Abstract
Congenital generalized lipodystrophy type 4 is an extremely rare autosomal recessive disorder. We report our clinical experience on two unrelated Turkish patients with congenital generalized lipodystrophy type 4. A 13-year-old girl (patient-1) presented with generalized lipodystrophy and myopathy. Further tests revealed ventricular and supraventricular arrhythmias, gastrointestinal dysmotility, atlantoaxial instability, lumbosacral scoliosis, and metabolic abnormalities associated with insulin resistance. A 16-year-old girl (patient-2) with congenital generalized lipodystrophy type 4 was previously reported. Here, we report on her long term clinical follow-up. She received several course of anti-arrhythmic treatments for catecholaminergic polymorphic ventricular tachycardia and rapid atrial fibrillation. An implantable cardioverter defibrillator was also placed. A homozygous PTRF mutation, c.259C > T (p.Gln87*), was identified in patient-1. Congenital generalized lipodystrophy type 4 was caused by homozygous PTRF c.481–482insGTGA (p.Lys161Serfs*41) mutation in patient-2. Our data indicate that patients with congenital generalized lipodystrophy type 4 should be meticulously evaluated for cardiac, neuromuscular, gastrointestinal and skeletal diseases, as well as metabolic abnormalities associated with insulin resistance.
Keywords: Arrhythmia, Lipodystrophy, Insulin resistance, Myopathy, PTRF
1. Introduction
Congenital generalized lipodystrophy (CGL) is a rare autosomal recessive disorder characterized by near total absence of adipose tissue at birth or shortly thereafter (Garg, 2011). Until now, mutations in four different genes have been linked to CGL. Mutations in 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2) (Agarwal et al., 2002), Berardinelli-Seip congenital lipodystrophy 2 (BSCL2) (Magre et al., 2001) and caveolin 1 (CAV1) (Kim et al., 2008) genes were identified in CGL type 1, type 2 and type 3, respectively. Simha et al. (Simha et al., 2008) reported a novel subtype of CGL associated with muscular weakness and cervical spine instability in two Mexican American siblings. Afterwards, mutations in the polymerase I and transcript release factor (PTRF) gene, encoding a caveolar-associated protein that is essential for formation of caveolae and proper localization, were reported by Hayashi et al. (Hayashi et al., 2009), resulting in a similar phenotype which was called as CGL type 4.
In addition to generalized lipodystrophy and myopathy, CGL type 4 presents gastrointestinal dysmotility, skeletal abnormalities, and cardiac arrhythmias (Simha et al., 2008; Hayashi et al., 2009; Shastry et al., 2010). Rajab et al. (Rajab et al., 2010) reported mutations in PTRF in eight families with CGL and myopathy of whom five members had died from sudden cardiac death during their teenage years. Electrocardiography (ECG) studies from these patients revealed long-QT interval, bradycardia, as well as supraventricular and ventricular tachycardias. Our previous report suggested that CGL type 4 was associated with catecholaminergic polymorphic ventricular tachycardia (CPVT) (Shastry et al., 2010). CPVT is characterized by stress induced ventricular tachycardia, and has a mortality rate of 30% in symptomatic patients when not treated (Kontula et al., 2005).
Here, we report our clinical experience on two young Turkish girls with CGL type 4. Both affected subjects underwent genetic, laboratory and radiological studies. We also report long-term clinical follow-up of one patient regarding metabolic abnormalities and persistent cardiac arrhythmias.
2. Clinical report
2.1. Patient-1
This 13-year-old Turkish girl was admitted with complaints of difficulty on walking and atypical appearance. She was born after full-term gestation via spontaneous vaginal delivery with a birth weight of 3600 g. Parents were reportedly not consanguineous; however they were from the same Anatolian town with a population of around 7000 people. Her parents first noticed increased musculature when she was 9 months of age. She was discovered to have elevated CK levels when she was 15 months old. Developmental milestones were mildly delayed. She was able to hold her head up at 4 months. She was able to walk at 18 months. There was no family history for lipodystrophy or muscular dystrophy.
