Background:
Currently, 8 different brands of a silicone gel–filled breast implant are commercially available in Korea. But the superiority of short-term safety has not been established.
Methods:
A total of 709 patients (1,418 breasts) received an implant-based augmentation mammaplasty. We compared a 1-year incidence of complications and complication-free survival among the breast implants. Moreover, we performed a subgroup analysis of the patient cohorts by excluding cases associated with a periareolar incision, revision or reoperation, and anatomic implants.
Results:
In our series, 1-year incidences of complications were 0.55% (1/182), 3.14% (5/159), 5.19% (7/135), and 6.58% (10/152) in patients receiving the BellaGel/BellaGel SmoothFine, the Mentor CPG, the Matrix, and the Motiva Ergonomix, respectively. Moreover, the incidence of capsular contracture was 0.55%, 0.63%, 1.32%, and 3.70% in patients receiving the BellaGel/BellaGel SmoothFine, the Mentor CPG, the Motiva Ergonomix, and the Matrix, respectively. Furthermore, a complication-free survival was 24.82 ± 0.18, 22.23 ± 1.09, 22.15 ± 0.77, and 22.12 ± 1.07 months in patients receiving the BellaGel/BellaGel SmoothFine, the Motiva Ergonomix, the Mentor CPG, and the Matrix, respectively, except for the 2 other remaining products used for a smaller number of patients. However, a subgroup analysis showed no significant differences in a 1-year incidence of complication and complication-free survival among the BellaGel SmoothFine, the Motiva Ergonomix, and the Naturgel.
Conclusion:
It is impossible to draw a definite conclusion about the superiority of the short-term safety among the silicone gel–filled breast implants that are commercially available in Korea.
INTRODUCTION
Since the US Food and Drug Administration approval of the fifth generation of cohesive gel breast implants in 2006, there has been an increase in the use of textured surface ones, both round and anatomically shaped, for augmentation mammaplasty. This is followed by published studies showing that surface texturing is closely associated with a reduced occurrence of capsular contracture (CC).1,2 According to a meta-analysis of 7 randomized controlled trials (RCTs), surface texturing had a protective effect against CC.3 Another meta-analysis of 6 prospective, randomized controlled studies showed that textured implants caused a lower incidence of CC.4 This is also supported by a systematic review implying a correlation between textured implants and a lower incidence of CC.5 Finally, the use of anatomically shaped silicone gel–filled breast implant was associated with a decreased occurrence of CC.6 It is, therefore, of paramount importance to make an evidence-based approach to implant-based augmentation mammaplasty and to discuss the effects of implant fill material or its shell on the postoperative occurrence of CC.7,8
To date, the incidence, severity, and long-term sequelae of local complications of an implant-based augmentation mammaplasty have been studied only in a limited scope.7,9–12 Moreover, differences in study design and methodology have made it difficult to directly compare the results between the studies in this series.13 Furthermore, it is imperative that the safety of an implant-based augmentation mammaplasty be rigorously assessed from perspectives of stakeholders in plastic surgery.14
According to Adams et al,15 most cases of CC occur within 1 year of augmentation mammaplasty. In addition, Schaub et al5 performed a systematic review of the 16 previously published studies, where patients were followed up for at least 1 year. Time to the occurrence of a certain medical event, such as a patient’s death, recurrence of a malignancy, or revision to an implant, serves as an outcome measure in some studies; the corresponding data were referred to as survival data and then analyzed based on the length of delay from the time origin to the occurrence of an event of interest.16 From this context, a 1-year, complication-free survival of a silicone gel–filled breast implant serves as an outcome measure.17
Given the above background, we conducted this study to examine a 1-year incidence of complications and complication-free survival of silicone gel–filled breast implants that are commercially available in Korea.
METHODS
Study Patients and Setting
We conducted a multicenter, retrospective study in 709 patients (1,418 breasts) who underwent augmentation mammaplasty using silicone gel–filled breast implants, at 2 local clinics in Korea (BS The Body Plastic Surgery Clinic in Busan and V Plastic Surgery Clinic in Daegu) between January 29, 2017, and March 2, 2018. Inclusion and exclusion criteria for the current study are summarized in Table 1.
Table 1.
Criteria for Selecting Patients for the Current Study
| Eligibility Criteria |
|---|
| Inclusion criteria |
| (1) Women ≥22 years old |
| (2) Women who underwent primary or revision augmentation mammaplasty using silicone gel–filled breast implants for esthetic purposes |
| (3) Women with an adequate amount of tissue for coverage of the breast implant |
| (4) Women with availability of follow-up data |
| Exclusion criteria |
| (1) Women with unilateral or bilateral presence of premalignant breast lesions |
| (2) Women with breast cancer genes 1 or 2 (BRCA1 or BRCA2) mutations with no history of taking bilateral mastectomy |
| (3) Women with untreated malignancies |
| (4) Women with an inadequate amount of or inappropriate tissue for coverage of the breast implant because of radiation-induced damage, vascular compromise, or impaired wound healing |
| (5) Women with abscess or infection |
| (6) Women who had a history of taking any drugs that may interfere with blood clotting or raise risks of developing postoperative complications |
| (7) Women with underlying medical conditions that may raise risks for developing postoperative complications [eg, obesity (BMI ≥40), diabetes mellitus, autoimmune disease, chronic lung, severe cardiovascular disease connective tissue, or rheumatoid disease) |
| (8) Women who are pregnant or breastfeeding |
| (9) Women with medical conditions that may interfere with wound healing (eg, active infectious collagen disease) |
| (10) Women with active fever (temperature >38°C) |
| (11) Women with severe lung disease (eg, chronic obstructive pulmonary disease) |
| (12) Women with cystic fibrosis |
| (13) Women with active cutaneous or systemic infections |
| (14) Women undergoing radiotherapy or chemotherapy within 6 mo preoperatively |
| (15) Women lost to follow-up |
| (16) Women who are deemed to be ineligible for the current analysis according to our judgment |
BMI, body mass index.
