Abstract
Background
Emerging evidence suggests that a subset of coronavirus disease 2019 (COVID-19) patients may present with or develop cerebrovascular disease during the course of hospitalization. Whereas ischemic stroke in COVID-19 patients has been well described, data on intracranial hemorrhage (ICH) in these patients is still limited. We, therefore, conducted a rapid systematic review of current scientific literature to identify and consolidate evidence of ICH in COVID-19 patients.
Methods
A systematic search of literature was conducted between November 1, 2019, and August 14, 2020, on PubMed and China National Knowledge Infrastructure (CNKI) to identify eligible studies.
Results
A total of 23 studies describing ICH in 148 COVID-19 patients were included. The pooled incidence of ICH in COVID-19 patients was 0.7% (95% CI 0.5–0.9), with low levels of inter-study heterogeneity observed (I2 = 33.6%, Cochran’s Q = 12.05, p = 0.149). Most of the patients were elderly male patients (65.8%) with comorbidities, the most common being systemic hypertension (54%). Hemorrhage involving multiple cranial compartments was reported in 9.5% of cases. Single compartments were involved in the rest, with intraparenchymal hemorrhage (IPH) being the most common variety (62.6%) and intraventricular hemorrhage (IVH) the least common (1.4%). Half of these patients were on some form of anticoagulation. Overall, the mortality rate in the COVID-19 patients with ICH was about 48.6%.
Conclusion
Although relatively uncommon among COVID-19 patients, ICH is associated with a high mortality rate. Early identification of patients at risk of developing ICH, particularly with comorbid conditions and on anticoagulant therapy, may be important to improve outcomes.
Keywords: COVID-19, Intracranial hemorrhage, Hemorrhagic stroke
Introduction
Recent reports have highlighted the relationship between coronavirus disease 2019 (COVID-19) and cerebrovascular disease (CVD). Past history of CVD has been associated with poor outcomes among COVID-19 patients [1–3]. On the other hand, a subset of these patients develops CVD during the course of hospitalization [4, 5]. Whereas ischemic CVD, which has been attributed to a hypercoagulable state characterized by micro- and macrovascular thrombotic angiopathy [6, 7], is more common and is described in literature [4, 5, 8, 9], reports on hemorrhagic CVD in these patients are few and scattered [10–13]. We, therefore, conducted a rapid systematic review of current scientific literature to identify and consolidate data on the incidence, age and sex distribution, clinical presentation, types, and clinical outcomes of intracranial hemorrhage (ICH) in COVID-19 patients.
Methods
A rapid systematic review of scientific literature was conducted to consolidate currently available data on intracranial hemorrhage (ICH) in COVID-19 patients. Rapid reviews accelerate the process of evidence synthesis while maintaining a systematic approach.
Literature search strategy
A comprehensive and systematic search of literature from November 1, 2019, to August 14, 2020, was conducted on the Medline (PubMed interface) and China National Knowledge Infrastructure (CNKI) to identify studies eligible for inclusion. The electronic search was carried out Boolean operators and using the strategy as follows: (COVID-19) AND (((((((stroke) OR (hemorrhagic stroke)) OR (intracerebral hemorrhage)) OR (subarachnoid hemorrhage)) OR (cerebrovascular disease)) OR (neurological manifestation))). No language restriction was applied. When the articles were published by the same study group and there was an overlap of the search period, only the most recent article was included to avoid duplication of data. The PubMed function “related articles” was used to extend the search. Also, we searched major infectious disease, neurology, and general medicine journals reporting articles about COVID-19 infection to identify additional studies. We then performed hand-search of the bibliography of included studies, to detect other potentially eligible investigations.
Eligibility criteria
The search results were screened by title and abstract, with those of potential relevance evaluated by full text. Studies were deemed eligible for inclusion if they fulfilled the following criteria: (1) were case reports/case series/cohort studies, (2) included patients with a reverse transcriptase polymerase chain reaction (RT-PCR)-confirmed COVID-19 diagnosis, (3) monitored the patients for development of complications during the course of admission, and (4) reported clear extractable data on hemorrhagic stroke.