Physical examination showed generalized lack of subcutaneous fat, acromegaloid appearance, large tongue, umbilical prominence, muscular hypertrophy and prominent veins in the limbs. Weight and height were at 25th percentiles, and head circumference was normal for age. “Mounding” and Percussion Induced Rapid Contractions (PIRCs) could be elicited in response to taps at the deltoid, biceps and forearm muscles with reflex hammer (Fig. 1a). She exhibited good strength of 5/5 in extremities. Deep tendon reflexes were normal. No acanthosis nigricans was visible.
Fig. 1.

1a: “Mounding” and Percussion Induced Rapid Contractions (PIRCs) elicited in response to taps at the biceps muscle with reflex hammer (arrow). 1b: Whole body MRI, TSE T1 weighted coronal (i), TSE T2 fat suppressed coronal (ii) and TSE T1 axial images of the thorax (iii), abdomen (iv) and mid-thighs (v) show near total loss of subcutaneous and intra-abdominal fat. Bone marrow fat is preserved. Liver shows increased signal intensity due to hepatic steatosis. 1c: Figure shows a homozygous exon 1 PTRF mutation, c.259C > T (p.Gln87*) in patient-1. Her parents and a sibling were carriers for the heterozygous mutation c.259C > T. 1d: Lateral radiographs of the cervical spine during extension (i) and flexion (ii) show atlantoaxial instability. The atlantoaxial space is measured as 3.5 mm in flexion. 1e: AP (i) and lateral (ii) radiographs of the thoracolumbar spine show lumbosacral scoliosis. 1f: Holter recordings reveal non-sustained ventricular tachycardia episodes (i and ii), and supraventricular tachycardia episodes consistent with atrial tachycardia (iii). All arrhythmia episodes are observed when patient’s baseline sinus heart rate is increased. 1g: During the treadmill stress test, non-sustained ventricular tachycardia (i) develops at 6th minute of the exercise on Bruce Protocol at stage 3 with a sinus heart rate of approximately 140 beats per minute. Afterwards, atrial tachycardia (ii) develops at 7th minute of the exercise on Bruce Protocol at stage 3.
Blood tests showed an elevated CK level of 1496 IU/L (normal range: 29–168 IU/L) and normal levels of AST (33 IU/L, normal range: <50 IU/L) and ALT (47 IU/L, normal range: <50 IU/L). Her fasting serum triglycerides were 321 mg/dL (normal range: 40–150 mg/dL), HDL cholesterol: 22 mg/dL (normal range: >50 mg/dL), and LDL cholesterol: 37 mg/dL (normal range: 30–160 mg/dL). Her fasting glucose level was 107 mg/dL (normal range: 70–100 mg/dL). Fasting C-peptide level was 4.34 ng/mL (normal range: 1.1–4.4 ng/mL), and insulin level was 8.64 μIU/mL (normal range: <25 μIU/mL). HOMA score was 2.29. Serum leptin level was undetectable (<0.1 ng/mL; normal range: 3.4–13 ng/mL).
Generalized lipodystrophy was demonstrated by whole body magnetic resonance imaging (MRI) (Fig. 1b). Genotyping revealed a homozygous PTRF null mutation, c.259C > T in exon 1 (p.Gln87*). Her parents and a sibling were carriers for the heterozygous mutation c.259C > T (Fig. 1c).
Atlantoaxial instability was detected on cervical spine X-ray (Fig. 1d). Gastrointestinal dysmotility was evident on barium radiographs. X-rays of the thoraco-lumbar spine revealed moderate lumbosacral scoliosis (Fig. 1e). Resting ECG was normal with a normal QT interval. Echocardiogram did not show any structural abnormalities. Holter monitoring revealed several runs of asymptomatic non-sustained ventricular tachycardia and supraventricular tachycardia episodes consistent with atrial tachycardia (Fig. 1f). During the treadmill stress test (Fig. 1g), non-sustained ventricular tachycardia developed at 6th minute of the exercise on Bruce Protocol at stage 3 with a sinus heart rate of approximately 140 beats per minute. Afterwards, atrial tachycardia developed at 7th minute of the exercise on Bruce Protocol at stage3. She was commenced on propranolol (4 mg/kg/day).