Informed consent was waived due to its retrospective nature. The current study was approved by the Internal Institutional Review Board of the Korea National Institute of Bioethics Policy and was conducted in compliance with the relevant ethical guidelines. All procedures described herein were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Evidence-based Approach to an Implant-based Augmentation Mammaplasty
On history taking and physical examination, the patients were evaluated for whether they were in healthy conditions. Moreover, they were also screened for underlying diseases, such as hypertension or diabetes mellitus. For preoperative assessment, we analyzed the optimal degree of soft-tissue coverage or location of the pocket for placement of a breast implant; the volume, type, size and dimension of a breast implant; the optimal location for the inframammary fold (IMF); and the location of a surgical incision, as described by Tebbetts and Adams.18 For prophylactic use of antibiotics, the patients were intravenously given cefazolin, or ofloxacin if allergic to penicillin. Under general anesthesia and intravenous sedation, surgery was performed by 2 board-certified specialists in plastic surgery who used almost the same operative technique, uniformly administered antibiotics, applied similar dressings, used the same postoperative instructions, and scheduled follow-up visits in a consistent manner. Thus, there was consistency in the patient care regardless of their surgical skills and time lapse. Types of surgical incision were determined based on our preference, along with the patients’ characteristics.19 Options of surgical incision include periareolar incision, IMF incision, and axillary incision. Following this, a breast implant was inserted into a pocket depending on their types, the degree of augmentation, types of the patients’ body, and our recommendations. Incisions were closed using layered sutures in the breast tissue, followed by the use of a skin adhesive or a surgical tape to close the skin.
Postoperative course was meticulously monitored at 1, 2, 3, and 4 weeks, 3, 6, 9, and 12 months postoperatively and, thereafter, during a regular follow-up period. Moreover, the patients were also recommended to receive further evaluation on magnetic resonance imaging scans at 3 years postoperatively and at a 2-year interval, and thereafter, in accordance with the US Food and Drug Administration labeling recommendation.17
Analysis of Baseline Characteristics of the Patients
Demographic, baseline, and clinical characteristics of the patients were evaluated through a retrospective review of medical records. These include age, sex, round of surgery (primary and revision breast augmentation), smoking status (never, current, and former smokers), body mass index, the type and volume of breast implant, and the type of surgical approach.
The patients were evaluated for overall and cumulative incidences of postoperative complications. Potential postoperative complications include CC, implant malposition, breast deformation or asymmetry, wound or skin problems, infection, hematoma or hemorrhage, implant rupture, seroma, abscess, silicone granuloma or implant extrusion, rupture, double capsule, folding, upside-down rotation, and breast implant–associated anaplastic large cell lymphoma.9,17
Analysis of Complication-free Survival of a Silicone Gel–filled Breast Implant
As stated earlier, a 1-year, complication-free survival of a silicone gel–filled breast implant serves as an outcome measure for the current study. We calculated the percentage of silicone gel–filled breast implants that are persistently present without undergoing revision or removal without revision.17 Thus, we estimated cumulative survival period defined as time-to-events.
Subgroup Analysis
It has been reported that an implant-based augmentation mammaplasty via a periareolar incision produces a higher rate of CC.20,21 Moreover, some “Core” studies have shown that the rate of CC was significantly higher in secondary cases than in primary ones.6,12,22,23 Furthermore, the use of anatomical implants was closely associated with a decreased rate of CC.24 Based on these previously published studies, we performed a subgroup analysis of the patient cohorts by excluding cases associated with a periareolar incision, revision or reoperation, and anatomic implants. Then, we compared a 1-year incidence of complications and complication-free survival between the breast implants.
Statistical Analysis of the Patient Data
All data were expressed as mean ± SD, mean ± standard error, or the number of patients with percentage, where appropriate. The overall complication-free survival was expressed as mean ± standard error, and it was estimated with 95% CIs. The cumulative complication-free survival was compared between the breast implants, for which statistical significance was analyzed using the log-rank test. Moreover, the corresponding Kaplan–Meier survival and hazards were plotted as a curve. Statistical analysis was done using the SPSS version 18.0 for windows (SPSS Inc., Chicago, IL). A P value of <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Patients
All the patients (n = 709; 1,418 breasts) were women with a mean age of 34.48 ± 8.54 years old. All surgical procedures were performed with a dual-plane technique. The patients were followed up for a mean period of 7.54 ± 5.32 months, whose baseline characteristics are represented in Table 2.
Table 2.
Baseline Characteristics of the Patients
| Variables | Values |
|---|---|
| Age (y) | 34.48 ± 8.54 |
| Sex (male-to-female ratio) | 0:709 |
| Follow-up period (mo) | 7.54 ± 5.32 |
| Height (cm) | 162.23 ± 5.04 |
| Weight (kg) | 51.26 ± 5.51 |
| Round of surgery | |
| Primary augmentation mammaplasty | 654 (92.24) |
| Revision augmentation mammaplasty | 20 (2.82) |
| Reoperation | 35 (4.94) |
| Smoking status | |
| Never smokers | 640 (90.28) |
| Former smokers | 2 (0.28) |
| Current smokers | 67 (9.44) |
| Volume of breast implant (mL) | |
| Right side | |
| ≤245 | 31 (4.37) |
| 250–295 | 287 (40.48) |
| 300–345 | 325 (45.84) |
| ≥350 | 65 (9.17) |
| Left side | |
| ≤245 | 54 (7.62) |
| 250–295 | 324 (45.70) |
| 300–345 | 279 (39.35) |
| ≥350 | 51 (7.19) |
| Surgical approach | |
| Axillary incision | 2 (0.28) |
| IMF incision | 690 (97.32) |
| Crescent periareolar incision | 13 (1.83) |
| IMF incision in the right breast and periareolar mastopexy of the left breast | 1 (0.15) |
| Crescent periareolar incision in the right breast and IMF incision in the left breast | 1 (0.15) |
| Vertical augmentation mastopexy | 2 (0.28) |
Values are mean ± SD or the number of patients with percentage, where appropriate.
The patients underwent augmentation mammaplasty using breast implants; these include the BellaGel/BellaGel SmoothFine (HansBiomed Co. Ltd., Seoul, Korea) (n = 182), the Mentor CPG (Mentor Worldwide LLC, Santa Barbara, CA) (n = 159), the Motiva Ergonomix (Establishment Labs Holdings Inc., Alajuela, Costa Rica) (n = 152), the Matrix (GC Aesthetics PLC, Apt Cedex, France) (n = 135), the Naturgel (Groupe Sebbin SAS, Boissy-l’Aillerie, France) (n = 61), and the Natrelle 410/510 (Allergan Inc., Irvine, Calif.) (n = 20). Distribution of breast implants by the manufacturer is shown in Figure 1 and Table 3. Baseline characteristics of the patients depending on the brand of breast implant are represented in Table 4.
Fig. 1.
