phase proteins.12 , 26
Regarding the above, it is suggested that LMWH administered in the early stages of SARS-CoV2 infection can exert a positive effect not only in terms of preventing thrombosis but also reducing systemic and pulmonary inflammation and limiting viral invasion.7 , 13 Other nonanticoagulant actions of heparin include its antiviral role (experimental models), decreased collagen deposits and antiarrhythmic properties (animal models), as well as modulation of endothelial dysfunction, improvement of microvascular dysfunction, and mitigation of pulmonary coagulopathy.26 , 27
In patients who remain completely immobilized, there may be an additional benefit with intermittent pneumatic compression in addition to drug thromboprophylaxis. This therapy should also be considered if there is severe thrombocytopenia (platelets <25,000 to 50,000 × 10⁹/L)2 , 25 , 28 , 29
The use of extended ambulatory thromboprophylaxis (from 14 to 45 days) should be considered in patients at high risk of VTE, independent of COVID-19 infection, and that includes reduced mobility, previous thromboembolic events, comorbidities (eg, active cancer) and Elevated D-dimer (>2 times normal value). Thromboprophylaxis for patients who are quarantined for mild COVID-19, but with significant comorbidities, or patients without COVID-19 but who are functionally severely limited by quarantine is not recommended. These patients should be advised to remain active at home.1 , 2 , 25
Anticoagulation
The presence of comorbidities (eg, cardiovascular disease, obesity), SIC score> 4, elevation of D-dimer (>6 times the normal value), C-reactive protein, troponins and other DIC markers; is associated to a worse prognosis in hospitalized patients with severe COVD-19, reaching a hospital mortality of 42% (Table 7).25
In this population, initial anticoagulant treatment with LMWH has been shown to reduce mortality by 48% at 7 days and 37% at 28 days and achieve a significant improvement in the arterial oxygen pressure/inspired fraction of O2 (PaO2/FiO2) by mitigating the formation of microthrombi and associated pulmonary coagulopathy, also decreasing complementary inflammation.26 , 30 , 31
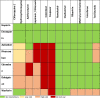
Anticoagulation should be considered if there is evidence of VTE or if the patient is anticoagulated, unless they have thrombocytopenia (<50,000 × mm × or active bleeding). The selected drug depends on kidney and liver function, platelet count, and gastrointestinal function. Parenteral anticoagulation is recommended in critically ill patients, as it can be temporarily suspended and has no interactions with drugs considered for the treatment of COVID-19. Given the exposure of health personnel with the use of unfractionated heparin by taking paraclinics and dose adjustment, the use of LMWH is preferred in these patients. The benefits of direct oral anticoagulants include no need for routine monitoring and easy outpatient management, however, potential risks may include their use in the presence of clinical deterioration and the lack of availability of a reversal agent in all institutions. In patients who are going to be discharged, the use of direct oral anticoagulants and LMWH should be preferred, avoiding frequent tests for INRs. The potential for drug interactions with potential treatments for COVID-19 should always be evaluated1 , 5 , 7 , 14 , 18 (Table 8 ).
TABLE 8.
 |
A 30%-50% decrease in platelet count from the start of heparin treatment (4-14 days) should suggest heparin-induced thrombocytopenia. The foregoing makes it necessary to suspend this anticoagulant treatment, and may explain some cases of limb ischemia that have been observed in cases of COVID-19.15
Conclusions
There are different ways in which the COVID-19 pandemic can affect the prevention and treatment of thrombotic or thromboembolic diseases. First, the direct effect of COVID-19 or the indirect effect related to the cytokine storm that precipitates the onset of the systemic inflammatory response syndrome and predisposes to the development of thrombotic events; second, the interventions available to treat COVID-19 (eg, lopinavir/ritonavir, remdesivir, bevacizumab, tocilizumab, sarilumab, fingolimod, chloroquine/hydroxychloroquine, interferon, azithromycin) may have drug interactions with antiplatelets and/or anticoagulants; and third, the pandemic, due to the redistribution of resources and social distancing recommendations, can adversely affect the care of patients without COVID-19 but who present thrombotic events and the fear of acquiring COVID-19 or presenting complications leads to not receiving or suspending the anticoagulant treatment.1
The protocols for thromboprophylaxis, anticoagulation, and additional considerations for the management of coagulopathy and bleeding should be implemented in each institution following the most current national and international recommendations.
Footnotes
The authors report no conflict of interest.
References
- 1.Bikdeli B, Madhavan MV, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccia M, Aronne L, Celia B. COVID-19 and coagulative axis: review of emerging aspects in a novel disease. Monaldi Arch Chest Dis. 2020;90:271–276. doi: 10.4081/monaldi.2020.1300. [DOI] [PubMed] [Google Scholar]
- 3.Song JC, Wang G, Zhang W. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7:19. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2. 2020; 1–21
- 5.Günertem E, Akay T, Aliyev A. Treatment and prophylaxis strategies for deep vein thrombosis during COVID-19 outbreak. Turk J Vasc Surg. 2020;29:203–207. doi: 10.9739/tjvs.2020.734. [DOI] [Google Scholar]
- 6.Connors J, Levy J. COVID-19 and its implications for thrombosis and anticoagulation. Blood. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivas D, Roldán V, Esteve-Pastor MA. Recomendaciones sobre el tratamiento antitrombótico durante la pandemia COVID-19. Posicionamiento del Grupo de Trabajo de Trombosis Cardiovascular de la Sociedad Española de Cardiología. Rev Esp Cardiol. 2020;73:749–757. doi: 10.1016/j.recesp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Bai H, Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 in patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.China CDC The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 12.Marietta M, Ageno W, Artoni A. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thachil J, Tang N, Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Hajizadeh N, Moore EE. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hematology [internet]. Washington, DC. US; 2020. COVID-19 resources: COVID-19 and Coagulopathy: Frequently Asked Questions. 2020 Apr. [Cited: 2020 sept 27]. Available from : https://www.hematology.org/covid-19/covid-19-and-coagulopathy.
- 19.Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192:E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covid Treatment Group. Thromboprophylaxis and anticoagulation in COVID-19 infection. Imperial College Healthcare – NHS. V 0.1 08.04.2020
- 21.Klok FA, Kruip MJHA, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Yan X, Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middeldorp S, Coppens M, van Haaps TF. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spyropoulos AC, Levy JH, Ageno W. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18:1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menezes-Rodrigues FS, Padrão Tavares JG, Pires de Oliveira M. Anticoagulant and antiarrhythmic effects of heparin in the treatment of COVID-19 patients. J Thromb Haemost. 2020;18:2073–2075. doi: 10.1111/jth.14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NICE guidelines venous thromboembolism: reducing the risk for patients in hospital. Available at: http://www.nice.org.uk/guidance/cg92
- 29.Schünemann HJ, Cushman M, Burnett AE. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Li Y, Yang B. Low-molecular-weight heparin treatment for acute lung injury / acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2018;11:414–422. [Google Scholar]
- 31.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]