2.2. Patient-2
This 11-year-old Turkish girl was first referred to our clinic because of scoliosis and atypical appearance and has been reported previously (Shastry et al., 2010). Blood tests revealed a CK level of 2337 IU/L (normal range: 29–168 IU/L). Serum leptin level was 0.58 ng/mL (normal range: 3.4–13 ng/mL). Near total lack of fat was demonstrated by whole body MRI (Fig. 2a). She was diagnosed with CGL type 4 caused by a homozygous insertion, c.481–482insGTGA in exon 2 of PTRF which was predicted to result in a frame shift making an abnormal protein p.Lys161Serfs*41. Her parents and a sibling were carriers for the heterozygous mutationc.481–482insGTGA (Fig. 2b).
Fig. 2.
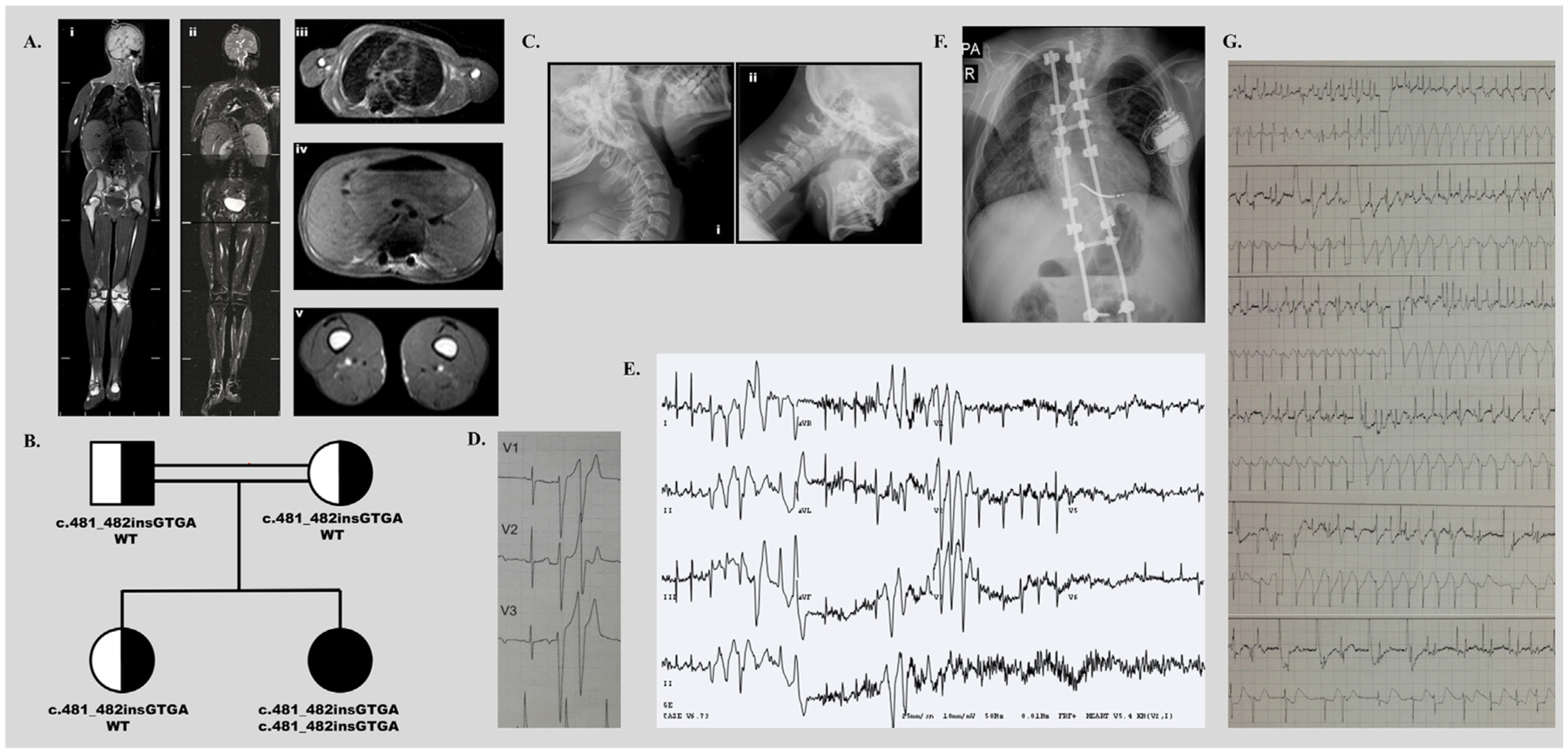
2a: Whole body MRI, TSE T1 weighted coronal (i), TSE T2 fat suppressed coronal (ii) and TSE T1 axial images of the thorax (iii), abdomen (iv) and mid-thighs (v) show near total loss of subcutaneous and intra-abdominal fat. Bone marrow fat is preserved. Also evident is left sided thoracic scoliosis. 2b: A homozygous insertion, c.481–482insGTGA), in exon 2 of PTRF was predicted to result in a frame shift making an abnormal protein p.Lys161Serfs*41 in patient-2. Her parents and a sibling were carriers for the heterozygous mutation c.481–482insGTGA. 2c: Lateral radiographs of the cervical spine during extension (i) and flexion (ii) show atlantoaxial instability. 2d: Holter monitor tracing showing baseline normal sinus rhythm with two consecutive beats of ventricular premature contractions. 2e: Bidirectional VT develops after 9 min of exercise on Bruce Protocol at stage 4 with a sinus heart rate of approximately 200 beats per minute, suggesting CPVT. 2f: The ICD is placed. Also evident is left sided thoracic scoliosis. The rods and screws of the corrective surgery for thoracic scoliosis are also seen. 2g: The ICD recording shows that the patient develops rapid atrial fibrillation. The device applies several shocks of 34.6 J to convert her arrhythmia to normal sinus rhythm. Instead, she experiences a CPVT episode triggered by the erroneous ICD shocks. After the 6th shock, the device gets exhausted and the arrhythmia terminates spontaneously. (Top) Atrial electrogram. (Bottom) Ventricular electrogram.