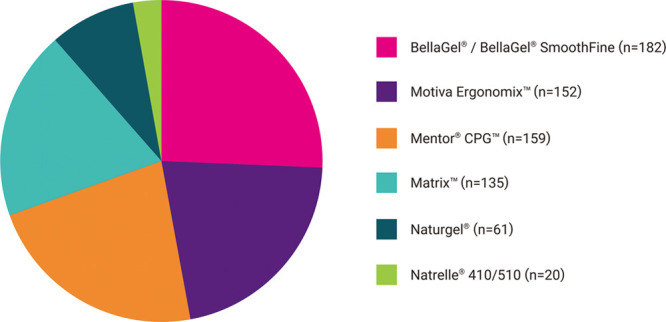
Distribution of the breast implant by the manufacturer. The patients underwent augmentation mammaplasty using the BellaGel/BellaGel SmoothFine (HansBiomed Co. Ltd., Seoul, Korea) (n = 182), the Mentor CPG (Mentor Worldwide LLC, Santa Barbara, CA) (n = 159), the Motiva Ergonomix (Establishment Labs Holdings Inc., Alajuela, Costa Rica) (n = 152), the Matrix (GC Aesthetics PLC, Apt Cedex, France) (n = 135), the Naturgel (Groupe Sebbin SAS, Boissy-l’Aillerie, France) (n = 61), or the Natrelle 410/510 (Allergan Inc., Irvine, CA) (n = 20).
Table 3.
Distribution of the Breast Implants by Manufacturer
| Manufacturer | Trade Name | n (%) |
|---|---|---|
| BellaGel | 4 (0.56) | |
| HansBiomed Co. Ltd. | BellaGel SmoothFine | 178 (25.11) |
| Mentor Worldwide LLC | Mentor CPG | 159 (22.43) |
| GC Aesthetics PLC | Matrix | 135 (19.04) |
| Establishment Labs Holdings Inc. | Motiva Ergonomix | 152 (21.44) |
| Groupe Sebbin SAS | Naturgel | 61 (8.60) |
| Allergan Inc. | Natrelle 410/510 | 20 (2.82) |
Values are the number of patients with percentage.
Table 4.
Baseline Characteristics of the Patients Depending on the Breast Implant
| Variables | Values | |||||
|---|---|---|---|---|---|---|
| BellaGel/BellaGel SmoothFine (n = 182) | Mentor CPG (n = 159) | Motiva Ergonomix (n = 152) | Matrix (n = 135) | Naturgel (n = 61) | Natrelle 410/510 (n = 20) | |
| Age (y) | 33.15 ± 8.29 | 31.96 ± 8.32 | 36.67 ± 7.77 | 36.66 ± 8.06 | 33.69 ± 9.78 | 37.55 ± 9.47 |
| Sex (male-to-female ratio) | 0:182 | 0:159 | 0:152 | 0:135 | 0:61 | 0:20 |
| Follow-up period (mo) | 7.98 ± 4.80 | 7.91 ± 4.83 | 7.17 ± 5.36 | 6.78 ± 4.81 | 8.38 ± 8.42 | 5.90 ± 3.49 |
| Height (cm) | 163.04 ± 5.06 | 161.86 ± 5.09 | 161.75 ± 5.37 | 162.35 ± 4.69 | 161.69 ± 5.12 | 162.35 ± 3.03 |
| Weight (kg) | 51.16 ± 5.47 | 51.80 ± 5.68 | 50.58 ± 5.34 | 51.75 ± 5.62 | 50.11 ± 5.13 | 53.28 ± 5.51 |
| Round of surgery | ||||||
| Primary augmentation mammaplasty | 170 (93.41) | 151 (94.97) | 130 (85.53) | 124 (91.85) | 59 (96.72) | 20 (100.00) |
| Revision augmentation mammaplasty | 1 (0.55) | 3 (1.89) | 8 (5.26) | 8 (5.93) | 0 (0.00) | 0 (0.00) |
| Reoperation | 11 (6.04) | 5 (2.75) | 14 (9.21) | 3 (2.22) | 2 (3.28) | 0 (0.00) |
| Smoking status | ||||||
| Never smokers | 166 (91.21) | 153 (84.07) | 144 (94.74) | 120 (88.89) | 39 (63.93) | 18 (90.00) |
| Former smokers | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 20 (32.79) | 0 (0.00) |
| Current smokers | 16 (8.79) | 6 (3.77) | 8 (5.26) | 15 (11.11) | 2 (3.28) | 2 (10.00) |
| Volume of breast implant (mL) | ||||||
| Right side | ||||||
| ≤245 | 0 (0.00) | 10 (6.29) | 10 (6.58) | 2 (1.48) | 6 (9.84) | 3 (15.00) |
| 250–295 | 36 (19.78) | 99 (62.26) | 44 (28.95) | 71 (52.59) | 26 (42.62) | 11 (55.00) |
| 300–345 | 119 (65.38) | 36 (22.64) | 84 (55.26) | 53 (39.26) | 29 (47.54) | 4 (20.00) |
| ≥350 | 26 (14.29) | 14 (8.81) | 14 (9.21) | 9 (6.67) | 0 (0.00) | 2 (10.00) |
| Left side | ||||||
| ≤245 | 3 (1.65) | 14 (8.81) | 19 (12.50) | 2 (1.48) | 12 (19.67) | 4 (20.00) |
| 250–295 | 59 (32.42) | 97 (61.01) | 47 (30.92) | 83 (61.48) | 29 (47.54) | 9 (45.00) |
| 300–345 | 101 (55.49) | 34 (21.38) | 78 (51.32) | 41 (30.37) | 20 (32.79) | 5 (25.00) |
| ≥350 | 18 (9.89) | 14 (8.81) | 8 (5.26) | 9 (6.67) | 0 (0.00) | 2 (10.00) |
| Surgical approach | ||||||
| Axillary incision | 2 (1.10) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| IMF incision | 179 (98.35) | 154 (96.86) | 147 (96.71) | 130 (96.30) | 61 (100.00) | 19 (95.00) |
| Crescent periareolar incision | 0 (0.00) | 4 (2.52) | 4 (2.63) | 5 (3.70) | 0 (0.00) | 0 (0.00) |
| IMF incision in the right breast and periareolar mastopexy of the left breast | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.00) |
| Crescent periareolar incision in the right breast and IMF incision in the left breast | 0 (0.00) | 1 (0.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Vertical augmentation mastopexy | 1 (0.55) | 0 (0.00) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
Values are mean ± SD or the number of patients with percentage, where appropriate.