Data extraction
Data extraction was conducted by two independent reviewers (I.C and B.N). For each study, the following information was extracted: the surname of the first author and the year of publication, country where the study was performed, the type of study (case report/case series/cohort), sample size, demographic characteristics, number of patients with intracranial hemorrhage, type of intracranial hemorrhage, anticoagulation prior to onset of hemorrhagic event, comorbidities, and mortality rate. Any variances arising during this were resolved by a consensus.
Synthesis of findings
Synthesis of results was carried out in two steps. First, findings on all eligible studies reporting intracranial hemorrhage in COVID-19 patients were presented in the form of a summary of findings table (Table 1) accompanied by a narrative description. Thereafter, a pooled analysis incorporating only cohort studies in which all hospitalized patients were studied within a specified period of time was conducted to estimate pooled incidence of intracranial hemorrhage in COVID-19 patients using the Meta-Analyst (software version 5.26.14, Center for Evidence-Based Medicine, Brown University, Providence, USA). A random effects model was applied. The magnitude of heterogeneity among the included studies was assessed using the chi-square test (Chi2) and I-squared statistic (I2). For the Chi2 test, a Cochrane’s Q p value of < 0.10 was considered significant. An I2 of < 40% was considered not significant. Additionally, a leave-one-out sensitivity analysis was performed to assess the robustness of the results and to further probe the sources of inter-study heterogeneity.
Table 1.
Characteristics of the included studies
| Author | Setting | Type of study | Sample size | No. with hemorrhagic cerebrovascular disease | Type of hemorrhagic event | Age and sex composition of the patient(s) | Initial symptoms (neurologic vs respiratory) | Time interval between COVID-19 and stroke | Comorbid conditions | Anticoagulation prior to stroke? | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-olama 2020 | UAE | Case report | 1 | 1 | IPH (lobar) and SDH | M, 36 years | Respiratory | 5 days | None | None | Survival |
| Al-Saiegh 2020 | USA | Case series | 2 | 1 | SAH (PICA aneurysm rupture) | M, 31 years | Respiratory | 1 week | None | None | Survival |
| Haddadi 2020 | Iran | Case report | 1 | 1 | IPH (bilateral basal ganglia) | F, 54 years | Respiratory | 5 days | DM, HTN | None | Survival |
| Heman-Acka 2020 | USA | Case series | 2 | 2 | IPH (lobar) |
F, 58 years M, 46 years |
Respiratory | 19 and 13 days, respectively |
DM, SLE HTN, OSA |
Heparin for VV-ECMO (within therapeutic range) | Death in both patients |
| Mao 2020 | China | Cohort | 214* | 1 | IPH (lobar) | M, 60 years | Respiratory | 10 days | HTN | None | Death |
| Morassi 2020 | Italy | Case series | 6 | 2 | IPH (cerebellar and cerebral lobar) |
M, 57 years M, 57 years |
Respiratory | 7 days and 22 days, respectively |