Atlantoaxial instability was evident (Fig. 2c). She had tight heel cords due to shortening of the Achilles tendon. Scoliosis was severe on radiological studies (Fig. 2a and f). She underwent surgeries for scoliosis and shortening of the Achilles tendon during follow-up (at age 13). No arrhythmias or untoward events were reported and the patient recovered well from these surgeries. She had gastrointestinal dysmotility. Her triglyceride (109 mg/dL, normal range: 40–150 mg/dL) and LDL cholesterol (63 mg/dL, normal range: 30–160 mg/dL) levels were within the normal ranges; however HDL cholesterol level was low (23 mg/dL, normal range: >50 mg/dL). Thereafter, she developed mild hypertriglyceridemia at age 15. Her recent fasting glucose level was 106 mg/dL (normal range: 70–100 mg/dL), C-peptide level was 6.32 ng/mL (normal range:1.1–4.4 ng/mL), and insulin level was 28.46 μIU/mL (normal range: <25 μIU/mL) with a HOMA score of 7.45.
At 12 years of age, she first complained of intermittent palpitations that initiated a cardiac evaluation. On ECG, there was no evidence of QT prolongation. Echocardiogram was normal. 24-h ECG monitoring revealed non-sustained episodes of VT and polymorphic VES beats (Fig. 2d). An exercise study was conducted which revealed several runs of asymptomatic bidirectional VT that strongly resembled CPVT. She was commenced on propranolol (4 mg/kg/day) which resulted in resolution of palpitations for 3 months. However, repeat Holter monitoring showed episodes of CPVT. The patient was switched to metoprolol, but CPVT episodes didn’t resolve. The exercise stress test showed CPVT on stage 4 under the beta blocker therapy during her follow-up (Fig. 2e). ICD implantation was performed (Fig. 2f). During a follow up of 12 months on ICD, no device-related local complication was observed. Several short traces of CPVT were recorded, all of which converted to sinus rhythm spontaneously without any need of ICD shock. The records showed that she experienced several episodes of rapid atrial fibrillation. One of these episodes initiated ICD shocks which triggered a CPVT episode. She received 6 subsequent shocks of 34.6 J, which didn’t resolve the arrhythmia each time. After the 6th shock, the device got exhausted and the arrhythmia terminated spontaneously (Fig. 2g). ICD set-up was reviewed and adjusted accordingly. Verapamil (6 mg/kg/day) and flecainide (100 mg/m2/day) were added to her anti-arrhythmic treatment.
This case study was approved by the Dokuz Eylul University Ethics Review Panel. Both patients and parents provided written informed consent.
