Summary:
Reports of women with breast implants who suffer a wide variety of systemic symptoms have become more and more prevalent over the past several years. This entity has become known as breast implant illness in conventional news and social media outlets but has vague and nonspecific diagnostic criteria. As a result, the phenomenon is difficult to both identify and treat. The reported patient is a 76-year-old woman who underwent breast reconstruction with a latissimus dorsi flap and textured silicone implant 20 years before the onset of symptoms, which included debilitating joint pain. She had previously maintained an active lifestyle, but symptoms progressed to a point where she was unable to perform basic tasks. A full rheumatologic and connective tissue workup was done, but other than elevated markers of inflammation, it showed no diagnosable disease entity. After a lengthy discussion with her primary care physician and plastic surgeon, she decided to undergo removal of the implant. Following explantation, her symptoms quickly improved, and within several weeks, she was able to return to her active lifestyle.
INTRODUCTION
The safety of breast implants has been in question for decades, culminating in a moratorium on their use in cosmetic augmentation issued by the Food and Drug Administration (FDA) in January 1992. While this was lifted 14 years later due to a lack of evidence proving a link to systemic disease, there has been a recent resurgence in reports of some of the same symptoms, both in academic journals and in mainstream news outlets.1–3 The term “breast implant illness” has become popular in news media and social media and describes a patient with silicone breast implants who experiences one or more of a variety of symptoms, including fatigue, headaches, joint aches, chronic pain, sleep disturbance, depression, and others.4 It is not an official medical diagnosis and does not carry any consistent laboratory or radiographic findings. The vagueness in diagnostic criteria, heterogeneity of symptoms, and lack of consistent objective data makes this entity difficult to diagnose, treat, and understand. In this article, we present a case of a patient who experienced symptoms consistent with breast implant illness, which improved after removal of her implant. We hope that sharing her case will not only contribute data to a growing body of literature on this topic but will also provide other practitioners with information that can guide management for patients with similar presentations.
CASE REPORT
The patient was a 76-year-old woman with a history of right breast infiltrating mucinous colloid carcinoma, who underwent a right simple mastectomy and immediate reconstruction with a latissimus dorsi flap and placement of a textured, moderate profile, silicone implant. Over the subsequent several years, she developed symptoms of capsular contracture—the implant began to ride higher on her chest and became firm. She opted not to undergo corrective surgery, and over the course of the following 20 years, her symptoms did not change. During this time, she maintained an active lifestyle, regularly playing tennis, cross training, and swimming. Her medical history is also significant for a thyroidectomy for a benign multinodular goiter with subsequent hypothyroidism, an appendectomy as a child, and several eye surgeries.
In 2018, she began to have right shoulder pain that progressively worsened and decreased her activity level. She was referred to an orthopedic surgeon who diagnosed right shoulder impingement that failed to improve with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and physical therapy. The pain continued to worsen and began to involve the right elbow and wrist, as well as the left shoulder and bilateral hips. A magnetic resonance imaging of the right shoulder showed evidence of adhesive capsulitis, and serial ultrasound-guided steroid injections to each shoulder provided transient symptom relief. Two to 3 months after the onset of symptoms, her hip pain had progressed to the point that she was unable to sit for >10–15 minutes at a time and wrist pain prevented her from performing basic functions such as opening a jar. Hip x-rays and magnetic resonance imaging of the spine failed to show any concerning findings.
The first rheumatologic evaluation was approximately 4 months after the onset of the initial symptoms. A full rheumatologic laboratory workup was done, revealing persistently elevated markers of inflammation but no positive results on various autoimmune assays. Of note, she also had elevated levels of both mercury and silicone. A presumed exclusionary diagnosis of polymyalgia rheumatica was made, and she was started on oral steroids, but only experienced mild improvement. During the subsequent months, she was intermittently on and off oral steroids as prescribed by her rheumatologist to treat her symptoms conservatively. During the months following initial evaluation of these symptoms, she continued physical therapy, without appreciable improvement. At this point, she was referred to plastic surgery for evaluation of the implant as a possible source for her pain and discomfort. On evaluation, the reconstructed breast had a grade 3 capsular contracture and no palpable lymphadenopathy. After a thorough discussion with the patient and her primary care doctor, the decision was made to pursue removal of the implant and capsulectomy. She was taking steroids for several weeks leading up to her surgery, so it was the recommendation of her rheumatologist to administer stress-dose steroids at the time of surgery and taper the dose postoperatively.