HTN None in the 2nd patient |
Prophylactic 4000 IU enoxaparin daily subcutaneously |
Death in both patients |
| Muhammad 2020 | Germany | Case report | 1 | 1 | IPH (lobar) with ventricular extension (pericallosal aneurysm) | F, 60 years | Neurologic | Not reported | Not reported | None | Survival |
| Romero-Sánchez 2020 | Spain | Cohort | 841* | 3 | Not reported | Not reported | Respiratory | Not reported | Not reported | Not reported | Not reported |
| Scullen 2020 | USA | Cohort | 27** | 3 | IPH (lobar) | Not reported | Respiratory | Not reported | Not reported | Therapeutic heparin (for elevated D-dimers) | Not reported |
| Sharif-Razavi 2020 | Iran | Case report | 1 | 1 | MCH (IPH [lobar], IVH & SAH | M, 79 years | Respiratory | 3 days | None | None | Not reported |
| Hernández-Fernández 2020 | Spain | Cohort | 1683* | 5 | IPH (lobar, 4 patients; basal ganglia bleed, 1 patient); SAH (2 patients) | 51, 61, 64, 68 and 69 years; 1 female and 4 males | Respiratory in 4 patients; neurologic in 1 patient | 12 days (median) | HTN (4 patients); DM and dyslipidemia (2 patient) | 3 on enoxaparin 1 mg/kg/twice a day | Death in 2 patients (40%) |
| Karadas 2020 | Turkey | Cohort | 239* | 2 | IPH (lobar) | Not reported | Respiratory | Not reported | Not reported | Not reported | Not reported |
| Li 2020 | China | Cohort | 219* | 1 | IPH (lobar) | M, 60 years | Respiratory | 10 days | HTN | Not reported | Death |
| Pinna 2020 | USA | Case series | 650* | 8 | IPH (lobar) (4 patients); SAH (non-aneurysmal) (4 patients) | Not reported | Respiratory (6 patients); neurologic (2 patients) | Not reported | Not reported | Not reported | Not reported |
| Pons-Escoda 2020 | Spain | Cohort | 112** | 7 | IPH (lobar, 4 patients); basal ganglia hemorrhage, 3 patients) |
6 males; 1 female Age: 49–78 years |
Respiratory | Not reported | HTN (4 patients); DM (2 patients); hypercholesterolemia (1 patient) | Not reported | Not reported |
| Reddy 2020 | USA | Case series | 12 | 2 | IPH (lobar) |
F, 60 years M, 48 years |
Not reported | 2 and 20 days, respectively | HTN and DM | Not reported | Death |
| Sweid 2020 | USA | Case series | 22 | 3 | SAH (PICA, PComm, and ACA aneurysms) | Not reported | Respiratory | 3 days, 9 days and 9 days, respectively | Not reported | None | Not reported |
| Varatharaj 2020 | UK | Cohort | 153** | 9 | IPH (lobar) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Altschul 2020 | USA | Cohort* | 5227 | 35 | SDH (17 patients); SAH (2 patients); MCH (7 patients); IPH (lobar) (9 patients) |
21 males, 14 females Median age: 67 years |
Not reported | Not reported | HTN (25 patients); DM (10 patients); CHF (6 patients); CAD (2 patients) | Anticoagulation in 7 patients | Death in 16 patients (45.7%) |
| Dogra 2020 | USA | Cohort* | 3824 | 33 | IPH (lobar) |
26 males, 7 females Mean age: 61.6 years |
Respiratory, 29 patients; neurologic, 4 patients | 17 days (median) | HTN (12 patients); DM (10 patients); CAD (4 patients); dyslipidemia (12 patients) | Anticoagulation in 22 patients | Death in 14 patients (42.4%) |