3. Discussion
CGL type 4 caused by PTRF mutations represents an extremely rare form of generalized lipodystrophy which is characterized by unique clinical characteristics as shown in our patients. CGL type 4 is an autosomal recessive disorder. The coexistence of myopathy, arrhythmias, gastrointestinal dysmotility and skeletal abnormalities should prompt a diagnosis of CGL, type 4, in a case of generalized lack of fat.
It is well known that generalized lipodystrophy is associated with severe insulin resistance (Garg, 2011). On the other hand, Hayashi et al. (Hayashi et al., 2009) reported no metabolic abnormalities associated with insulin resistance in their original report. In contrast, both of our patients exhibited signs of insulin resistance such as IFG, hypertriglyceridemia, low HDL cholesterol levels, and hepatic steatosis. Patient-2 had no metabolic abnormalities associated with insulin resistance apart from low HDL cholesterol when she was first diagnosed with CGL type 4. However, she developed metabolic abnormalities in the course of the disease. Several further case studies also reported some evidence of insulin resistance in CGL type 4 (Shastry et al., 2010; Murakami et al., 2013). Metabolic abnormalities may be less severe in patients with CGL type 4 compared to other subtypes of CGL such as CGL type 1 and CGL type 2 (Garg, 2011).
The underlying mechanism of CPVT in patients with CGL type 4 is unknown. However, it is very likely that CPVT occurs as a result of disrupted caveolae formation in patients with CGL type 4. Various cardiac ion channels are closely associated with caveolae immunoreactivity (Lohn et al., 2000). Recent evidence suggests that caveolae play an important role in modulating sodium current in the heart (Besse et al., 2011). Caveolin 3 is the major component of caveolae and is highly expressed in the cardiac muscles (Tang et al., 1996). It has been demonstrated that caveolin 3 co-localizes with the cardiac sodium channel (Yarbrough et al., 2002). Studies have shown that caveolin 3 regulates functions of ion channels such as SCN5A-encoded cardiac sodium channels (SCN5A) via several mechanisms (Head et al., 2005; Feron and Kelly, 2002). In previous studies, beta adrenergic receptor stimulation was reported to increase current densities of caveolar SCN5A (Frohnwieser et al., 1997; Palygin et al., 2008). Supporting these data, caveolin 3 mutations were reported in patients with both type 9 long QT syndrome (Vatta et al., 2006) and sudden infant death syndrome (Cronk et al., 2007). PTRF is a highly abundant caveolae component which is suggested to have an essential role in caveolae formation (Chadda and Mayor, 2008). It has been shown that no morphologically detectable caveolae was present in mice lacking PTRF (Liu et al., 2008). Sward et al. (Sward et al., 2013) reported increased pulmonary artery pressure and increased right ventricular mass in cavin-1/PTRF deficient mice.
Little is known about the treatment of CPVT in children. Beta-blockers are used traditionally; however, treatment failure is not uncommon. Newer options such as flecainide and left cardiac sympathetic denervation can be considered; however they are not well validated in children. Therefore, ICDs are usually needed despite numerous device-related complications (Roston et al., 2015). In patient-2, once beta blockers were not sufficient to completely stop CPVT episodes, we resorted to ICD implantation to prevent sudden cardiac death. After the placement of ICD, she experienced one CPVT episode triggered by erroneous ICD shock in response to rapid atrial fibrillation. Atrial fibrillation has been reported previously in a 27-year-old Japanese male with CGL type 4 (Hayashi et al., 2009), and both of our patients also developed atrial arrhythmias. An episode of rapid atrial fibrillation triggered false ICD shocks in patient-2. The device applied several shocks to convert her atrial fibrillation to normal sinus rhythm. Instead, the patient developed CPVT which didn’t respond to recurrent ICD shocks, and terminated spontaneously. It is not clear why her CPVT episode could not be terminated by the ICD shocks. On the other hand, the ICD shocks may have prevented degeneration to ventricular fibrillation (VF). It has been proposed that high adrenergic load, which preceded CPVT and which intensified because of the stressful effect of recurrent shocks, may be associated with the treatment failure (Marai et al., 2012). This observation highlights the importance of concomitant use of beta blockers along with ICD.