Upon opening the implant capsule, there was approximately 20 mL of thick brown fluid surrounding the implant, which was sent for culture, pathology, and cytology. The implant was grossly intact but had an oily film surrounding it, indicative of a possible silicone leak. The capsule was expectedly thickened and was removed in its entirety and sent for pathologic evaluation. The periprosthetic fluid was negative for CD30 (a cell membrane protein and tumor marker), and there was no abnormal T-cell population. The capsule comprised benign fibrous tissue.
Following removal of the implant, the patient had rapid improvement in her symptoms, which continued to improve over the subsequent months. She reported resolution of wrist and hip pain and significant improvement in the pain in her shoulders. At her 3-month follow-up, she had returned to activities such as swimming and pilates that she had been previously unable to do. She continued physical therapy during the postoperative period and underwent an additional steroid injection for residual shoulder pain, after which she noted that she was pain free for the first time since symptoms began a year earlier. Inflammatory markers, and blood levels of silicon and mercury, improved postoperatively (Figs. 1, 2).
Fig. 1.
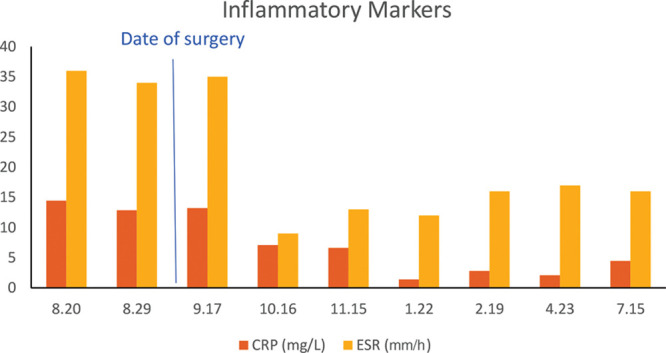
Inflammatory marker trend in perioperative and postoperative period.
Fig. 2.
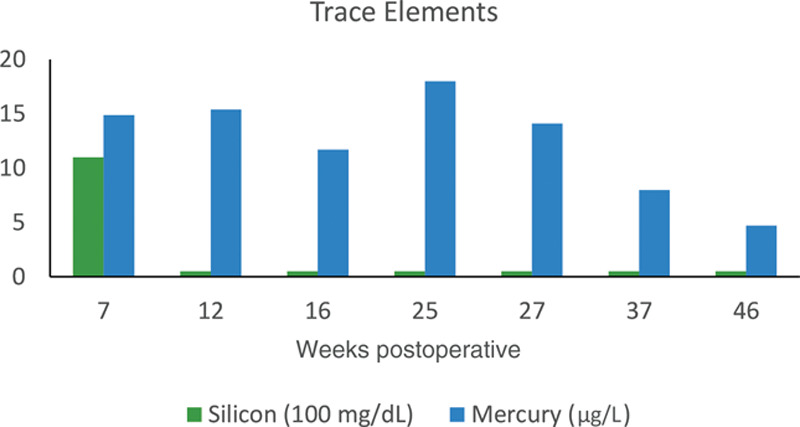
Trace element levels postoperatively.