| Mehpour 2020 | Iran | Case series | 10 | 1 | IPH (lobar) | 75 years | Not reported | Not reported | Not reported | Not reported | Not reported |
| Nawabi 2020 | Germany, France, and Switzerland | Cohort** | 18 | 18 | SDH (1 patient); IVH (3 patients); SAH (13 patients); IPH (lobar) (6 patients) |
9 males, 9 females Median age: 49.50 |
Respiratory, 16 patients; neurologic, 2 patients | Not reported | HTN (10 patients); DM (4 patients) | Anticoagulation in 8 patients | Death in 8 patients (44.4%) |
| Rothstein 2020 | USA | Cohort* | 844 | 8 | IPH (lobar) (5 patients); SAH (3 patients) |
4 males, 4 females Mean age 57 ± 7 |
Respiratory symptoms in all | 25 days | HTN (6 patients); DM (3 patients); dyslipidemia (5 patients); CAD (3 patients); obesity (3 patients) | Anticoagulation for VV-ECMO in 4 patients | Death in 6 patients (76%) |
MCH multicompartmental hemorrhage, IPH intraparenchymal hemorrhage, IVH intraventricular hemorrhage SAH subarachnoid hemorrhage, PICA posterior inferior cerebellar artery, PComm posterior communicating artery, SDH subdural hemorrhage, M male, HTN hypertension, DM diabetes mellitus, SLE systemic lupus erythematosus, OSA obstructive sleep apnea
*All hospitalized COVID-19 patients were included in the study
**Only COVID-19 patients with neurologic complications were included
Results
Study identification
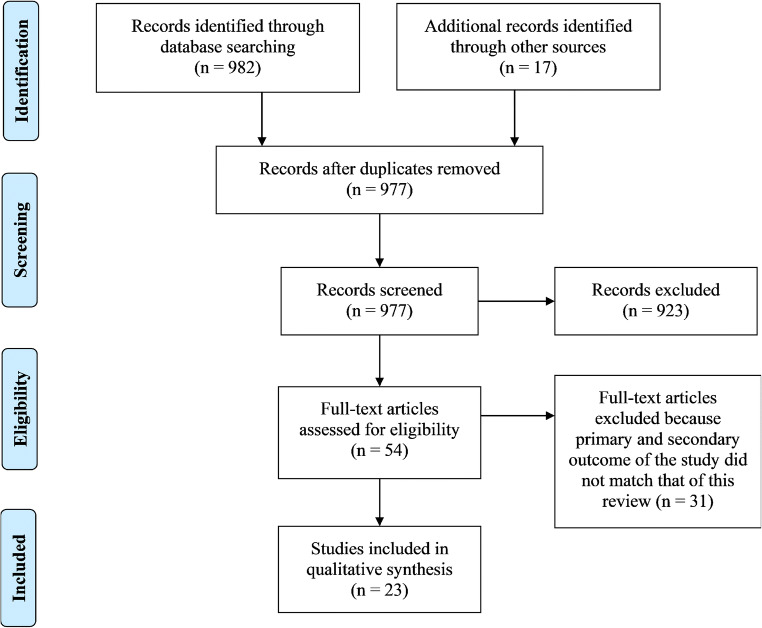
The initial search produced 999 potentially relevant articles. Following the removal of duplicates and primary screening, 54 articles were assessed by full text for eligibility in the meta-analysis. Of these, 31 were excluded because the primary and secondary outcome of the study did not match that of this review. Thus, a total of 23 articles were included in this systematic review and meta-analysis (Fig. 1 and Table 1).
Fig. 1.
PRISMA flow diagram indicating flow of studies through the review
Characteristics of the included studies
A total of 23 studies describing intracranial hemorrhage (ICH) in 148 COVID-19 patients were included [10–32]. Majority of the studies were from North America (USA, 9 studies) and Europe (8 studies). The rest were from the Middle East (4 studies) and Asia (2 studies). Of the included studies, twelve were cohort, six were case series, while the rest were case reports. Essential characteristics of the included are outlined in Table 1.
Data synthesis
Incidence of intracranial hemorrhage in COVID-19 patients
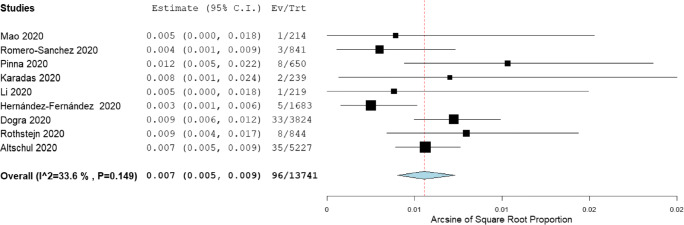
Nine cohort studies (n = 13,741 patients) reported data on the incidence of intracranial hemorrhage (ICH) in COVID-19 patients, with the incidence ranging from 0.3 to 1.2% [10, 13, 16, 19–22, 26, 28]. The pooled incidence of ICH across these nine studies was 0.7% (95% CI 0.5–0.9), with low levels of inter-study heterogeneity observed (I2 = 33.6%, Cochran’s Q = 12.05, p = 0.149) (Fig. 2). No significant changes in the pooled incidence were observed in the leave-one-out sensitivity analysis.