Results of Analysis of a 1-year Incidence of Complications and Complication-free Survival
As shown in Table 5, there were a total of 26 cases (3.67%) of postoperative complications; these include 9 cases (1.27%) of CC, 3 cases (0.42%) of IMF malposition, 2 cases (0.28%) of IMF malposition with rippling, 2 cases (0.28%) of psychological distress, 2 cases (0.28%) of dissatisfaction with size, 1 case (0.14%) of malrotation, 1 case (0.14%) of CC with seroma, 1 case (0.14%) of seroma, 1 case (0.14%) of asymmetry, 1 case (0.14%) of foreign body sensation, 1 case (0.14%) of infection, and 1 case (0.14%) of hematoma. Cumulative incidences of postoperative complications by the breast implant are summarized in Table 6 (χ2 = 14.316; df = 5; P = 0.014).
Table 5.
Incidences of Postoperative Complications by Breast Implant
| Variables | Values | |||||||
|---|---|---|---|---|---|---|---|---|
| BellaGel/BellaGel SmoothFine (n = 182) | Mentor CPG (n = 159) | Motiva Ergonomix (n = 152) | Matrix (n = 135) | Naturgel (n = 61) | Natrelle 410/510 (n = 20) | P | ||
| BellaGel (n = 4) | BellaGel SmoothFine (n = 178) | |||||||
| Total incidences | 0 (0.00) | 1 (0.55) | 5 (3.14) | 10 (6.58) | 7 (5.19) | 2 (3.28) | 1 (5.00) | |
| Malrotation | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.74) | 0 (0.00) | 1 (5.00) | ||
| IMF malposition | 0 (0.00) | 1 (0.63) | 2 (1.32) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| IMF malposition with rippling | 0 (0.00) | 0 (0.00) | 1 (0.66) | 1 (0.74) | 0 (0.00) | 0 (0.00) | ||
| CC | 0 (0.00) | 1 (0.55) | 1 (0.63) | 2 (1.32) | 5 (3.70) | 0 (0.00) | 0 (0.00) | |
| sCC with seroma | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) | ||
| Seroma | 0 (0.00) | 0 (0.00) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Asymmetry | 0 (0.00) | 0 (0.00) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Foreign body sensation | 0 (0.00) | 1 (0.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Infection | 0 (0.00) | 0 (0.00) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Psychological distress | 0 (0.00) | 1 (0.63) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Dissatisfaction with size | 0 (0.00) | 1 (0.63) | 1 (0.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Hematoma | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.64) | 0 (0.00) | 0.082 | |
Values are the number of patients with percentage.
CC, capsular contracture.
Table 6.
Cumulative Incidences of Postoperative Complications and TTE by Breast Implant
| Breast Implants | N | Censored Values | TTE (mo) |
|---|---|---|---|
| Total (n = 709) | 26 | 683 (96.33) | 27.11 ± 0.64 (25.85–28.37) |
| BellaGel/BellaGel SmoothFine (n = 182) | 1 | 181 (99.45) | 24.82 ± 0.18 (24.47–25.17) |
| Mentor CPG (n = 159) | 5 | 154 (96.86) | 22.15 ± 0.77 (20.64–23.66) |
| Motiva Ergonomix (n = 152) | 10 | 142 (93.42) | 22.23 ± 1.09 (20.10–24.36) |
| Matrix (n = 135) | 7 | 128 (94.81) | 22.12 ± 1.07 (20.03–24.21) |
| Naturgel (n = 61) | 2 | 59 (96.72) | 28.59 ± 1.03 (26.58–30.60) |
| Natrelle 410/510 (n = 20) | 1 | 19 (95.00) | 13.00 ± 0.87 (11.30–14.70) |
Values are represented as mean ± standard error with 95% CI or the number of patients with percentage, where appropriate.
N, incidence of postoperative complications; TTE, time-to-events.
As shown in Table 6, a complication-free survival was 28.59 ± 1.03, 24.82 ± 0.18, 22.23 ± 1.09, 22.15 ± 0.77, 22.12 ± 1.07, and 13.00 ± 0.87 months in the women receiving the Naturgel, the BellaGel/BellaGel SmoothFine, the Motiva Ergonomix, the Mentor CPG, the Matrix, and the Natrelle 410/510, respectively. Moreover, their cumulative survival rates are summarized in Table 7. The corresponding Kaplan–Meier survival and hazards are plotted as a curve as shown in Figures 2 and 3.
Table 7.
Cumulative Survival by Breast Implant
| Breast Implants | FU (mo) | N | n | Survival Rate |
|---|---|---|---|---|
| BellaGel/BellaGel SmoothFine (n = 182) | 8 | 95 | 1 | 0.990 ± 0.011 (0.969–1.000) |
| Mentor CPG (n = 159) | 7 | 64 | 1 | 0.984 ± 0.016 (0.954–1.000) |
| 10 | 44 | 1 | 0.962 ± 0.027 (0.911–1.000) | |
| 15 | 20 | 2 | 0.866 ± 0.069 (0.741–1.000) | |
| 17 | 15 | 1 | 0.808 ± 0.085 (0.657–0.993) | |
| Motiva Ergonomix (n = 152) | 2 | 150 | 1 | 0.993 ± 0.007 (0.980–1.000) |
| 6 | 73 | 1 | 0.980 ± 0.015 (0.951–1.000) | |
| 7 | 54 | 2 | 0.943 ± 0.029 (0.888–1.000) | |
| 8 | 44 | 2 | 0.901 ± 0.041 (0.824–0.984) | |
| 11 | 30 | 2 | 0.841 ± 0.056 (0.738–0.957) | |
| 15 | 16 | 1 | 0.788 ± 0.073 (0.657–0.945) | |
| 24 | 4 | 1 | 0.591 ± 0.179 (0.326–1.000) | |
| Matrix (n = 135) | 5 | 91 | 1 | 0.989 ± 0.011 (0.968–1.000) |
| 6 | 66 | 3 | 0.944 ± 0.027 (0.892–0.999) | |
| 12 | 24 | 1 | 0.905 ± 0.047 (0.818–1.000) | |
| 15 | 14 | 2 | 0.775 ± 0.094 (0.612–0.982) | |
| Naturgel (n = 61) | 2 | 60 | 1 | 0.983 ± 0.017 (0.951–1.000) |
| 6 | 25 | 1 | 0.944 ± 0.042 (0.866–1.000) | |
| Natrelle 410/510 (n = 20) | 10 | 4 | 1 | 0.750 ± 0.217 (0.426–1.000) |
Values are represented as mean ± standard error with 95% CI or the number of patients, where appropriate.
FU, time points of follow-up; N, number of risks; n, incidence of postoperative complications.
Fig. 2.