DISCUSSION
Reports of patients with silicone breast implants who developed connective tissue disease or rheumatologic symptoms began to appear in the decade leading up to the FDA ban on the devices. However, the first large-scale investigation to find a positive correlation was a 1996 retrospective cohort study in which nearly 400,000 women health professionals self-reported presence or absence of breast implants and connective tissue diseases.5 Critics of the study point out the biases on self-reporting, and indeed a follow-up study by the same group revealed an approximately 25% confirmation rate of connective tissue disease on review of medical charts.6 In subsequent years, a number of cohort, case control, and cross-sectional studies were published reporting no link between silicone implants and connective tissue diseases. These studies culminated in the 1999 report by the Institute of Medicine, which concluded that there was a paucity of evidence to establish a correlation.7
There has been a recent resurgence of publicity on the topic following a 2019 multicentered, 10-year cohort study involving nearly 100,000 patients.8 The data for this study were born from the FDA stipulation following the lift on the silicone implant moratorium, where Allergan and Mentor manufacturers were required to conduct large post-approval studies to gather more conclusive long-term data. The authors report a significant association between silicone breast implants and Sjogren syndrome, scleroderma, and rheumatoid arthritis, as well as higher rates of melanoma and stillbirth in women with silicone implants. This report garnered tremendous media attention2,3 and triggered a swift backlash from both the scientific community9 and the FDA,10 which released a formal statement disagreeing with the authors’ conclusions due to “significant shortcomings with the study’s methodology and how the data is presented and concluded, including inconsistencies in the data and potential sources of bias.”11
While there is no scientifically proven association between silicone implants and autoimmune or connective tissue disease, it is worthwhile to continue to study this topic. In this report, we present a case of a patient who would have escaped detection of a clinical problem in the majority of the above-mentioned investigations. She had symptoms that prompted an extensive rheumatologic workup but never had the clinical picture or serologic findings that would have met a distinct diagnostic entity. Furthermore, this ambiguity makes it difficult to understand the true mechanism of the role that the implant has played in her clinical picture. We present this case of a patient whose clinical picture improved dramatically after removal of her silicone breast implant, in hopes that our experience will aid the scientific community in better understanding this phenomenon.
Footnotes
Published online 26 May 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Rohrich RJ, Kaplan J, Dayan E.Silicone implant illness: science versus myth? Plast Reconstr Surg. 2019;144:98–109. [DOI] [PubMed] [Google Scholar]
- 2.Grady D, Rabin R.Reports of breast implant illnesses prompt federal review. New York Times. March 19, 2019:Health. [Google Scholar]
- 3.Burton T.Patients continue to report problems with breast implants. Wall Street Journal. March 25, 2019:Health. [Google Scholar]
- 4.Magnusson MR, Cooter RD, Rakhorst H, et al. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;1433S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma74S–81S. [DOI] [PubMed] [Google Scholar]
- 5.Hennekens CH, Lee IM, Cook NR, et al. Self-reported breast implants and connective-tissue diseases in female health professionals. A retrospective cohort study. JAMA. 1996;275:616–621. [PubMed] [Google Scholar]
- 6.Karlson EW, Lee IM, Cook NR, et al. Comparison of self-reported diagnosis of connective tissue disease with medical records in female health professionals: the Women’s Health Cohort Study. Am J Epidemiol. 1999;150:652–660. [DOI] [PubMed] [Google Scholar]
- 7.Bondurant S, Ernster V, Herdman R.Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants. In Safety of Silicone Breast Implants. 1999Washington, D.C.:National Academies Press; [PubMed] [Google Scholar]
- 8.Coroneos CJ, Selber JC, Offodile AC, II, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269:30–36. [DOI] [PubMed] [Google Scholar]
- 9.Colwell AS, Mehrara B.Editorial: US FDA breast implant postapproval studies-long-term outcomes in 99,993 patients. Ann Surg. 2019;269:39–40. [DOI] [PubMed] [Google Scholar]
- 10.Ashar BS.Assessing the risks of breast implants and FDA’s vision for the national breast implant registry. Ann Surg. 2019;269:37–38. [DOI] [PubMed] [Google Scholar]
- 11.Ashar BS.Statement from Binita Ashar, M.D., of the FDA’s Center for Devices and Radiological Health on agency’s commitment to studying breast implant safety. 2018. Available at https://www.fda.gov/news-events/press-announcements/statement-binita-ashar-md-fdas-center-devices-and-radiological-health-agencys-commitment-studying Accessed September 8, 2019.