Fig. 2.
Forest plot for the pooled incidence of intracranial hemorrhage in COVID-19 patients
Age and sex distribution of COVID-19 patients with intracranial hemorrhage
Majority of the COVID-19 patients with intracranial hemorrhage were male (65.8%). The reported age of these patients ranged from 31 to 78 years. Across all case reports and case series, only 16% of patients were < 50 years old. The mean or median age of the patients was > 50 years in all but one cohort study.
Types of intracranial hemorrhage among COVID-19 patients
Hemorrhage involving multiple cranial compartments (MCH) was reported in 14 cases (9.5%). Single compartments were involved in the rest, with intraparenchymal hemorrhage (IPH) being the most common variety (62.6%), followed by subarachnoid hemorrhage (SAH) (15.0%), subdural hemorrhage (SDH) (11.6%), and intraventricular hemorrhage (IVH) (1.4%). In patients with IPH, the most location of the bleed was the cerebral lobes (93.5%). Other sites included basal ganglia (5.4%) and cerebellum (1.1%).
Initial symptom (respiratory vs neurologic)
Majority (71%) of the patients were admitted due to respiratory symptoms of COVID-19 and developed the intracranial hemorrhage (ICH) in their course of admission. The interval between the onset of respiratory symptoms and diagnosis of ICH ranged from 2 to 25 days. The rest (21%) were admitted due to neurological symptoms, such as acute loss of consciousness and sudden onset severe headache, and were later confirmed to have COVID-19 through RT-PCR tests.
Comorbid conditions in the COVID-19 patients with intracranial hemorrhage
Majority of the patients had pre-existing illnesses such as hypertension [10–12, 16–19, 21, 22, 25, 27, 28], diabetes mellitus (DM) [10, 16–19, 25, 27, 28], hyperlipidemia [16, 19, 27, 28], coronary artery disease (CAD) [10, 16, 28], obesity [28], congestive heart failure (CHF) [10], obstructive sleep apnea (OSA) [18], and systemic lupus erythematosus (SLE) [18].
Anticoagulation prior to onset of intracranial hemorrhage
Data on anticoagulation in COVID-19 patients prior to onset of ICH was reported in 8 (n = 114 patients) [10, 11, 16, 18, 19, 25, 28, 29]. Overall, 58 patients (50.9%) were on some form of anticoagulation. The indication for anticoagulation was part of in-hospital treatment for COVID-19 (standard prophylaxis [11, 16, 18, 19], elevated D-dimers [16, 29], and extracorporeal membrane oxygenation (ECMO) [25, 28]) in majority of these patients (84%). The rest were on therapeutic anticoagulation for non-COVID-19 indications [10, 16].
Mortality in COVID-19 patients with intracranial hemorrhage
Across the 14 studies (n = 111 patients) (Table 1) that reported data on mortality in COVID-19 patients with ICH, the mortality rate was 48.6%.
Discussion
This review of the literature provides a comprehensive and systematic analysis of intracranial hemorrhage (ICH) in COVID-19 patients. The incidence of ICH was found to be 0.7% (95% CI 0.5–0.9), which is lower than the incidence of ischemic stroke which has been reported to develop in about 1.2% of these patients [33].
The role of the severe acute respiratory syndrome 2 (SARS-CoV-2) virus in the development of ICH in COVID-19 patients is still unclear. Majority of these patients had classic Framingham risk factors such as advanced age, being male, and pre-existing illnesses such as hypertension and diabetes mellitus, which are well-established risk factors for vascular degenerative changes, that could have predisposed them to the development of ICH [34, 35]. Further, a significant proportion of patients were on some form of anticoagulation therapy, which could have predisposed them to the development of ICH. This is consistent with a recent retrospective study of 3824 COVID-19 patients by Melmed and colleagues [36] in which anticoagulation was associated with a 5-fold increase (OR = 5.26, 95% CI 2.22–12.24) in the risk of ICH. The association between anticoagulation and risk of ICH in COVID-19 patients was confirmed. Some of the patients however had no prior illnesses or risk factors that could explain the ICH [14, 15], leading to speculations about possible causal role of the SARS-CoV-2 virus.