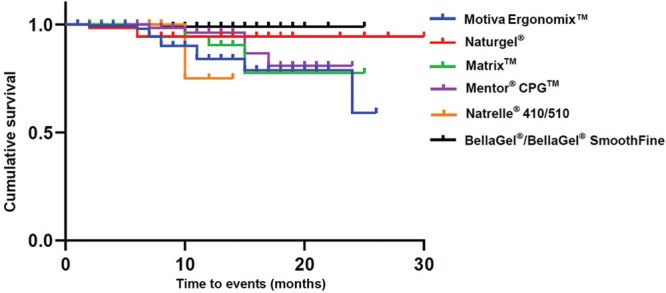
Kaplan–Meier survival by the breast implant.
Fig. 3.
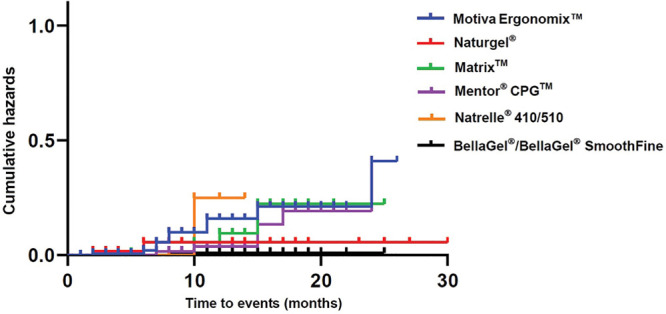
Kaplan–Meier hazards by the breast implant.
Results of Subgroup Analysis
In our series, the patients receiving the BellaGel SmoothFine (n = 166), the Motiva Ergonomix (n = 127), or the Naturgel (n = 52) met the criteria for subgroup analysis. Baseline characteristics of the patients included in the subgroup analysis are represented in Table 8.
Table 8.
Baseline Characteristics of the Patients by the Breast Implant on Subgroup Analysis
| Variables | BellaGel SmoothFine (n = 166) | Motiva Ergonomix (n = 127) | Naturgel (n = 52) |
|---|---|---|---|
| Age (y) | 32.75 ± 8.27 | 35.72 ± 7.53 | 32.58 ± 9.77 |
| Sex (male-to-female ratio) | 0:166 | 0:127 | 0:52 |
| Follow-up period (mo) | 7.91 ± 4.52 | 7.24 ± 5.37 | 7.83 ± 7.73 |
| Height (cm) | 162.37 ± 5.09 | 161.62 ± 5.39 | 162.23 ± 4.73 |
| Weight (kg) | 50.97 ± 5.39 | 50.37 ± 5.50 | 49.20 ± 5.54 |
| Round of surgery | |||
| Primary augmentation mammaplasty | 166 (100.00) | 127 (100.00) | 52 (100.00) |
| Smoking status | |||
| Never smokers | 152 (91.57) | 121 (95.28) | 32 (61.54) |
| Former smokers | 0 (0.00) | 0 (0.00) | 18 (34.62) |
| Current smokers | 14 (8.43) | 6 (4.72) | 2 (3.85) |
| Volume of breast implant (mL) | |||
| Right side | |||
| ≤245 | 0 (0.00) | 6 (4.72) | 3 (5.77) |
| 250–295 | 33 (20.00) | 35 (27.56) | 23 (44.23) |
| 300–345 | 108 (65.45) | 73 (57.48) | 26 (50.00) |
| ≥350 | 24 (14.55) | 13 (10.24) | 0 (0.00) |
| Left side | |||
| ≤245 | 3 (1.82) | 12 (9.45) | 9 (17.31) |
| 250–295 | 55 (33.33) | 41 (32.28) | 26 (50.00) |
| 300–345 | 92 (55.76) | 67 (52.76) | 17 (32.69) |
| ≥350 | 15 (9.09) | 7 (5.51) | 0 (0.00) |
| Surgical approach | |||
| Axillary incision | 2 (1.20) | 0 (0.00) | 0 (0.00) |
| IMF incision | 163 (98.19) | 126 (99.21) | 52 (100.00) |
| Vertical augmentation mastopexy | 1 (0.60) | 1 (0.79) | 0 (0.00) |
Values are mean ± SD or the number of patients with percentage, where appropriate.
As shown in Table 9, there were no significant differences in total incidences of complications between the breast implants (P = 0.416). Moreover, cumulative incidences of postoperative complications depending on the breast implant showed a significant difference; statistical significance was confirmed using the log-rank test (χ2 = 8.458; df = 2; P = 0.015) (Table 10).
Table 9.
Incidences of Postoperative Complications by Breast Implant on Subgroup Analysis
| Variables | BellaGel SmoothFine (n = 166) | Motiva Ergonomix (n = 127) | Naturgel (n = 52) | P |
|---|---|---|---|---|
| Total incidences | 1 (0.60) | 7 (5.51) | 2 (3.85) | 0.416 |
| Malrotation | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| IMF malposition | 0 (0.00) | 2 (1.57) | 0 (0.00) | |
| IMF malposition with rippling | 0 (0.00) | 1 (0.66) | 0 (0.00) | |
| CC | 1 (0.60) | 0 (0.00) | 0 (0.00) | |
| CC with seroma | 0 (0.00) | 0 (0.00) | 1 (1.92) | |
| Seroma | 0 (0.00) | 1 (0.78) | 0 (0.00) | |
| Asymmetry | 0 (0.00) | 1 (0.78) | 0 (0.00) | |
| Foreign body sensation | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Infection | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Psychological distress | 0 (0.00) | 1 (0.78) | 0 (0.00) | |
| Dissatisfaction with size | 0 (0.00) | 1 (0.78) | 0 (0.00) | |
| Hematoma | 0 (0.00) | 0 (0.00) | 1 (1.92) |
Values are the number of patients with percentage.
CC, capsular contracture.
Table 10.
Cumulative Incidences of Postoperative Complications and TTE by the Breast Implant on Subgroup Analysis
| Breast Implants | N | Censored Values | TTE (mo) |
|---|---|---|---|
| Total (n = 295) | 10 | 285 (96.33) | 25.29 ± 0.60 (24.12–26.46) |
| BellaGel SmoothFine (n = 166) | 1 | 165 (99.40) | 21.84 ± 0.16 (21.53–22.15) |
| Motiva Ergonomix (n = 127) | 7 | 120 (94.49) | 22.05 ± 1.03 (20.03–24.07) |
| Naturgel (n = 52) | 2 | 50 (96.15) | 25.53 ± 1.06 (23.44–27.61) |
Values are represented as mean ± standard error with 95% CI or the number of patients with percentage, where appropriate.
N, incidence of postoperative complications; TTE, time-to-events.
Cumulative survival period of the breast implant is shown in Table 10: the Naturgel (25.53 ± 1.06), the Motiva Ergonomix (22.05 ± 1.03), and the BellaGel SmoothFine (21.84 ± 0.16). Moreover, their cumulative survival rates are summarized in Table 11. The corresponding Kaplan–Meier survival and hazards are plotted as a curve as shown in Figures 4 and 5.