4. Conclusion
CGL type 4 is a unique form of generalized lipodystrophy associated with myopathy, cardiac arrhythmias, skeletal abnormities and gastrointestinal dysmotility. CGL type 4 is associated with metabolic abnormalities secondary to insulin resistance. Cardiac arrhythmias are a life-threatening condition, which should be screened in all patients with CGL type 4. The role of ICD for preventing sudden cardiac death caused by CPVT in patients with CGL type 4 remains unclear. Further follow-up of other patients may clarify the benefits and risks of ICD implantation to prevent CPVT.
Acknowledgements
The authors thank to Dr.Ozgur Aslan for his valuable comments on ICD recordings.
Footnotes
Competing interests
The authors declare that they have no competing financial interests.
References
- Agarwal AK, Arioglu E, De Almeida S, et al. , 2002. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet 31, 21–23. [DOI] [PubMed] [Google Scholar]
- Besse IM, Mitchell CC, Hund TJ, Shibata EF, 2011. A computational investigation of cardiac caveolae as a source of persistent sodium current. Front. Physiol 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadda R, Mayor S, 2008. PTRF triggers a cave. Cell 132, 23–24. [DOI] [PubMed] [Google Scholar]
- Cronk LB, Ye B, Kaku T, et al. , 2007. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm 4, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Kelly RA, 2002. Gaining respectability: membrane-delimited, caveolar-restricted activation of ion channels. Circ. Res 90, 369–370. [DOI] [PubMed] [Google Scholar]
- Frohnwieser B, Chen LQ, Schreibmayer W, Kallen RG, 1997. Modulation of the human cardiac sodium channel alpha-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J. Physiol 498 (Pt 2), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, 2011. Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab 96, 33132–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi YK, Matsuda C, Ogawa M, et al. , 2009. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest 119, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, et al. , 2005. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J. Biol. Chem 280, 31036–31044. [DOI] [PubMed] [Google Scholar]
- Kim CA, Delepine M, Boutet E, et al. , 2008. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab 93, 1129–1134. [DOI] [PubMed] [Google Scholar]
- Kontula K, Laitinen PJ, Lehtonen A, Toivonen L, Viitasalo M, Swan H, 2005. Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights. Cardiovasc Res. 67, 379–387. [DOI] [PubMed] [Google Scholar]
- Liu L, Brown D, McKee M, et al. , 2008. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn M, Furstenau M, Sagach V, et al. , 2000. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ. Res 87, 1034–1039. [DOI] [PubMed] [Google Scholar]
- Magre J, Delepine M, Khallouf E, et al. , 2001. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet 28, 365–370. [DOI] [PubMed] [Google Scholar]
- Marai I, Khoury A, Suleiman M, et al. , 2012. Importance of ventricular tachycardia storms not terminated by implantable cardioverter defibrillators shocks in patients with CASQ2 associated catecholaminergic polymorphic ventricular tachycardia. Am. J. Cardiol 110, 72–76. [DOI] [PubMed] [Google Scholar]
- Murakami N, Hayashi YK, Oto Y, et al. , 2013. Congenital generalized lipodystrophy type 4 with muscular dystrophy: clinical and pathological manifestations in early childhood. Neuromuscul. Disord 23, 441–444. [DOI] [PubMed] [Google Scholar]
- Palygin OA, Pettus JM, Shibata EF, 2008. Regulation of caveolar cardiac sodium current by a single Gsalpha histidine residue. Am. J. Physiol. Heart Circ. Physiol 294, H1693–H1699. [DOI] [PubMed] [Google Scholar]
- Rajab A, Straub V, McCann LJ, et al. , 2010. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 6, e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roston TM, Vinocur JM, Maginot KR, et al. , 2015. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ. Arrhythm. Electrophysiol 8, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A, 2010. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am. J. Med. Genet. A 152A, 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simha V, Agarwal AK, Aronin PA, Iannaccone ST, Garg A, 2008. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. Am. J. Med. Genet. A 146A, 2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward K, Sadegh MK, Mori M, Erjefalt JS, Rippe C, 2013. Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF. Physiol. Rep 1, e00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, et al. , 1996. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem 271, 2255–2261. [DOI] [PubMed] [Google Scholar]
- Vatta M, Ackerman MJ, Ye B, et al. , 2006. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114, 2104–2112. [DOI] [PubMed] [Google Scholar]
- Yarbrough TL, Lu T, Lee HC, Shibata EF, 2002. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ. Res 90, 443–449. [DOI] [PubMed] [Google Scholar]