Several hypotheses have subsequently been put forward. First, it has been postulated that SARS-CoV-2 is neutropic [37, 38] and can invade and directly damage cerebral blood vessels, facilitated by the overexpression of angiotensin converting enzyme 2 (ACE2) [39, 40], the viral entry protein for SARS-CoV-2, within vascular endothelium. This may result in endotheliitis, characterized histologically by diffuse endothelial damage and mononuclear infiltration [40]. This hypothesis is further supported by recent electron microscopic studies that have demonstrated the presence of viral inclusion particles within the endothelium, and viral RNA detection in cerebrospinal fluid [41, 42]. Second, entry of the SARS-CoV-2 virus into cells results in marked reduction in ACE-2 levels [43]. Since this protein usually catalyzes conversion of angiotensin II to counter-regulatory angiotensin 1-7 [44, 45], reduction in its levels results in enhanced and unopposed effects angiotensin II via the ACE-angiotensin II-AT1 receptor axis [43–45]. These effects, mediated by angiotensin II, vasopressin, and aldosterone, include vasoconstriction, water and sodium reabsorption, as well as vascular wall inflammation [45], all of which could contribute to development of ICH. This is supported by pre-clinical studies in which an inverse relationship between ACE2 levels and the occurrence of hypertension has been observed [46]. Clinical studies have also highlighted on adverse blood pressure changes in COVID-19 patients. For instance, Vicenzi et al. [47] in their study of 40 COVID-19 patients demonstrated significant rise in the systemic blood pressure with deterioration in the pulmonary function, even in patients without prior history of hypertension. Third, a subset of COVID-19 patients usually develops a systemic hyperinflammatory syndrome characterized by fulminant hypercytokinemia [48, 49], which may mediate vascular remodeling and predispose to ICH. The pro-inflammatory cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) are potent activators of matrix metalloproteinases (MMPs), a group of proteolytic enzymes that degrade elastin, collagen, and other components of the extracellular matrix (ECM) [50]. Such alterations result in loss of vascular wall integrity, increasing risk of rupture and hemorrhage, as has been well documented in other vascular degenerative diseases such as aortic aneurysms [51]. The cytokines may also activate the coagulation cascade, resulting in thrombotic microangiopathy (TMA) of the vasa-vasora which may result in arterial wall hypoxia, undermining vascular integrity and leading to rupture [52].
Our findings suggest that COVID-19 patients who develop ICH experience poor outcomes, with mortality rates of approximately 49%. This is largely reflective of the distribution of the various types of ICH observed in these patients, where intraparenchymal hemorrhage which are known to have less favorable outcomes [53] were the most common. Our findings are consistent with a recent retrospective cohort of 3824 COVID-19 patients in which ICH was associated with increased mortality (OR = 2.6, 95% CI 1.2–5.9) [36]. The mortality rate observed in this study is also consistent with recent studies in which COVID-19 patients with stroke were shown to have worse functional outcome and higher mortality rates than non-COVID-19 stroke patients [54, 55].
Our study was limited by the small number of included studies, some of which mainly case reports and case series. There was insufficient data to perform meta-regression on incidence of ICH. Larger studies are needed to corroborate these findings.
Conclusion
Although relatively uncommon among COVID-19 patients, ICH is associated with a high mortality rate. Early identification of patients at risk of developing ICH, particularly with comorbid conditions and on anticoagulant therapy, may be important to improve outcomes.