Table 11.
Cumulative Survival by the Breast Implant on Subgroup Analysis
| Breast Implants | FU (mo) | N | n | Survival Rate |
|---|---|---|---|---|
| BellaGel SmoothFine (n = 166) | 8 | 88 | 1 | 0.988 ± 0.011 (0.967–1.000) |
| Motiva Ergonomix (n = 127) | 6 | 60 | 1 | 0.983 ± 0.017 (0.951–1.000) |
| 7 | 46 | 1 | 0.962 ± 0.027 (0.911–1.000) | |
| 8 | 39 | 2 | 0.913 ± 0.042 (0.833–0.999) | |
| 11 | 25 | 1 | 0.876 ± 0.054 (0.776–0.989) | |
| 15 | 14 | 1 | 0.788 ± 0.073 (0.673–0.983) | |
| 24 | 3 | 1 | 0.591 ± 0.179 (0.238–1.000) | |
| Naturgel (n = 52) | 2 | 51 | 1 | 0.980 ± 0.019 (0.943–1.000) |
| 6 | 21 | 1 | 0.934 ± 0.049 (0.842–1.000) |
Values are represented as mean ± standard error with 95% CI or the number of patients, where appropriate.
FU, time points of follow-up; N, number of risks, n, incidence of postoperative complications.
Fig. 4.
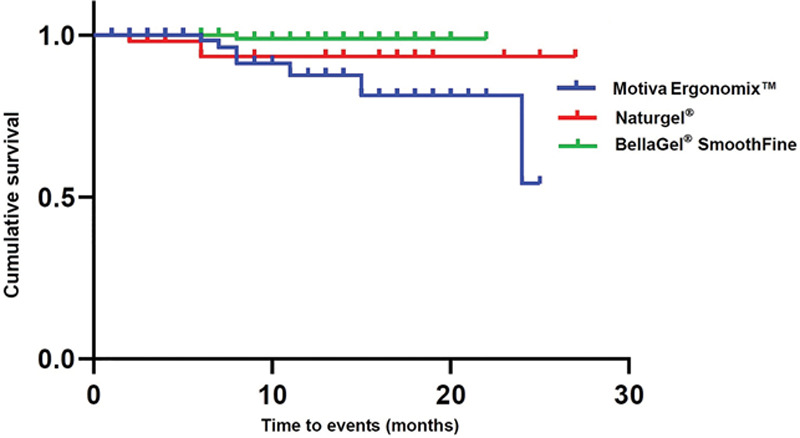
Kaplan–Meier survival by the breast implant on subgroup analysis.
Fig. 5.
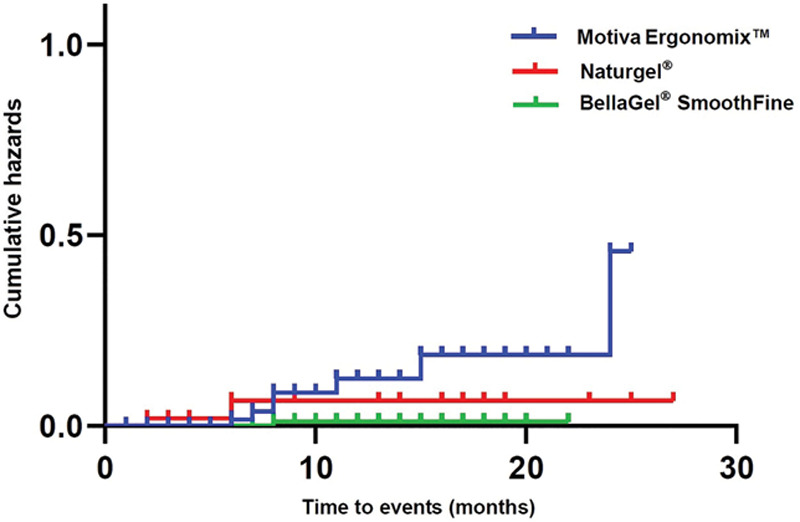
Kaplan–Meier hazards by the breast implant on subgroup analysis.
DISCUSSION
CC is the most common complication of an implant-based augmentation mammaplasty, whose incidence has been reported to range from 4% to 5% during the first 2-year follow-up period.25,26 To date, it has been suggested that the rate of CC has a significant correlation with the length of time spent since placement of a breast implant.26,27 However, there are also other studies showing occurrences of >90% of the total CC cases within the first 12 postoperative months.25,28 It is widely known that patients with CC are treated with reoperation. Although all of them are not indicated in reoperation, their symptoms, such as discomfort due to or distortion of a breast implant, would provide a sufficient reason for reoperation.27–31
Still, little is known about the etiology of CC, although it is presumed that multifactorial mechanisms might be involved in it.29 Involvement of multiple factors in the pathogenesis of CC has been suggested by possibility that there is a persistent presence of risk of developing CC over time, although short-term risk factors, such as bacterial infection, surgical technique, drains, and antibiotics, disappear with time.26,28 According to some authors, there are certain patient-specific risk factors for developing CC; these include radiation exposure, an implant-based breast reconstruction, and a history of CC.25–27,32
Implant-specific properties, such as fill material, surface, and profile, have also been suggested to be related to CC, but this has shown inconsistent results.27
Locations of the implant pocket also serve as risk factors for developing CC. That is, a subglandular plane is commonly associated with an increased risk of developing CC when compared with a submuscular plane or dual plane.27 IMF, periareolar, and transaxillary incisions have their own merits and demerits.33 Of these, a periareolar incision is associated with a higher risk of developing CC when compared with an IMF incision.8,20 Presumably, this might be explained based on the hypothesis that a periareolar incision disrupts the ductal system to a greater extent when compared with an IMF incision and this promotes the colonization of bacterial flora on the surface of a breast implant.8
We compared 1-year incidences of complications, particularly focusing on the CC, between the commercially available breast implants. In our series, 1-year incidences of complications were 0.55% (1/182), 3.14% (5/159), 5.19% (7/135), and 6.58% (10/152) in the patients receiving the BellaGel/BellaGel SmoothFine, the Mentor CPG, the Matrix, and the Motiva Ergonomix, respectively. Moreover, the incidence of CC was 0.55%, 0.63%, 1.32%, and 3.70% in patients receiving the BellaGel/BellaGel SmoothFine, the Mentor CPG, the Motiva Ergonomix, and the Matrix, respectively. Furthermore, there were no cases of CC with seroma following the use of the BellaGel/BellaGel SmoothFine, the Mentor CPG, the Motiva Ergonomix, or the Matrix.