Data availability
Data is available at reader’s request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 2.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM et al (2020) Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 191:148–150 [DOI] [PMC free article] [PubMed]
- 5.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S, Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, Lavinio A, Besser M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viguier A, Delamarre L, Duplantier J, Olivot J-M, Bonneville F (2020) Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol 47(5):393–394 [DOI] [PMC free article] [PubMed]
- 10.Altschul DJ, Unda SR, de La Garza RR, Zampolin R, Benton J, Holland R, et al. Hemorrhagic presentations of COVID-19: risk factors for mortality. Clin Neurol Neurosurg. 2020;198:106112. doi: 10.1016/j.clineuro.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy ST, Reddy ST, Garg T, Shah C, Nascimento FA, Imran R, et al. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. 2020;77030:199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, González E, Redondo-Peñas I, Perona-Moratalla AB, del Valle-Pérez JA, Gracia-Gil J, Rojas-Bartolomé L, Feria-Vilar I, Monteagudo M, Palao M, Palazón-García E, Alcahut-Rodríguez C, Sopelana-Garay D, Moreno Y, Ahmad J, Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T et al (2020) Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry 91(8):1–3 [DOI] [PubMed]
- 15.Al-olama M, Rashid A, Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir. 2020;162(7):1495–1499. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, Lewis A, Melmed K, Hochman K, Horwitz L, Galetta S, Berger J. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddadi K, Ghasemian R, Shafizad M (2020) Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019. Cureus 12(4): e7869 [DOI] [PMC free article] [PubMed]
- 18.Heman-Ackah SM, Su YRS, Spadola M, Petrov D, Chen HI, Schuster J, Lucas T. Neurologically devastating intraparenchymal hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation: a case series. Neurosurgery. 2020;87(2):E147–E151. doi: 10.1093/neuros/nyaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Fernández F, Valencia HS, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C et al (2020) Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain J Neurol 143(10):3089–3103 [DOI] [PMC free article] [PubMed]
- 20.Karadaş Ö, Öztürk B, Sonkaya AR. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 2020;41(8):1991–1995. doi: 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 5(3):1–6 [DOI] [PMC free article] [PubMed]
- 22.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrpour M, Shuaib A, Farahani M, Hatamabadi H, Fatehi Z, Ghaffari M et al (2020) Coronavirus disease 2019 and stroke in Iran: a case series and effects on stroke admissions. Int J Stroke (online). 10.1177/1747493020937397 [DOI] [PMC free article] [PubMed]
- 24.Muhammad S, Petridis A, Cornelius JF, Hänggi D. Letter to editor: severe brain haemorrhage and concomitant COVID-19 Infection: a neurovascular complication of COVID-19. Brain Behav Immun. 2020;87:150–151. doi: 10.1016/j.bbi.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawabi J, Morotti A, Wildgruber M, Boulouis G, Kraehling H, Schlunk F, et al. Clinical and imaging characteristics in patients with SARS-CoV-2 infection and acute intracranial hemorrhage. J Clin Med. 2020;9(8):1–14. doi: 10.3390/jcm9082543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, Osteraas ND, Pellack DR, Asthana A, Fegan K, Patel V, Conners JJ, John S, Silva ID. Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415(May):116969. doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons-Escoda A, Naval-Baudín P, Majós C, Camins A, Cardona P, Cos M et al (2020) Neurologic involvement in COVID-19: cause or coincidence? A neuroimaging perspective. Am J Neuroradiol (online). 10.3174/ajnr.A6627 [DOI] [PMC free article] [PubMed]
- 28.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL (2020) Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke 51(9):e219–e222 [DOI] [PMC free article] [PubMed]
- 29.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437–e446. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR et al (2020) Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke 0(0):1–10 [DOI] [PMC free article] [PubMed]
- 32.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, Coles JP, Manji H, al-Shahi Salman R, Menon DK, Nicholson TR, Benjamin LA, Carson A, Smith C, Turner MR, Solomon T, Kneen R, Pett SL, Galea I, Thomas RH, Michael BD, Allen C, Archibald N, Arkell J, Arthur-Farraj P, Baker M, Ball H, Bradley-Barker V, Brown Z, Bruno S, Carey L, Carswell C, Chakrabarti A, Choulerton J, Daher M, Davies R, di Marco Barros R, Dima S, Dunley R, Dutta D, Ellis R, Everitt A, Fady J, Fearon P, Fisniku L, Gbinigie I, Gemski A, Gillies E, Gkrania-Klotsas E, Grigg J, Hamdalla H, Hubbett J, Hunter N, Huys AC, Ihmoda I, Ispoglou S, Jha A, Joussi R, Kalladka D, Khalifeh H, Kooij S, Kumar G, Kyaw S, Li L, Littleton E, Macleod M, Macleod MJ, Madigan B, Mahadasa V, Manoharan M, Marigold R, Marks I, Matthews P, Mccormick M, Mcinnes C, Metastasio A, Milburn-McNulty P, Mitchell C, Mitchell D, Morgans C, Morris H, Morrow J, Mubarak Mohamed A, Mulvenna P, Murphy L, Namushi R, Newman E, Phillips W, Pinto A, Price DA, Proschel H, Quinn T, Ramsey D, Roffe C, Ross Russell A, Samarasekera N, Sawcer S, Sayed W, Sekaran L, Serra-Mestres J, Snowdon V, Strike G, Sun J, Tang C, Vrana M, Wade R, Wharton C, Wiblin L, Boubriak I, Herman K, Plant G. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Y-K, Goh C, Leow AST, Tambyah PA, Ang A, Yap E-S et al (2020) COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis 50(3):587–595 [DOI] [PMC free article] [PubMed]
- 34.Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An SJ, Kim TJ, Yoon B-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An Update. J Stroke. 2017;19(1):3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melmed KR, Cao M, Dogra S, Zhang R, Yaghi S, Lewis A, Jain R, Bilaloglu S, Chen J, Czeisler BM, Raz E, Lord A, Berger JS, Frontera JA (2020) Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis (online). 10.1007/s11239-020-02288-0 [DOI] [PMC free article] [PubMed]
- 37.Yachou Y, El Idrissi A, Belapasov V, Ait BS (2020) Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci 41:2657–2669 [DOI] [PMC free article] [PubMed]
- 38.Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bermejo-Martin J, Almansa R, Torres A, Gonzalez-Revera M, Kelvin DJ. COVID-19 as cardiovascular disease: the potential role of chronic endothelial dysfunction [Internet] Cardiovasc Res. 2020;116:e132–e133. doi: 10.1093/cvr/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silhol F, Sarlon G, Deharo J-C, Vaïsse B. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: should we block the renin–angiotensin system? Hypertens Res. 2020;43(8):854–856. doi: 10.1038/s41440-020-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciulla MM. SARS-CoV-2 downregulation of ACE2 and pleiotropic effects of ACEIs/ARBs. Hypertens Res. 2020;43(9):985–986. doi: 10.1038/s41440-020-0488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 2019;44(6):1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicenzi M, Cosola RD, Ruscica M, Ratti A, Rota I, Rota F, et al. The liaison between respiratory failure and high blood pressure: evidence from COVID-19 patients. Eur Respir J. 2020;56:2001157. doi: 10.1183/13993003.01157-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A et al (2020) COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist 26(5-6):402–414 [DOI] [PubMed]
- 49.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 52.Martin JF, Booth RFG, Moncada S. Arterial wall hypoxia following thrombosis of the vasa vasorum is an initial lesion in atherosclerosis. Eur J Clin Investig. 1991;21(3):355–359. doi: 10.1111/j.1365-2362.1991.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 53.Al-Mufti F, Thabet AM, Singh T, El-Ghanem M, Amuluru K, Gandhi CD. Clinical and radiographic predictors of intracerebral hemorrhage outcome. Interv Neurol. 2018;7(1–2):118–136. doi: 10.1159/000484571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M et al (2020) Cerebrovascular complications of COVID-19. Stroke 51(9):e227–e231 [DOI] [PMC free article] [PubMed]
- 55.Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J et al (2020) Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke 51:e254–e258 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available at reader’s request.