A complication-free survival was 24.82 ± 0.18, 22.23 ± 1.09, 22.15 ± 0.77, and 22.12 ± 1.07 months in the patients receiving the BellaGel/BellaGel SmoothFine, the Motiva Ergonomix, the Mentor CPG, and the Matrix, respectively, except for the 2 other remaining products used for a smaller number of patients.
A subgroup analysis was performed for the patients receiving the BellaGel SmoothFine (n = 166), the Motiva Ergonomix (n = 127), or the Naturgel (n = 52). This showed no significant differences in total incidences of complications between the breast implants (P = 0.416). But there were significant differences in cumulative incidences of postoperative complications between the breast implants (χ2 = 8.458; df = 2; P = 0.015). Furthermore, cumulative survival period of the breast implant is 25.53 ± 1.06 months in the patients receiving the Naturgel, 22.05 ± 1.03 months in those receiving the Motiva Ergonomix, and 21.84 ± 0.16 months in those receiving the BellaGel SmoothFine.
Limitations of the current study are as follows:
We evaluated our clinical series of the patients who had been surgically treated at only 2 local clinics. The possibility of selection bias could not be completely ruled out.
We followed up the patients for a short period of time. But it is difficult to perform a long-term follow-up of patients receiving an implant-based augmentation mammaplasty, which might be due to the following reasons: first, patients are less likely to return to their plastic surgeons for a reexamination if they feel assured about their breast health. Second, patients who are dissatisfied with treatment outcomes tend to seek opinions from another surgeon.26
We included a small series of the patients in the current study. It has been reported, however, that only a small number of patients successfully complete a follow-up examination even in manufacturer-sponsored trials.33 Therefore, the possibility of underestimating incidences of complications of an implant-based augmentation mammaplasty could not be completely ruled out.26
The current results from analysis of a 1-year incidence of CC cannot be generalized. According to Maxwell et al,12,34 there was an approximately 1% annual increase in the incidence of CC of Baker grade III/IV from the previously reported 6-year incidences. Further long-term follow-up studies are, therefore, warranted to estimate the accurate incidence of CC of a silicone gel–filled breast implant.
We failed to control confounding variables that may contribute to the occurrence of CC. Since the emergence of the first breast implant, at least 240 styles and 8,300 models have been manufactured.35 It is, therefore, difficult to make a direct comparison between the breast implants from different manufacturers. This is because no clinical trials have been conducted to standardize the above-mentioned confounding variables.36
According to a review of literature on a breast implant, most of the studies are authored by surgeons who have acknowledged financial support from the corresponding manufacturers.37–39 This strongly suggests that they frequently focus on a single manufacturer; their results were not derived from an unbiased comparison of all available options and can be questioned for the presence of bias.40
CONCLUSIONS
Designs of contemporary silicone gel–filled breast implants share common characteristics: optimization of esthetic outcomes and minimization of postoperative complications. This endows them with a higher degree of patient satisfaction and a lower incidence of complications when compared with their predecessors.41 Moreover, studies have also been conducted to compare the outcomes between the silicone gel–filled breast implants.40,42–49 Most of the studies comparing between them are retrospective in nature. As compared with retrospective studies, prospective RCTs are more useful in providing more scientifically sound methods, and valuable results, but they are difficult to conduct. They require a large sample size, a sufficiently long period of follow-up observation, and a high level of patient compliance. Moreover, there is also a possibility that trial devices would no longer be the first-line choice for an implant-based augmentation mammaplasty when such prospective RCTs are completed and their results are published in a literature if they are modified by the corresponding manufacturers.42 Finally, it deserves special attention that a study design bias might be introduced to several clinical research on a silicone gel–filled breast implant. Song et al50 performed a meta-analysis of the previously published studies about the use of magnetic resonance imaging or ultrasound in detecting breast implant ruptures, thus showing that results of such studies are flawed with methodologic shortcomings. From this context, our results might be of limited value and should be interpreted with caution.
Here, we describe a 1-year incidence of complications and complication-free survival of silicone gel–filled breast implants that are commercially available in Korea. It is impossible, however, to draw definite conclusions about the superiority of the short-term safety between them. After exclusion of cases of revision surgery or reoperation, periareolar incision, and anatomic implants, our results showed no significant differences in a 1-year incidence of complication and complication-free survival among the BellaGel SmoothFine, the Motiva Ergonomix, and the Naturgel. Further prospective, large-scale, multicenter, randomized controlled studies with a long period of follow-up and valid outcome measures are warranted to establish our results.
Footnotes
Published online 14 May 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was sponsored by the HansBiomed Co. Ltd., the manufacturer of the BellaGel/BellaGel SmoothFine.
REFERENCES
- 1.Calobrace MB, Capizzi PJ.The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg. 2014;134:6S–11S. [DOI] [PubMed] [Google Scholar]
- 2.Calobrace MB, Schwartz MR, Zeidler KR, et al. Long-term safety of textured and smooth breast implants. Aesthet Surg J. 2017;38:38–48. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley GP, Sigurdson LJ, Barnsley SE.Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. [DOI] [PubMed] [Google Scholar]
- 4.Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118:1224–1236. [DOI] [PubMed] [Google Scholar]
- 5.Schaub TA, Ahmad J, Rohrich RJ.Capsular contracture with breast implants in the cosmetic patient: saline versus silicone—a systematic review of the literature. Plast Reconstr Surg. 2010;126:2140–2149. [DOI] [PubMed] [Google Scholar]
- 6.Caplin DA.Indications for the use of MemoryShape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants. Plast Reconstr Surg. 2014;1343 Suppl27S–37S. [DOI] [PubMed] [Google Scholar]
- 7.Headon H, Kasem A, Mokbel K.Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg. 2005;54:343–351. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Findlay EJ.Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127:56–66. [DOI] [PubMed] [Google Scholar]
- 10.Baek WY, Lew DH, Lee DW.A retrospective analysis of ruptured breast implants. Arch Plast Surg. 2014;41:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderman A, Pusic A, Murphy DK.Prospective analysis of primary breast augmentation on body image using the BREAST-Q: results from a nationwide study. Plast Reconstr Surg. 2016;137:954e–960e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fryzek JP, Signorello LB, Hakelius L, et al. Local complications and subsequent symptom reporting among women with cosmetic breast implants. Plast Reconstr Surg. 2001;107:214–221. [DOI] [PubMed] [Google Scholar]
- 14.Deva AK, Cuss A, Magnusson M, et al. The “game of implants”: a perspective on the crisis-prone history of breast implants. Aesthet Surg J. 2019;39Supplement_1S55–S65. [DOI] [PubMed] [Google Scholar]
- 15.Adams WP, Jr, Rios JL, Smith SJ.Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;118:46S–52S. [DOI] [PubMed] [Google Scholar]
- 16.Biau DJ, Hamadouche M.Estimating implant survival in the presence of competing risks. Int Orthop. 2011;35:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung JY, Jeong JP, Moon DS, et al. Short-term safety of augmentation mammaplasty using the BellaGel implants in Korean Women. Plast Reconstr Surg Glob Open. 2019;7:e2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tebbetts JB, Adams WP.Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2005;116:2005–2016. [PubMed] [Google Scholar]
- 19.Lista F, Ahmad J.Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg. 2013;132:1684–1696. [DOI] [PubMed] [Google Scholar]
- 20.Wiener TC.Relationship of incision choice to capsular contracture. Aesthetic Plast Surg. 2008;32:303–306. [DOI] [PubMed] [Google Scholar]
- 21.Newman AN, Davison SP.Effect of Keller funnel on the rate of capsular contracture in periareolar breast augmentation. Plast Reconstr Surg Glob Open. 2018;6:e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear SL, Murphy DAllergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;133:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens WG, Calobrace MB, Harrington J, et al. Nine-year core study data for Sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J. 2016;36:404–416. [DOI] [PubMed] [Google Scholar]
- 24.Rocco N, Rispoli C, Moja L, et al. Different types of implants for reconstructive breast surgery. Cochrane Database Syst Rev. 2016:CD010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg. 2003;51:531–539. [DOI] [PubMed] [Google Scholar]
- 26.Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117:757–767; discussion 768. [DOI] [PubMed] [Google Scholar]
- 27.Araco A, Gravante G, Araco F, et al. A retrospective analysis of 3,000 primary aesthetic breast augmentations: postoperative complications and associated factors. Aesthetic Plast Surg. 2007;31:532–539. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson JM, Gatti ME, Schaffner AD, et al. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32:456–462. [DOI] [PubMed] [Google Scholar]
- 29.Adams WP., Jr.Capsular contracture: what is it? what causes it? how can it be prevented and managed? Clin Plast Surg. 2009;36:119, vii–126, vii. [DOI] [PubMed] [Google Scholar]
- 30.Spear SL, Low M, Ducic I.Revision augmentation mastopexy: indications, operations, and outcomes. Ann Plast Surg. 2003;51:540–546. [DOI] [PubMed] [Google Scholar]
- 31.Spear SL, Murphy DK, Slicton A, et al. Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;1207 Suppl 18S–16S; discussion 17S. [DOI] [PubMed] [Google Scholar]
- 32.Cordeiro PG, McCarthy CM.A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg. 2006;118:832–839. [DOI] [PubMed] [Google Scholar]
- 33.Spear S, Erwin E, Venturi M.Breast augmentation. Plast Reconstr Surg. 2006;118:188s–196s. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32:709–717. [DOI] [PubMed] [Google Scholar]
- 35.Barr S, Hill EW, Bayat A.Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. J Mech Behav Biomed Mater. 2017;75:75–81. [DOI] [PubMed] [Google Scholar]
- 36.Derby BM, Codner MA.Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg. 2015;135:113–124. [DOI] [PubMed] [Google Scholar]
- 37.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123. [DOI] [PubMed] [Google Scholar]
- 38.Kinney BM, Jeffers LL, Ratliff GE, et al. Silicone gel breast implants: science and testing. Plast Reconstr Surg. 2014;1341 Suppl47S–56S. [DOI] [PubMed] [Google Scholar]
- 39.Haws MJ, Schwartz MR, Berger LH, et al. Sientra portfolio of Silimed brand shaped implants with high-strength silicone gel: a 5-year primary augmentation clinical study experience and a postapproval experience-results from a single-surgeon 108-patient series. Plast Reconstr Surg. 2014;1341 Suppl38S–46S. [DOI] [PubMed] [Google Scholar]
- 40.Henderson PW, Nash D, Laskowski M, et al. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg. 2015;39:724–732. [DOI] [PubMed] [Google Scholar]
- 41.Chao AH, Garza R, III, Povoski SP.A review of the use of silicone implants in breast surgery. Expert Rev Med Devices. 2016;13:143–156. [DOI] [PubMed] [Google Scholar]
- 42.Niechajev I, Jurell G, Lohjelm L.Prospective study comparing two brands of cohesive gel breast implants with anatomic shape: 5-year follow-up evaluation. Aesthetic Plast Surg. 2007;31:697–710. [DOI] [PubMed] [Google Scholar]
- 43.Jewell ML, Jewell JL.A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J. 2010;30:51–65. [DOI] [PubMed] [Google Scholar]
- 44.Al-Ajam Y, Marsh DJ, Mohan AT, et al. Assessing the augmented breast: a blinded study comparing round and anatomical form-stable implants. Aesthet Surg J. 2015;35:273–278. [DOI] [PubMed] [Google Scholar]
- 45.Doren EL, Pierpont YN, Shivers SC, et al. Comparison of Allergan, Mentor, and Sientra contoured cohesive gel breast implants: a single surgeon’s 10-year experience. Plast Reconstr Surg. 2015;136:957–966. [DOI] [PubMed] [Google Scholar]
- 46.Atlan M, Bigerelle M, Larreta-garde V, et al. Characterization of breast implant surfaces, shapes, and biomechanics: a comparison of high cohesive anatomically shaped textured silicone, breast implants from three different manufacturers. Aesthetic Plast Surg. 2016;40:89–97. [DOI] [PubMed] [Google Scholar]
- 47.Hidalgo DA, Weinstein AL.Intraoperative comparison of anatomical versus round implants in breast augmentation: a randomized controlled trial. Plast Reconstr Surg. 2017;139:587–596. [DOI] [PubMed] [Google Scholar]
- 48.Martin SV, Ho W, Khan K.An extended 7-year review of textured breast implants for primary breast augmentation: Allergan versus Mentor. Ann Breast Surg. 2019;3:14. [Google Scholar]
- 49.Nam SY, Lee M, Shin BH, et al. Characterization of BellaGel SmoothFine implant surfaces and correlation with capsular contracture. J Biomater Nanobiotechnol. 2019;10:196–211. [Google Scholar]
- 50.Song JW, Kim HM, Bellfi LT, et al. The effect of study design biases on the diagnostic accuracy of magnetic resonance imaging for detecting silicone breast implant ruptures: a meta-analysis. Plast Reconstr Surg. 2011;127:1029–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


